Abstract
Background:
Cisplatin is widely used as radio sensitizer in head and neck cancer (HNC) and carcinoma cervix (CaCx). This study aims to see comparative nephrotoxicity of cisplatin in HNC and in CaCx without obstructive uropathy treated by concurrent chemoradiotherapy (CCRT).
Materials and Methods:
Fifty patients of HNC and 50 patients of CaCx stage II/III without obstructive uropathy were included in this study. Cisplatin 50 mg intravenous weekly was given before EBRT with adequate hydration and premedication in both groups. Before chemotherapy; blood urea, serum creatinine, and glomerular filtration rate (GFR) were measured. GFR was measured using 99mTc diethylene triamine pentacaetic acid (DTPA) renogram study.
Results:
At the end of 4th week, blood urea level 41-45 mg% was in 40 and 4% in HNC and CaCx, respectively (P = 0.018). At the end of 3rd and 4th week, blood urea level >45 mg% was 10 and 6% in HNC cases, respectively. At the end of 4th week, serum creatinine level 1.1-1.5 mg% was 50 and 8% in HNC and CaCx, respectively (P = 0.047). Serum creatinine level >1.5 mg% was 6, 8, and 22% in HNC at the end of 2nd, 3rd, and 4th week, respectively. GFR <80 ml/min at the end of 4th week was 14% in HNC and only 2% in CaCx. GFR <100ml/min was significant at the end of 4th week (P = 0.04). Univariate analysis showed significant relation between reduced oral fluid intake and reduced GFR (P < 0.001).
Conclusion:
In HNC, during concurrent chemoradiation, as the 3rd-4th week is reached, oral mucosal reactions increase and affect oral intake which further add to the cisplatin-induced nephrotoxicity. In CaCx without obstructive uropathy, renal function impairment is less severe as oral intake of water and liquid is not much impaired.
Keywords: Carcinoma cervix, cisplatin-induced nephrotoxicity, diethylene triamine pentacaetic acid scan, glomerular filtration rate, head and neck cancer
Introduction
Cisplatin is widely used as radio sensitizer in head and neck cancer (HNC) and carcinoma cervix (CaCx). Dose-related and cumulative renal insufficiency, including acute renal failure, is the major dose-limiting toxicity of cisplatin. This study aims to see comparative nephrotoxicity of cisplatin in HNC and in CaCx without obstructive uropathy treated by concurrent chemoradiotherapy (CCRT). To best of our knowledge, such study is not available in any published literature.
Materials and Methods
Over a 2-years period from April 2011 to March 2013, 50 patients of CaCx and 50 patients of HNC, selected to receive treatment with CCRT were prospectively included in this study. The group allocation was purely random. All patients who were included in the study were histopathologically proved and registered cancer cases at the regional cancer institute.
Inclusion criteria for CaCx were histopathologically proven squamous cell carcinoma (SCC) cervix, International Federation of Gynecology and Obstetrics (FIGO) stage II and III without obstructive uropathy, age of 30-50 years, and Eastern Cooperative Oncology Group (ECOG) performance status 0-2. Inclusion criteria for HNC were histopathologically proven locally advanced SCC, patients of age of 40-60 years, and ECOG performance status 0-2. Exclusion criteria included any histology other than SCC; patients with hematological, cardiac, renal, or liver function abnormalities; patients having hypersensitivity to cisplatin; patients with any uncontrolled intercurrent illness. Pregnant women and patients who did not give consent for study was also excluded from study.
Both the groups were treated by CCRT. Patients of CaCx, fulfilling inclusion criteria, were planned for external beam radiotherapy (EBRT) delivered by 60Co teletherapy machine (Theratron 780C and E) and high dose rate intracavitary brachy therapy (HDR ICBT) by GAMMAMED-12i using 192Ir. Patients of HNC, fulfilling inclusion criteria, were planned for EBRT delivered by 60Co teletherapy machine (Theratron 780C and E). Total prescribed dose for CaCx was 50Gy by EBRT and 32.81Gy to point A by HDR ICBT. Total dose for HNC was 66-70Gy. Dose schedule of EBRT was 2Gy per fraction and 5 fractions in a week.
All the patients received four to six cycles of weekly cisplatin 50mg intravenous (IV) in 500 cc of 5% dextrose and normal saline over 1 h. Premedication consists of dexamethasone 8 mg IV, ranitidine 50 mg IV, and a 5-hydroxytryptamine type 3 (5HT3)-receptor antagonist IV as antiemetic with hydration for 2 h before and after chemotherapy with D5-NS at 150 cc/h.
Complete blood count and renal function tests were done prior to each cycle of chemotherapy. Renal function tests were included blood urea level, serum creatinine level, and glomerular filtration rate (GFR) measured by 99mTc diethylene triamine pentacaetic acid (DTPA). After injection into the venous system, the compound is excreted by the kidneys and its progress through the renal system can be tracked with a gamma camera.
During treatment, symptomatic treatment was given in patients suffering from side effects of CCRT. Adequate hydration and urinary output was maintained. In HNC patients who encountered oral mucosal reactions, Ryle's tube feeding was done. Fresh blood transfusion and buildup therapy was also given in needed patients.
Statistical analysis was done by Statistical Package for Social Sciences (SPSS) software version 10.0. Unpaired sample t-test and Chi-square test were used to calculate the significance level. Value of P < 0.05 was considered significant. Univariate analysis was performed to calculate the significance of decreased fluid intake during 3rd week of CCRT on GFR level.
Results
The patient characteristics at enrollment into the study are depicted in Table 1. The patients were well-balanced between the two groups. At the end of 4th week in HNC group, blood urea level was 31-40 mg% in 44% patients, 41-45 mg% in 40%, and >45 mg% in 6% patients; while in CaCx group it was in the range of 31-35 mg% in 42%, 36-40 mg% in 8%, and 41-45 mg% in 4% [Table 2].
Table 1.
Patient characteristics at enrollment into the study and estimated oral fluid intake of the patients during concurrent chemoradiotherapy 3rd week onwards
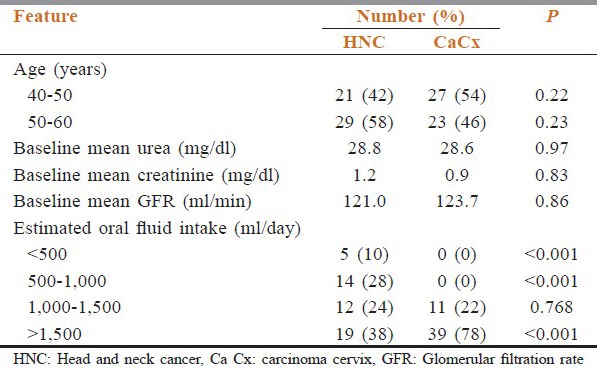
Table 2.
Levels of blood urea, serum creatinine and GFR at baseline and during radiotherapy
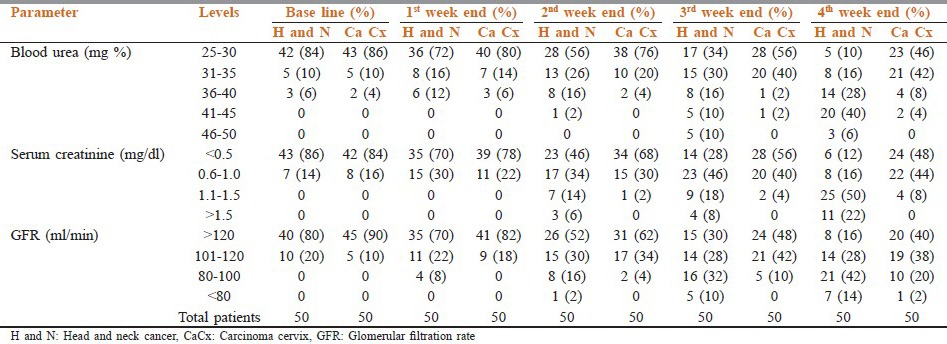
Serum creatinine level at the end of 4th week in HNC group was 1.1-1.5 mg% in 50% cases, while the same level in CaCx was seen in only 8% of cases. More than 1.5mg% level was seen in 22% of HNC group only [Table 2].
GFR at 4th week end in HNC group was in the range of 80-100ml/min in 42% of cases, while in CaCx same range was seen in only 20% of cases. More than 80 ml/min was seen in 14% of HNC group and 2% of CaCx cases [Table 2].
At the end of 4th week, blood urea level 41-45mg% was 40 and 4% in HNC and CaCx, respectively (P = 0.018). At the end of 3rd and 4th week, blood urea level >45 mg% was 10 and 6% in HNC cases only, respectively. At the end of 4th week, serum creatinine level 1.1-1.5 mg% was 50 and 8% in HNC and CaCx, respectively (P = 0.047). Serum creatinine level >1.5mg% was 6, 8, and 22% in HNC at the end of 2nd, 3rd, and 4th week, respectively. GFR <80 ml/min at the end of 4th week was 14% in HNC and only 2% in CaCx. GFR < 100 ml/min was significant at the end of 4th week (P = 0.04).
The estimated oral fluid intake of the patients during CCRT 3rd week onwards is shown in Table 1. It was significantly lower in patients of HNC as compared to CaCx (P < 0.001). Univariate analysis showed significant relation between reduced oral fluid intake and reduced GFR (P < 0.001).
Discussion
As time passed, experimental work and clinical experience in radiology accumulated and the basic radiobiological factors playing role in tumor and normal tissue to radiation became amenable to radiation oncologists. With this gain in radiobiological background, many radiation oncologists dared to evaluate clinical effectiveness of various chemotherapeutic agents used concurrently with radiotherapy as radio sensitizers.
Locally advanced HNC is a great challenge for oncologists. The most aggressive nonsurgical treatment is the combination of chemotherapy and radiation. The concurrent administration of chemotherapy and radiation has improved outcomes in a variety of clinical scenarios. These include locally advanced nasopharyngeal carcinomas, advanced unresectable cancers, organ preservation in locally advanced larynx and base of tongue cancers, and in high-risk postoperative patients.[1,2,3,4,5] Thus, CCRT is accepted as a standard option for these patients. Meta-analysis of chemotherapy on head and neck cancer (MACH-NC) demonstrated that the addition of chemotherapy concurrently to radiation therapy resulted in a 19% reduction in the risk of death and an overall 6.5% improvement in 5-year survival compared to treatment with EBRT alone (P < 0.0001).[6] While many regimens have been tested in these settings, high-dose cisplatin has been the most commonly studied agent.[7] It should be noted that the addition of chemotherapy concurrent with radiation is associated with a marked increase in both acute and late treatment effects. The dose-limiting toxicity is usually severe oral mucositis.
At present, the integration of radiosensitizing cisplatin-based chemotherapy concurrent with radiotherapy is considered the accepted standard in the management of high-risk patients with carcinoma of the cervix. In 1999, five large prospective randomized trials performed by the Gynecologic Oncology Group (GOG), Radiation Therapy Oncology Group (RTOG), and the South-West Oncology Group (SWOG) demonstrated significant survival advantage and superiority in reducing risk of death by 28-52% in cisplatin-based therapy given concurrently with pelvic radiotherapy when compared to either radiotherapy alone or radiotherapy in concurrent with non-platinum containing chemotherapy.[8,9,10,11,12] It was stated that cisplatin-based chemoradiotherapy also decreased the relative risk of recurrence and the mortality. Based on the results of these five randomized clinical trials, which consistently showed improved survival in patients treated with cisplatin-based CCRT, the National Cancer Institute (NCI) of the United States announced that “Strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with EBRT in women who require radiation therapy for treatment of cervical cancer” in 1999.[13] Although recently reported meta-analysis studies also demonstrated improved local control rates and survival with cisplatin-based chemotherapy concurrent to radiation therapy. Most widely accepted concurrent chemoradiation protocol is the combination of radiation and cisplatin administered once a week at a dose of 40 mg/m2 for 6 weeks.[14,15]
Cisplatin is one of the most commonly used drugs for CCRT. Through interactions with nucleophilic sites on deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), cisplatin introduces intra- and interstrand crosslinks; thereby, distorting the DNA structure and blocking nucleotide replication and transcription. Active in both hypoxic and well-oxygenated cells, several potential mechanisms for cisplatin-mediated radiation sensitization were reported and summarized by Wilson and coworkers.[16] It has been proposed that radiation induces free radicals and subsequently the formation of toxic platinum intermediates, which increase cell killing.[17] Moreover, ionizing radiation can increase cellular uptake of platinum.[18] Damage to DNA by ionizing radiation that typically would be repairable can become fixed and lethal through cisplatin's free electron-scavenging capacity. This inhibition of DNA repair leads to an increased incidence of cell cycle arrest and apoptotic cell death after radiation.
The main side effects of cisplatin are myelosuppression, gastrointestinal toxicity, nephrotoxicity, neurotoxicity, and ototoxicity. Dose-related and cumulative renal insufficiency, including acute renal failure, is the major dose-limiting toxicity of cisplatin. Cisplatin-induced renal cell death involves multiple pathways including oxidant stress, activation of intrinsic and extrinsic apoptotic cascades, and endonucleases.[19] Nephrotoxicity has been noted in 28-36% of patients treated with a single dose of 50 mg/m2. It is first noted during the 2nd week after a dose and is manifested by elevations in blood urea and serum creatinine levels and/or a decrease in creatinine clearance and GFR. Nephrotoxicity becomes more prolonged and severe with repeated courses of the drug. Renal function must return to normal before another dose of cisplatin can be given. The administration of cisplatin using a 4-6 h infusion with intravenous hydration, and mannitol has been used to reduce nephrotoxicity. However, nephrotoxicity still can occur after utilization of these procedures. Unfortunately, many of the same pathways mentioned above also contribute to the cytotoxic actions of cisplatin on tumor cells. Therefore, strategies intended to reduce cisplatin-induced nephrotoxicity may have the unintended consequences of reducing the antitumor actions of cisplatin. The design of preventive strategies must carefully consider this risk.[19]
In HNC, during CCRT, as the 3rd-4th week is reached, oral mucosal reactions increase and affect oral intake. In our study, we found significantly lower estimated oral fluid intake at this time in HNC patients as compared to CaCx during CCRT (P < 0.001). Univariate analysis showed significant relation between reduced oral fluid intake and reduced GFR (P < 0.001). During summer in our area, maximum temperature reaches 48-49°C that can further add to dehydration and leads to impaired renal function along with cisplatin-induced nephrotoxicity. In CaCx without obstructive uropathy, renal function impairment is less severe as oral intake of water and liquid is not much impaired. It is suggested that all patients of HNC should be treated with Ryle's tube feeding from 2nd week end onwards so that adequate hydration can be maintained and nephrotoxicity may be avoided.
Conclusion
In HNC, during concurrent chemoradiation, as the 3rd-4th week is reached, oral mucosal reactions increase and affect oral intake which further add to the Cisplatin induced nephrotoxicity. In CaCx without obstructive uropathy, renal function impairment is less severe as oral intake of water and liquid is not much impaired.
Acknowledgment
The support of Department of Radiation Oncology, Acharya Tulsi Regional Cancer Treatment and Research Institute, Bikaner, Rajasthan, India is gratefully acknowledged.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, le Maître A, Maillard E, Bourhis J MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,3416 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36:999–1004. doi: 10.1016/s0360-3016(96)00430-0. [DOI] [PubMed] [Google Scholar]
- 8.Stehman FB, Bundy BN, Kucera PR, Deppe G, Reddy S, O’Connor DM. Hydroxyurea, 5-fluorouracil infusion, and cisplatin adjunct to radiation therapy in cervical carcinoma: A phase I-II trial of the Gynecologic Oncology Group. Gynecol Oncol. 1997;66:262–7. doi: 10.1006/gyno.1997.4761. [DOI] [PubMed] [Google Scholar]
- 9.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 10.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and paraaortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 11.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 12.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 13.Washington: United States Department of Public Health; 1999. NCI Clinical Announcement: Concurrent Chemoradiation for Cervical Cancer. [Google Scholar]
- 14.Rose PG. Chemoradiotherapy for cervical cancer. Eur J Cancer. 2002;38:270–8. doi: 10.1016/s0959-8049(01)00352-5. [DOI] [PubMed] [Google Scholar]
- 15.Einstein MH, Novetsky AP, Garg M, Hailpern SM, Huang GS, Glueck A, et al. Survival and toxicity differences between 5-day and weekly cisplatin in patients with locally advanced cervical cancer. Cancer. 2007;109:48–53. doi: 10.1002/cncr.22369. [DOI] [PubMed] [Google Scholar]
- 16.Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation. Semin Radiat Oncol. 2006;16:2–9. doi: 10.1016/j.semradonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Richmond RC. Toxic variability and radiation sensitization by dichlorodiammineplatinum (II) complexes in Salmonella typhimurium cells. Radiat Res. 1984;99:596–660. [PubMed] [Google Scholar]
- 18.Yang LX, Douple EB, Wang HJ. Irradiation enhances cellular uptake of carboplatin. Int J Radiat Oncol Biol Phys. 1995;33:641–6. doi: 10.1016/0360-3016(95)00202-A. [DOI] [PubMed] [Google Scholar]
- 19.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin Nephrotoxicity. Toxins (Basel) 2010;2:2490–518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]


