Abstract
Background:
Transthoracic esophagectomy (TTE) and transhiatal esophagectomy (THE) are the two most common surgical approaches for carcinoma esophagus. Several studies have shown lymph nodal involvement to be one of the most important prognostic factors in carcinoma esophagus.
Aims:
The primary objective of this study was to explore the effectiveness of the ratio of positive lymph nodes to excised lymph nodes, namely the metastatic lymph nodal ratio (MLNR) as a prognostic factor in the survival of patients with carcinoma esophagus.
Settings and Design:
Retrospective analysis of a prospective database.
Materials and Methods:
A review of the operated esophageal cancer patients treated at a tertiary cancer center in South India between January 2002 and December 2006. Statistical analysis was done with the help of SPSS version 17 software (SPSS Inc., Chicago, IL). Proportions were compared using the Chi-square test. Survival data was generated using life table methods. Differences in survival estimates were compared using log-rank test.
Results and Conclusions:
Our study emphatically showed that the survival outcomes of patients with squamous cell carcinoma of the esophagus can be discriminated based on the MLNR groups, and it can be a reliable prognostic indicator. The overall survival for patients undergoing TTE, or THE for the entire cohort of patients was however not statistically significant. Whether a more aggressive TTE is a better esophageal cancer operation or whether MLNR is the factor that can significantly impact survival regardless of the technique is an issue that would require further investigation.
Keywords: Metastatic lymph nodal ratio, prognosis, transhiatal esophagectomy, transthoracic esophagectomy
Introduction
Esophageal cancer is ranked as one of the most deadly malignancies affecting humans with an estimated 5-year survival is about 14%. Among the various modalities of treatment; surgery has a definite role in the management of esophageal malignancies. The two most common approaches of surgery for carcinoma esophagus include transthoracic esophagectomy (TTE) and transhiatal esophagectomy (THE).
Lymph node metastasis in esophageal cancer is widely believed to be one of the most powerful prognostic indicators. More recent studies have explored the impact of lymph node metastases further, and investigators seem to agree that it is not only a question of the presence or absence of nodal disease, but more importantly, how many lymph nodes are involved with disease. The ratio of a number of positive nodes to the total number of nodes harvested, namely the metastatic lymph nodal ratio (MLNR) would, therefore, be of greater relevance.[1,2] We, in this study, have attempted to determine the effectiveness of MLNR in predicting the survival among the operated patients of squamous cell carcinoma (SCC) of the esophagus. The comparison between the approaches, THE and TTE have been raging on for decades. We have then attempted to use MLRN as a variable in establishing the noninferiority of THE over TTE.
Materials and Methods
This study was undertaken based on a retrospective review of the operated esophageal cancer patients treated at a tertiary cancer center in South India between January 2002 and December 2006. All patients were re-staged in accordance to the 7th edition of American Joint Committee on Cancer (AJCC) cancer staging manual.
The inclusion criteria for the present study were:
Histologically confirmed SCC of the thoracic esophagus
Patients who underwent upfront surgery (Either TTE with three field lymphadenectomy [TTE] or THE).
A total of 555 patients were diagnosed with carcinoma of the esophagus during the study period, of which 111 patients underwent curative surgery. Ninety-eight patients met the inclusion criteria for the study and were further taken up for the further analysis.
The three analytical steps were as follows:
Step 1: Grouping of patients
Grouping according to MLNR: The patients were classified into three revised nodal categories: (MLNR0 = 0), (MLNR1 ≥ 0 to ≤ 0.1), and (MLNR2 ≥ 0.1). The number of patients in pN0, pN1 and pN2 were 28.40 and 30, respectively
Grouping according to treatment modalities: We classified patients into two treatment subgroups: R × 1 = TTE and R × 2 = THE. The number of patients in R × 1 and R × 2 were 60 and 38 respectively.
Of the 98 patients, 4 patients were not included due to the reasons explained subsequently, making our effective cohort of 94 patients (58 patients in TTE subgroup and 36 patients in THE subgroup).
Step 2
To establish the effectiveness of using MLNR classifiers in predicting survival, we did an analysis of the pT2 (n = 20) and the pT3 (n = 74) subgroups in this cohort of patients. We did not analyze the pT1 (n = 3) and pT4 (n = 1) subgroups as there were very few patients in both these subgroups.
Step 3
After analyzing the utility of MLNR classifiers in predicting the survival of carcinoma esophagus patients, we then tried to extrapolate the MLNR to the treatment subgroups, namely TTE and THE and analyze the effectiveness MLNR in predicting the noninferiority of these two treatment modalities.
Statistical analysis
Statistical analysis was performed with the help of SPSS version 17 software (SPSS Inc., Chicago, IL) proportions were compared using the Chi-square test. Survival data was generated using life table methods. Differences in survival estimates were compared using log-rank test. Prognostic factors in the treatment groups were analyzed with the aid of Cox proportionate univariate and multivariate regression analysis.
Results
General patient characteristics
This study included 94 patients of whom 43 (45.7%) were males and 51 (54.3%) were females. The median age was 49.66 years (range: 21-69 years). The most common location of the tumor was in the lower thoracic esophagus (n = 61 [64.9%]) followed by middle thoracic (n = 31 [33%]) and upper thoracic esophagus.(n = 2 [2.1%]) [Table 1].
Table 1.
Patient characteristics
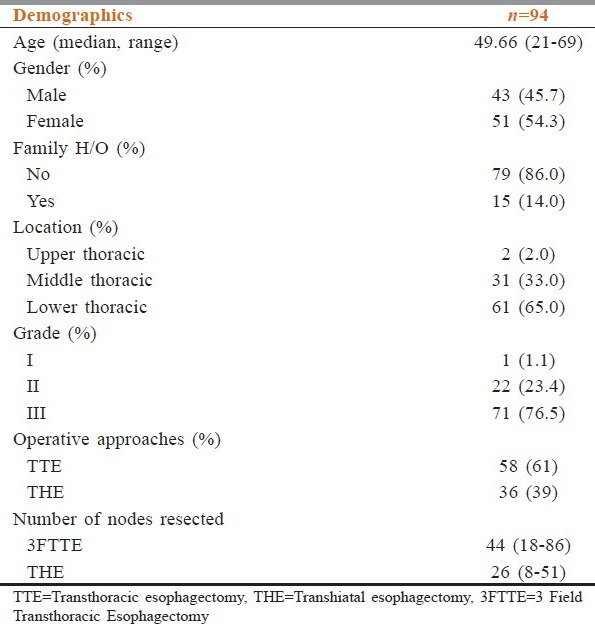
The operative technique was dictated by the location of the tumor, performance status of the patient as well as preference of the surgeon. Majority of patients (61.7%) underwent a TTE with three field lymphadenectomy, whereas 38.3% underwent esophagectomy via transhiatal approach. The average number of nodes resected by TTE was 44 (range: 18-86 nodes) and THE was 26 (8-51 nodes).
Metastatic lymph nodal ratio classifiers and pT staging
In the pT2 subgroup, the overall survival (OS) difference was statistically significant between the three MLNR subgroups (P = 0.05). The survival between the three MLNR categories also discriminated well the pT3 subgroup (P = 0.002) [Table 2 and Figure 1].
Table 2.
MLNR and survival analysis
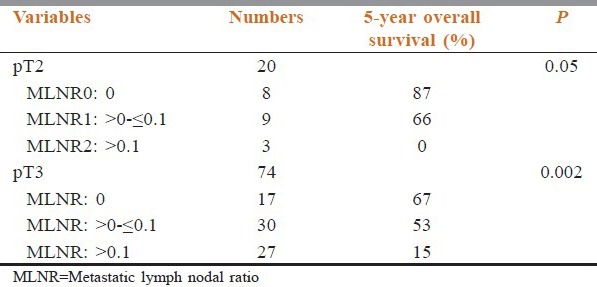
Figure 1.
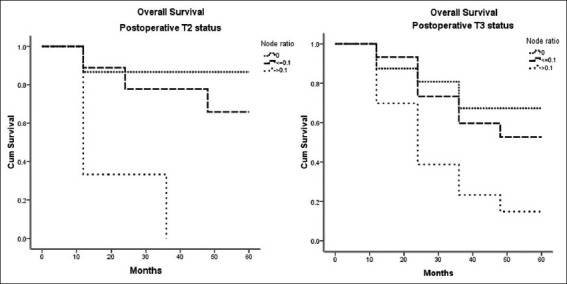
Overall survival graphs of the pT2 and pT3 patients the between the three metastatic lymph nodal ratio subgroups
Transthoracic esophagectomy versus transhiatal esophagectomy (for the entire cohort of 94 patients)
On the head to head comparison between 3 field transthoracic esophagectomy (3FTTE) (R × 1) and THE (R × 2), the 5-year OS of patients was not statistically significant (P = 0.389). The proportion of patients surviving at the end of 5-year of follow-up after having undergone TTE was 51% and that of THE was 40%.
Metastatic lymph nodal ratio classifiers and treatment sub-groups (R × 1 and R × 2)
For the MLNR0 subgroup, the cumulative proportion of patients surviving at the end of 5-year of follow-up in the TTE was 93% and in THE was 38%, which was of statistical significant (P = 0.025). For the MLNR1 subgroup, the cumulative proportion of patients surviving at the end of 5-year of follow-up in the 3FTTE was 51% and in THE was 66%%, which was not statistically significant (P = 0.145). For the MLNR2 subgroup, the cumulative proportion of patients surviving at the end of 5-year of follow-up in the TTE was 4% and in ‘THE’ was 20%, which was also not statistically significant (P = 0.862) [Table 3 and Figure 2].
Table 3.
MLNR and the two approaches of surgery
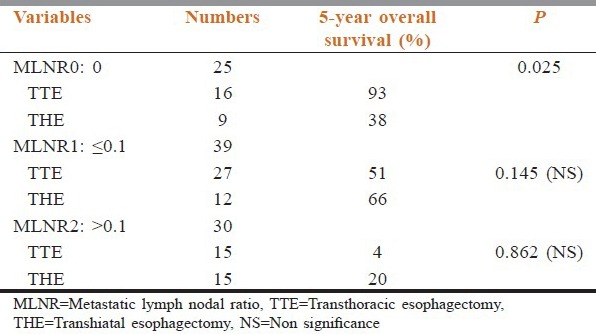
Figure 2.
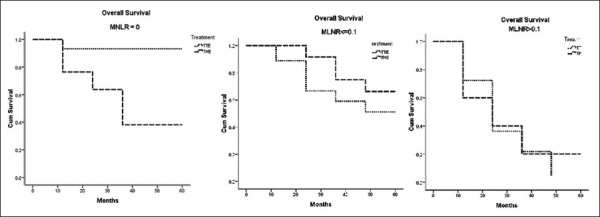
Overall survival graphs of the transthoracic esophagectomy and the transhiatal esophagectomy patients between the three metastatic lymph nodal ratio subgroups
Discussion
Surgical resection has traditionally remained the treatment of choice for carcinoma esophagus. But despite improvements in the operative techniques and the extent of lympadenectomy the OS continues to remain poor.[3]
Lymph nodal involvement is considered to be one of the most important prognostic factors in carcinoma esophagus.[4] Studies have clearly indicated that increasing number positive nodes, leads to graver the prognosis.[5,6] The current staging of carcinoma esophagus has included the number of metastatic lymph nodes for consideration of node classification. The question pertaining to the minimum number of lymph nodes that should be dissected during the performance of a lymphadenctomy in esophagectomy has been and continued a point of debate. The number of lymph nodes ranged anywhere from 6[7] to 23.[8] as the appropriate cut off for “adequate” lymphadenectomy. Greenstein et al.[9] and Yang et al.[10] recommended 18 nodes as the minimum number of resectable lymph nodes, whereas a consensus conference of the International Society for the Diseases of the Esophagus in 1995 suggested that accurate pathological staging of esophageal carcinoma requires resection of at least 15 nodes.[11] This issue assumes greater significance as the number of positive metastatic nodes is affected by the total number of nodes dissected, which is a definite confounding factor.
It was hence felt that an additional nodal evaluation in the form of MLNR would add to the prognostication of patients with potentially insufficient lymphadenectomy. The main goal of this study was to find the effectiveness of the ratio of positive lymph nodes to excised lymph nodes (MLNR) as prognostic factors in survival of patients with carcinoma esophagus.
Eloubeidi et al. reported that increasing MLNR was associated with a poorer prognosis.[12] Nigro et al. showed patients with an MLNR < 0.1 fared significantly better than those who had an MLNR ≥ 0.1.[6] Bollschweiler et al. reported that MLNR only became significant if it exceeded 0.20 (P < 0.01).[13]
Wilson et al. classified 144 patients into 4 groups according to the MLNR: 0, ≤25, >25–≤50, and > 50%.[14] Though an increasing MLNR was associated with a worsening 5-year survival in their study, statistical significance was not achieved (P = 0.153).
Feng et al. a retrospective analysis of 132 patients (>70 years) with esophageal SCC reported MLNR staging predicted survival similar to the 2010 AJCC N classification and felt that it should be considered an alternative to current N staging.[15] A similar view was echoed by some authors who, in fact, stated that patients are undergoing surgery for carcinoma esophagus should be staged according to MLNR because this more accurately predicted survival than current staging systems.
We were able to elucidate differences in survival of patients based upon the categorization of their MLNR as zero, less than 0.10 and greater than 0.10 which was statistically significant. After proving the significance of MLNR, we went one step further and evaluated the role of MLNR in the modality of surgery either TTE or THE. Both these approaches of esophagectomy have their respective advantages and disadvantages which have been elucidated in many previous studies.
A meta-analysis by Rindani included almost 5500 patients from 44 series published demonstrated a comparable 5-year survival between TTE and THE. Another meta-analysis by Hulscher, which involved over 7527 patients from 50 studies also showed no survival differences between TTE and THE. A more recent meta-analysis by Boshier also demonstrated no difference in 5-year OS between both the treatment groups. The authors however advised to view the finding of equivalent survival with caution as the extent of lymphadenectomy and the reported surgical quality was suboptimal in both groups and the TTE group had significantly more advanced cancers. A concern on the quality of treatment was echoed in a subsequent meta-analysis done by Yang et al., which also showed no significant differences of survival rate and postoperative morbidity and mortality between TTE resection group and non TTE resection group.
In our study, the 5-year OS for patients undergoing TTE or THE for the entire cohort of patients was not statistically significant. Though this to some extent stated the equality between the two procedures, we further extrapolated MLNR to the two treatment subgroups. For the MLNR0 subgroup, the 5-year OS of TTE was better than that of patients undergoing ‘THE’ (P = 0.025) For the MLNR1 and MLNR2 subgroups, the survival difference between the two treatment approaches were not statistically significant. The median number of lymph nodes dissected in the TTE group was 44, whereas it was 26 in the THE group [Table 1].
Although more extensive lymphadenectomy improves the surgical staging, the true impact of the same on survival is still controversial and is in part due to the effect of stage migration and distant disease relapse. The 7th edition of the AJCC staging manual on esophageal cancer has recommended resection of as many lymph nodes as possible and that more nodes should be dissected with increasing pT stage.(≥10 for T1, ≥20 for T2, and ≥30 for T3 and T4) It is therefore imperative from an oncological standpoint that, irrespective of the surgical technique, every effort must be made to resect as many regional lymph nodes as possible, as long as the resultant morbidity is acceptable.
The limitations in our study include the small number of patients in our study group. The numbers of patients in the pT1 and pT4 subgroups were too small to be included in the analysis. As this data is only from a single institution, we do believe that, more data from other centres needs to be amalgamated to validate our observation.
Conclusion
The OS of patients with SCC of the esophagus can be discriminated based on 3 groups: MLNR0, MLNR1 and MLNR2 and our study clearly has emphatically shown that it can be used as a reliable independent prognostic indicator. The OS for patients undergoing 3FTTE or THE for the entire cohort of patients was however not statistically significant. Whether a more aggressive TTE is a better esophageal cancer operation or whether MLNR by itself is the factor that can significantly impact survival regardless of the technique is an issue that would require further investigation. It is however also important that the decision regarding the approach to surgery for each patient must be individualized taking into account all the parameters that can possibly impact the final outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, et al. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–41. doi: 10.1097/SLA.0b013e3181656d07. [DOI] [PubMed] [Google Scholar]
- 2.Dhar DK, Hattori S, Tonomoto Y, Shimoda T, Kato H, Tachibana M, et al. Appraisal of a revised lymph node classification system for esophageal squamous cell cancer. Ann Thorac Surg. 2007;83:1265–72. doi: 10.1016/j.athoracsur.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Refaely Y, Krasna MJ. Multimodality therapy for esophageal cancer. Surg Clin North Am. 2002;82:729–46. doi: 10.1016/s0039-6109(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 4.Hagen JA, DeMeester SR, Peters JH, Chandrasoma P, DeMeester TR. Curative resection for esophageal adenocarcinoma: Analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520–30. doi: 10.1097/00000658-200110000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korst RJ, Rusch VW, Venkatraman E, Bains MS, Burt ME, Downey RJ, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115:660–69. doi: 10.1016/S0022-5223(98)70332-0. [DOI] [PubMed] [Google Scholar]
- 6.Nigro JJ, DeMeester SR, Hagen JA, DeMeester TR, Peters JH, Kiyabu M, et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960–8. doi: 10.1016/S0022-5223(99)70377-6. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Hu C, Zhang H, Ping Y, Chen LQ. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol. 2010;17:784–90. doi: 10.1245/s10434-009-0818-5. [DOI] [PubMed] [Google Scholar]
- 8.Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: An international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239–46. doi: 10.1002/cncr.23309. [DOI] [PubMed] [Google Scholar]
- 10.Yang HX, Xu Y, Fu JH, Wang JY, Lin P, Rong TH. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: Data from China. Ann Surg Oncol. 2010;17:1901–11. doi: 10.1245/s10434-010-0948-9. [DOI] [PubMed] [Google Scholar]
- 11.Fumagalli U, Akiyama H, DeMeester T. Resective surgery for cancer of the thoracic esophagus: Results of a consensus conference held at the VIth World Congress of the International Society for diseases of the esophagus. Dis Esophagus. 1996;9:30–8. [Google Scholar]
- 12.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S: The importance of tumor length and lymph node status. Cancer. 2002;95:1434–43. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 13.Bollschweiler E, Baldus SE, Schröder W, Schneider PM, Hölscher AH. Staging of esophageal carcinoma: Length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–63. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Rosato EL, Chojnacki KA, Chervoneva I, Kairys JC, Cohn HE, et al. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146:11–5. doi: 10.1016/j.jss.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JF, Huang Y, Chen L, Zhao Q. Prognostic analysis of esophageal cancer in elderly patients: Metastatic lymph node ratio versus 2010 AJCC classification by lymph nodes. World J Surg Oncol. 2013;11:162. doi: 10.1186/1477-7819-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]


