Abstract
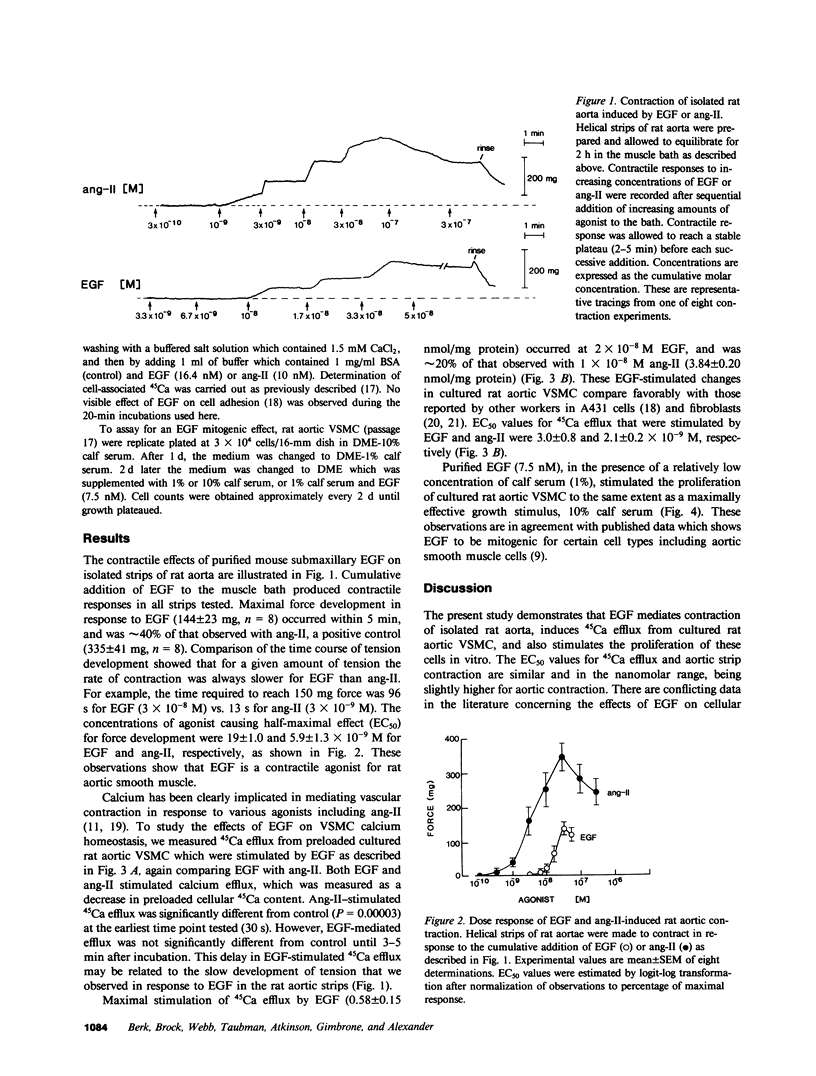
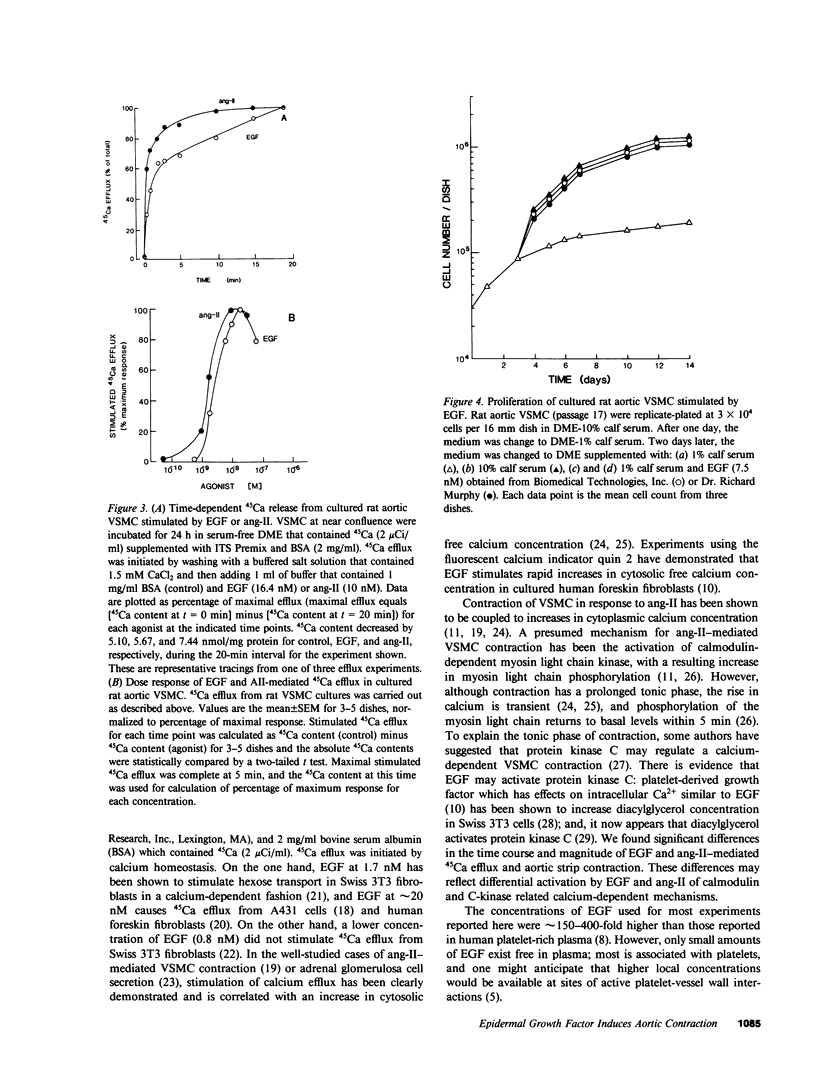
Atherosclerotic arteries have enhanced reactivity to vasoconstrictors, which suggests that features of the atherosclerotic process itself may result in this abnormal responsiveness. Since vascular smooth muscle proliferation is a prominent feature of atherosclerosis, we postulated that vasoactive agonists and smooth muscle mitogens may share certain common cellular mechanisms of action which potentially contribute to this hyperreactivity. To test this hypothesis, we studied the effects of epidermal growth factor (EGF), a well-characterized mitogen, on rat aortic vascular smooth muscle, both in intact aortic strips and in culture. EGF caused contraction (EC50 = 19 nM) of rat aortic strips which maximally was equivalent to 40% of that induced by angiotensin II, a potent vasoconstrictor. EGF increased 45Ca efflux (EC50 = 3 nM) from cultured rat aortic smooth muscle cells, which was an effect shared by angiotensin II and thought to reflect increased cytosolic-free calcium concentration. EGF (7.5 nM) also stimulated growth of these cultured cells to the same extent as 10% calf serum. These results demonstrate that EGF is both a vasoconstrictor and mitogen for rat aortic smooth muscle cells. The similarities in the effects of EGF and angiotensin II suggest that certain common intracellular mechanisms of action may exist for vasoactive agonists and growth factors which may contribute to the altered vasoreactivity of atherosclerotic vessels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Gimbrone M. A., Jr, Alexander R. W. Angiotensin II stimulates phosphorylation of the myosin light chain in cultured vascular smooth muscle cells. J Biol Chem. 1981 May 25;256(10):4693–4696. [PubMed] [Google Scholar]
- Chinkers M., McKanna J. A., Cohen S. Rapid rounding of human epidermoid carcinoma cells A-431 induced by epidermal growth factor. J Cell Biol. 1981 Feb;88(2):422–429. doi: 10.1083/jcb.88.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Foster R., Rasmussen H. Angiotensin-mediated calcium efflux from adrenal glomerulosa cells. Am J Physiol. 1983 Sep;245(3):E281–E287. doi: 10.1152/ajpendo.1983.245.3.E281. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Hirabayashi K., Giguère L., Tauber J. P. Factors controlling the proliferative rate, final cell density, and life span of bovine vascular smooth muscle cells in culture. J Cell Biol. 1981 Jun;89(3):568–578. doi: 10.1083/jcb.89.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Ku D. D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982 Nov 5;218(4572):576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Rozengurt E. Serum rapidly mobilizes calcium from an intracellular pool in quiescent fibroblastic cells. Biochem Biophys Res Commun. 1983 Jul 18;114(1):240–247. doi: 10.1016/0006-291x(83)91619-4. [DOI] [PubMed] [Google Scholar]
- Luchi R. J., Chahine R. A., Raizner A. E. Coronary artery spasm. Ann Intern Med. 1979 Sep;91(3):441–449. doi: 10.7326/0003-4819-91-3-441. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Efflux of 45Ca2+ from human fibroblasts in response to serum or growth factors. J Cell Physiol. 1983 Oct;117(1):23–29. doi: 10.1002/jcp.1041170105. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schwartz S. M. Cellular proliferation in atherosclerosis and hypertension. Proc Soc Exp Biol Med. 1983 May;173(1):1–13. doi: 10.3181/00379727-173-41601. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Tomoike H., Nabeyama S., Yamamoto H., Araki H., Nakamura M., Ishii Y., Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983 Aug 5;221(4610):560–562. doi: 10.1126/science.6408736. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15(1-3):198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Regulation of vascular tone, molecular mechanisms. Prog Cardiovasc Dis. 1981 Nov-Dec;24(3):213–242. doi: 10.1016/0033-0620(81)90029-3. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Nishino H., Iwashima A. Ca2+-dependent stimulation of hexose transport by A23187, 12-O-tetradecanoylphorbol-13-acetate and epidermal growth factor in mouse fibroblasts. Biochem Biophys Res Commun. 1983 Dec 16;117(2):637–642. doi: 10.1016/0006-291x(83)91248-2. [DOI] [PubMed] [Google Scholar]


