Abstract
Glycyrrhizic acid (GA) is a natural product with favorable antitumor activity. But, glycyrrhetinic acid monoglucuronide (GAMG) showed stronger antitumor activity than GA. It is of our interest to generate and identify novel compounds with regulation telomerase for cancer therapy. So, in this study, 18α-GAMG was synthesized via biotransformation. In vitro studies showed that it displayed potent anticancer activity and high selectivity on tumor liver cell SMMC-7721 versus human normal liver cell L-02. The further results in vivo confirmed that it could significantly improve pathological changes of N,N-diethylnitrosamine (DEN)-induced rat hepatic tumor. Western blot and immunofluorescence results indicated that the expression of p65-telomerase reverse transcriptase (TERT) was clearly down-regulated treated with it. Taken together, this study for the first time identified an active compound with high selectivity on tumor liver cell in mice. Furthermore, the title compound could inhibit the expression of protein p65 and TERT. These data support further studies to assess the rational design of more efficient p65 modulators in the future.
Telomerase is closely related to the occurrence and development of human cancer1,2,3. The latest research results of Juli Feigon et al show that telomerase synthesis is regulated by protein p65, when cut off the tail of p65, synthesis of telomerase is very limited4. The level of telomerase reverse transcriptase (hTERT) expression is the rate-limiting factor of telomerase complex, and most human somatic cells do not show detectable telomerase activity due to lacking expression of hTERT5,6,7,8. Therefore, if we can find active small molecule inhibiting the expression of p65 and hTERT, synthesis of telomerase is expected to be blocked, which will lead to tumor cells unexcessive proliferation.
Natural products or their derivatives play a major role in drug discovery9. Licorice is a well-known herb plant and has been widely used in traditional Chinese medicine for thousands of years. Glycyrrhizic acid (GA) is a natural product extracted from licorice root and is known to possess a wide range of pharmacological effects such as anti-inflammation and anti-tumor10,11,12. GA also has emerged as an attractive drug candidate for cancer therapy because of its inhibiting abnormal cell proliferation, tumor formation and growth13,14,15.
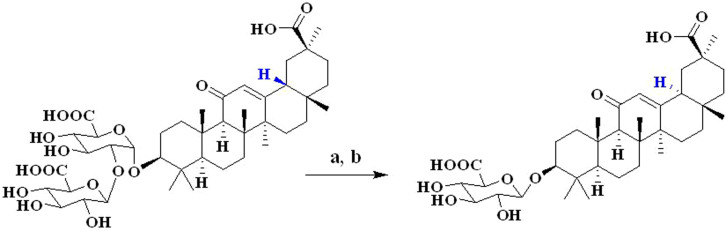
GA could be transformed into glycyrrhetinic acid monoglucuronide (GAMG) via biotransformation (Figure 1). Compared to GA, GAMG showed stronger pharmacological antitumor and anti-inflammatory activities16,17,18. In our previous study19, GAMGs were synthesized and we also found that GAMGs displayed higher anticancer activity than GA.
Figure 1. Synthesis of 18α-GAMG.
Reagents and conditions: (a) β-glucuroidase; (b) NaOH solution (5.0 M), 90°C, reflux, 12 h.
GAMGs can be divided into isomers of 18α-GAMG and 18β-GAMG (Figure 2). In this study, compound 18α-GAMG with high selectivity activity against human liver cancer (SMMC-7721) versus human normal liver cell (L-02) in vitro was discovered (Table 1, Table 2). In order to further evaluate anticancer activity of compound 18α-GAMG in vivo, effects of it on N,N-diethylnitrosamine (DEN)-induced rat hepatic tumor was studied. Based on above, focusing on protein p65 and TERT, regulation function of the title compound was explored.
Figure 2. Structures of 18α-GAMG and 18β-GAMG.
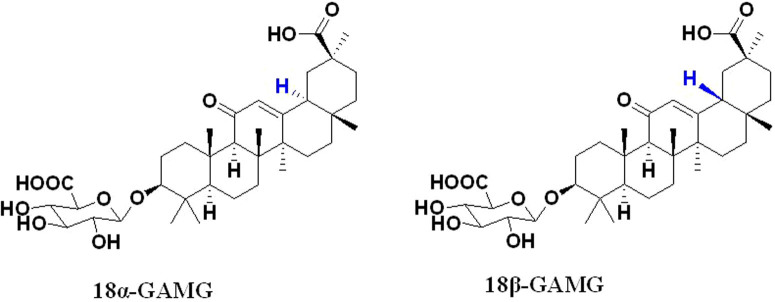
Table 1. Antiproliferative activity of title compound 18α-GAMG against SGC-7901, SMMC-7721 and MGC-803 cell lines.
| IC50/µM a, b | |||
|---|---|---|---|
| Comp. | SGC-7901 | SMMC-7721 | MGC-803 |
| 18α-GAMG | 1.51 ± 0.20c | 0.31 ± 0.05c | 0.97 ± 0.17c |
| AMD | 0.91 ± 0.11 | 0.70 ± 0.13 | 1.10 ± 0.09 |
aEach IC50 value is the mean ± SEM from three experiments (n = 3).
bThe standard deviation (SD) of three time independent tests.
cp < 0.005 vs control.
Table 2. IC50 values of title compound 18α-GAMG against human normal cell lines L-02 and GES-1proliferationa.
aMTT assays were used for evaluation, and values were expressed as mean IC50 of the triplicate experiment.
bThe standard deviation (SD) of three time independent tests.
Results
Anticancer activity in vitro
At first, compound 18α-GAMG was evaluated for its antiproliferative activity against human liver cancer (SMMC-7721), gastric cancer (SGC-7901) and gastric cancer (MGC-803) cell lines. The cells were allowed to proliferate in presence of tested material for 48 h, and the results were reported in terms of IC50 values (Table 1). It is obvious from Table 1 that compound 18α-GAMG exhibited high activity against SMMC-7721 cell with IC50 value of 0.31 ± 0.05 µM, surpassing that of the positive control Doxorubicin (AMD). We subsequently conducted a proliferative inhibition assay with human normal gastric mucosa cell (GES-1) and human normal liver cell (L-02). As given in Table 2, title compound 18α-GAMG manifested obvious non-toxic effect on GES-1 and L-02 with IC50 value about 3.0 mM. The data indicated the title compound with high selective activity against tumor cells versus human somatic cells (Tables 1–2).
Anticancer activity evaluation in vivo
The results of tables 1 and 2 showed that title compound 18α-GAMG with good selectivity on SMMC-7721 cell versus L-02 cell in vitro (IC50 value 0.31 µM vs about 3.0 mM). In order to further evaluate its anticancer activity in vivo, the effect on DEN-induced rat hepatic tumor was explored.
Microscopic features of the liver were examined. In the control group, as expected, integral liver cell structure, hepatic lobular architecture and hepatic nuclei were clearly observed (Figure 3 Control). In the DEN model group, normal liver lobular structure was completely destroyed. The tumor cells showed low differentiation and evident atypia (including multinuclear, karyopyknosis, nuclear vacuoles, etc.), with larger nuclei and nucleoli, chromatin rough, more M phase and less cytoplasm than the normal cells. In addition, tumor giant cells and cancer nests infiltrating surrounding tissues were also observed (Figure 3 Model). In the experimental group, 18α-GAMG treatment markedly abated pathological changes of hepatic lobules. The hepatic cells exhibited evidently reduced atypia, high differentiation and full cytoplasm. The cells were also observed similar multinuclear, karyopyknosis and nuclear vacuoles to those of normal cells. These results showed that title compound 18α-GAMG could significantly improve pathological changes of DEN-induced rat hepatic tumor (Figure 3 A).
Figure 3. Effect of compound 18α-GAMG (A) on pathological changes of DEN-induced rat hepatic tumor (HE staining ×200)a.
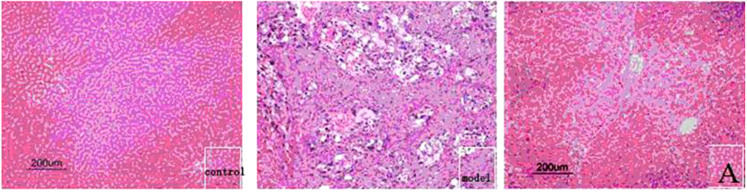
a Animals were randomly divided into 3 groups (n = 12 per group): control group, DEN model group and 18α-GAMG group A.
Cell Cycle Analysis
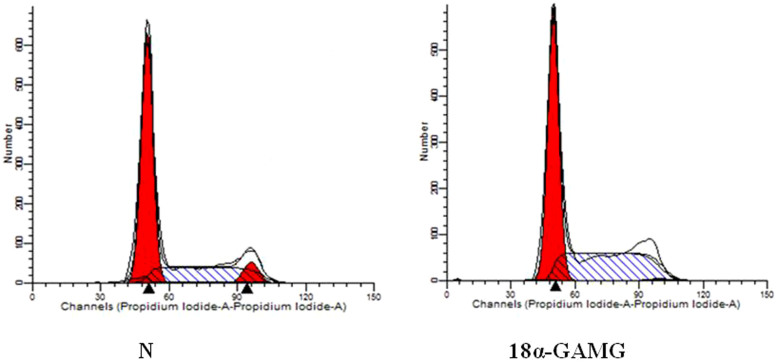
To understand whether the cell cycle arrest lead to decrease cancer cell proliferation, we used flow cytometric analysis to measure the effect of compound 18α-GAMG on induction of cell cycle. As shown in Figure 4, the cells in S phase in the SMMC-7721 control group accounted for about 30.38%, while after cells treated with compound 18α-GAMG for 48 h, the ratio was approximately 42.16% (Table 3). This showed that the cells were arrested in S phase.
Figure 4. Cells cycle analysis by flow cytometry treated with SMMC-7721 cell lines.
Table 3. Anti-proliferation rate of SMMC-7721 cells treated with 18α-GAMG*.
| Group | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|
| Normal | 62.91 ± 0.51 | 30.38 ± 0.20 | 6.70 ± 0.22 |
| 18α-GAMG-0.31 μM | 56.84 ± 0.60** | 42.16 ± 0.53*** | 1.00 ± 0.08** |
SMMC-7721 were incubated with PI and examined by flow cytometry. N, normal; 18α-GAMG (0.31 μM), n = 3.
*Results are the mean ± SD from three independent experiments.
**p < 0.05, *** p < 0.01 vs control.
Inhibition of p65 and TERT proteins
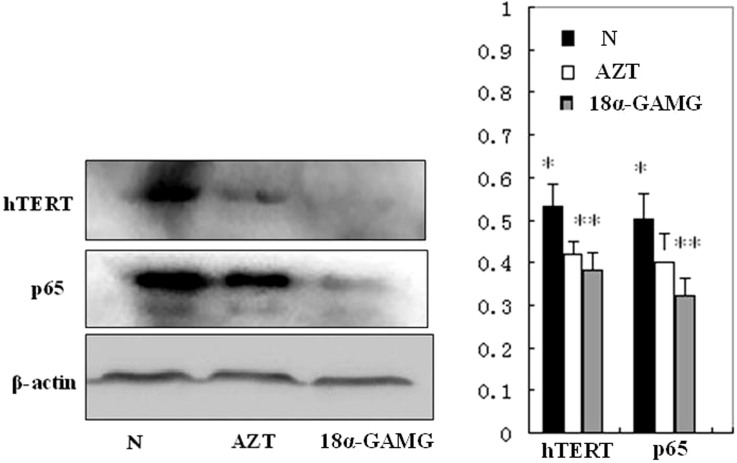
As shown in Figure 5, compared with normal control group, p65 and hTERT proteins were expressed at lower level in SMMC-7721 cells treated with title compound 18α-GAMG. The results suggested that p65 and hTERT was clearly down-regulated within 48 h when the cells exposed to title compound.
Figure 5. Title compound 18α-GAMG inhibited expression of p65 and hTERT.
Western blotting showed inhibition of p65 and hTERT protein expression in response to 18α-GAMG treatment for 48 h in SMMC-7721. AZT (3.0 mmol/L), positive control; Compound 18α-GAMG (0.31 μM). n = 3. Results are the mean ± SD from three independent experiments. * p < 0.05, ** p < 0.01 vs control.
Immunofluorescence analysis
The last research showed that the p65 promoted cell proliferation through shifting to the nucleus2. Immunofluorescence analysis showed that expression of p65 and hTERT had been down-regulated obviously treated with compound 18α-GAMG in SMMC-7721 cells nucleus (Figure 6).
Figure 6. Immunofluorescence analysis of p65 and hTERT protein treated with title compound*.
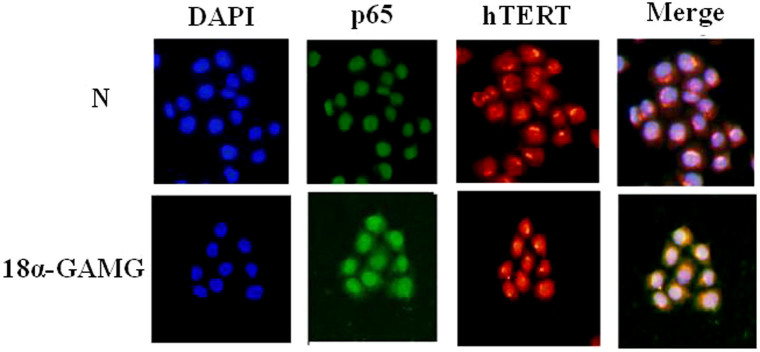
* Title compound 18α-GAMG (0.31 μM). Results was obtained under medium antibody concentration, the results were similar from three independent experiments.
Discussion
Compound 18α-GAMG was synthesized from 18β-glycyrrhizin (18β-GA) by biotransformation and alkaline isomerization (Figure 1). 18β-GA was selectively hydrolyzed by Aspergillus sp Ts-1, a kind of β-glucuronidase, to produce 18β-GAMG. The isomerization reaction was monitored by 13C-NMR spectroscopy (Figure S1), and the structure of 18α-GAMG was elucidated by 1H-NMR and 13C-NMR data (see Supplementary Information). A solution of 18β-GAMG (4.02 g, 6.0 mmol) in NaOH solution (5.0 M, 100 mL) was heated and stirred for 12 h at 90°C. After the reaction mixture was cooled to < 5°C, the pH was adjusted to 2.5 with concentrated HCl. After 12 h, the mixture was filtrated, washed with water, over Na2SO4 and concentrated, which was purified by flash column chromatography (elution with 0 to 30% MeOH in CHCl3) and crystallization from ethanol/EtOAc to give the desired product 18α-GAMG as white crystalline powder (2.83 g, 71% yield). Mp 229−231°C; [α]D20 = +24 (c = 1.0, MeOH); 1H NMR (300 MHz, DMSO-d6) δ 0.65 (s, 3H, 28-CH3), 0.77 (s, 3H, 24-CH3), 0.92 (s, 3H, 23-CH3), 0.98 (s, 3H, 25-CH3), 1.04 (s, 3H, 26-CH3), 1.16 (s, 3H, 29-CH3), 1.33 (s, 3H, 27-CH3), 2.27 (overlapped, 9-H), 3.01 (t, 1H, J = 8.4 Hz, 4′-H), 3.07 (dd, 1H, J = 6.5, 9.7 Hz, 3-H), 3.15 (t, 1H, J = 9.0 Hz, 3′-H), 3.30 (t, 1H, J = 9.8 Hz, 2′-H), 3.58 (d, 1H, J = 9.7 Hz, 5′-H), 4.24 (d, 1H, J = 7.8 Hz, 1'-H), 5.33 (s, 1H, 12-H); 13C NMR (75 MHz, DMSO-d6) δ 15.7, 16.45, 16.49, 17.1, 18.2, 20.4, 20.6, 25.7, 26.3, 27.5, 28.4, 31.4, 33.2, 35.1, 35.3, 36.2, 36.7, 38.3, 39.1, 39.8, 41.6, 43.4, 44.7, 54.3, 59.9, 71.6, 73.7, 75.6, 76.1, 87.9, 105.6, 123.1, 166.1, 170.6, 179.5, 198.7. TOF-HRMS: m/z [M + Na]+ calcd for C36H54NaO10: 669.3609; found: 669.3600.
It is of our interest to utilize rational chemical approaches to generate and identify novel compounds with regulation telomerase synthesis for cancer therapy. As is known to all, GA is a natural product with favorable antitumor activity10,11,12,13. Based on the results of our preliminary study19, anticancer activity of 18α-GAMG was superior to that of 18β-GAMG (Figure 2), so we focused on 18α-GAMG in this study. At first, proliferative inhibition assay in vitro was carried out, the result indicated that compound 18α-GAMG showed high activity against SMMC-7721 cells and obvious non-toxic effect on GES-1 and L-02 cell lines (Tables 1–2). This result reflected that title compound with selective activity against tumor cell SMMC-7721 versus human normal liver cell L-02. In order to further verify the anticancer activity of compound 18α-GAMG in vivo, rat model of DEN-induced hepatic tumor was established in this study to investigate its effect on hepatic tumor. Animals were randomly divided into 3 groups (n = 12 per group): control group, DEN model group and 18α-GAMG group. The result showed that title compound could significantly improve pathological changes of DEN-induced rat hepotic tumor (Figure 3).
In order to understand whether the reduced cancer cell proliferation was due to cell cycle arrest, we then used flow cytometric analysis to measure the effect of title compound 18α-GAMG on induction of cell cycle, we found that the fraction of SMMC-7721 cells staying in the G0/G1 phase was increased, whereas that in the S and G2/M phase decreased by stimulation of title compound (Figure 4). So the results showed that title compound could inhibit proliferation of SMMC-7721 cells, at least in part, via influencing cell cycle arrest.
The catalytic core of telomerase comprises hTERT and the essential protein p65. Among them, the p65 also stimulates conformational changes in other parts of telomerase RNA (TER), and it stabilizes hTERT in catalytically active conformation20. Interaction of the p65 C-terminal domain with TER is necessary and sufficient for the hierarchical assembly of the TERT-TER-p65 catalytic core2,21. In order to test whether titled compound modulates the expression of p65 and hTERT, we performed western blot assay and immunofluorescence analysis, the results indicated that the expression of p65-hTERT protein was clearly down regulated teated with title compound (Figures 5–6).
In summary, title compound 18α-GAMG as anticancer agent in vitro and in vivo was discovered. Flow cytometry assays indicated that it could suppress cancer cells proliferation through inducing cell cycle arrest in S phase. Our experiments preliminarily demonstrated that title compound could down-regulated the expression of protein p65 and hTERT, lead to inhibit SMMC-7721 cells proliferation. These results are of help in the rational design of more efficient p65 modulators in the future.
Methods
Cell proliferation assays
Briefly, target tumor cells were grown to log phase in RPMI 1640 medium supplemented with 10% fetal bovine serum. After diluting to 3 × 104 cells mL−1 with the complete medium, 100 μL of the obtained cell suspension was added to each well of 96-well culture plates. The subsequent incubation was performed at 37°C, 5% CO2 atmosphere for 24 h before subjecting to antiproliferation assessment. Tested samples at pre-set concentrations were added to 6 wells with AMD co-assayed as a positive reference. After 48 h exposure period, 25 μL of PBS containing 2.5 mg·mL−1 of 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) was added to each well. After 4 h, the medium was replaced by 150 μL DMSO to dissolve the purple formazan crystals produced. The absorbance at 570 nm of each well was measured on an ELISA plate reader (MH Medical, China). The data represented the mean of three experiments in triplicate and were expressed as means ± SD using non-parametric test. The IC50 value was defined as the concentration at which 50% of the cells could survive.
Animals
Kunming mice (SPF, male or female, 20 ± 2 g) were purchased from the experimental animal center of China Pharmaceutical University. Animals were housed in a temperature (22 ± 2°C) and relatively humidity (50%)-controlled room on a 12 h light/dark cycle, given free access to food and water, and acclimatized for at least one week prior to use. All the animal experiments were performed in accordance with the Regulations of the Experimental Animal Administration issued by the State Committee of Science and Technology of China. Efforts were made to minimize the number of animals used and their suffering. Animals were maintained in accordance with the Guides of Center for Developmental Biology, Anhui Medical University for the Care and Use of Laboratory Animals and all experiments used protocols approved by the institutions' subcommittees on animal care.
In vivo tumor model induced by DEN
SD rats, male, 120 ~ 160 g. 120 rats were divided randomly into two groups containing 12 normal rats and 108 DEN model rats. Rats were gavaged daily with DEN 8 mg/kg, once a day, 6 days a week. Normal saline rats were given the same volume, molding to 10 week. 60 model rats were randomly divided into 5 groups, 12 rats in each group, continued to give DEN; normal rats were given normal saline, molding to 16 week, stopped giving DEN. 18α-GAMG treatment group from the tenth week, 50 mg·kg−1 was orally given, once a day, for 10 consecutive weeks. Similar parts of left lobe of liver were taken and fixed by 4% polyformaldehyde solution, embedded in paraffin, sliced and HE stained, for histopathological observation.
Cell cycle analysis
For cell cycle analysis, we performed Cell Cycle Kit (Beyotime, China). SMMC-7721 cells were washed three times by cold phosphate buffer saline (PBS, 0.1 M, pH 7.4), and then cells were fixed in 70% ethanol at −20°C for 12 h. After fixation, cells were washed with cold PBS and stained with 0.5 mL of propidium iodide (PI) staining buffer, which contain 200 mg·mL−1 RNase A, 50 μg·mL−1 PI, at 37°C for 30 min in the dark. Analyses were performed on FACScan flow cytometer (Becton Dickinson, United States). The experiments were repeated three times.
Western blotting
Mouse anti-TERT monoclonal antibody was purchased from Abcam (Cambridge, UK). Secondary antibodies for goat anti-rabbit immunoglobulin G (IgG) horse radish peroxidase (HRP), goat anti-mouse IgG HRP was purchased from Santa Cruz Biotechnology (California, USA). β-actin antibody was obtained from Santa Cruz Biotechnology (California, USA). 3′-azido-deoxythymidine (AZT) as telomerase inhibitor was obtained from Sigma-Aldrich (poole, UK). And iCRT, a non-special β-catenin inhibitor, was produced by Merck Millipore Company (Darmstadt, Germany).
Human SMMC-7721 cells were lysed with RIPA lysis buffer (Beyotime, China). Whole extracts were prepared, and protein concentration was detected using a BCA protein assay kit (Beyotime, China). Total protein (30 or 50 mg) from samples were separated by SDS-PAGE and blotted onto a PVDF membrane (Millipore Corp, Billerica, MA, USA). After blockade of nonspecific protein binding, nitrocellulose blots were incubated for 1 h with primary antibodies diluted in TBS/Tween20 (0.075%) containing 3% Marvel. Mouse monoclonal antibody recognizing TERT (Abcam, UK) was used 1:500 as was anti-β-actin (Santa Cruz, USA). Horseradish peroxidase conjugated anti-mouse and anti-rabbit antibodies were used as secondary antibodies correspondingly. After extensive washing in TBS/Tween-20, the blots were processed with distilled water for detection of antigen using the enhanced chemiluminescence system. Proteins were visualized with ECL-chemiluminescent kit (ECL-plus, Thermo Scientific).
Immunofluorescence analysis
SMMC-7721 cells were fixed in ice-cold ethanol and washed three times with PBS for 3 min. The cells on the cover slips were blocked with 0.1% Triton X-100 and H2O2 for 6 min, rinsed with distilled water, and washed three times with PBS for 3 min. The cells were then blocked with rabbit serum for 10 min. The residual serum was removed, and the remaining content was incubated overnight with FITC-conjugated anti-hIgG at 4 uC. The cells were rinsed three times with PBS for 3 min each, and stained with hematoxylin for 1 min. The stained cells were washed with diluted ammonia water and rinsed two times for 3 min. The prepared specimen was sealed with glycerol and examined under a fluorescence microscope.
Statistical analysis
Statistical analysis was performed with SPSS Version 11.0 statistic software package. Data were expressed as means ± standard deviation (SD). Comparisons between groups were performed with analysis of non-parametric test. A value of P < 0.05 was considered statistically significant.
Author Contributions
X.H. designed the research; W.J. and Y.A. conducted the studies; H.X. and J.B. analyzed the data and prepared the manuscript; all authors read and approved the manuscript.
Supplementary Material
Supplemental Information
Acknowledgments
The authors wish to thank the National Natural Science Foundation of China (No.21272008); Science and Technological Fund of Anhui Province for Outstanding Youth (1408085J04).
References
- Bhattacharya S., Chaudhuri P., Jain A. K. & Paul A. Symmetrical bisbenzimidazoles with benzenediyl spacer: the role of the shape of the ligand on the stabilization and structural alterations in telomeric G-quadruplex DNA and telomerase inhibition. Bioconjugate Chem 21, 1148–1159 (2010). [DOI] [PubMed] [Google Scholar]
- Paul A. et al. Stabilization and structural alteration of the G-quadruplex DNA made from the human telomeric repeat mediated by Troger's base based novel benzimidazole derivatives. J Med Chem 55, 7460–7471 (2012). [DOI] [PubMed] [Google Scholar]
- Paul A. et al. Binding of gemini bisbenzimidazole drugs with human telomeric G-quadruplex dimers: effect of the spacer in the design of potent telomerase inhibitors. PLoS One 7, e39467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. et al. Structural Basis for Telomerase RNA Recognition and RNP Assembly by the Holoenzyme La Family Protein p65. Mol Cell 47, 16–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn W. C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med 5, 1164–1170 (1999). [DOI] [PubMed] [Google Scholar]
- Nakamura T. M. et al. Telomerase catalytic subunit homologs from fission yeast and human. Science 277, 955–959 (1997). [DOI] [PubMed] [Google Scholar]
- Meyerson M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997). [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S. et al. Expression profile of putative catalytic subunit of the telomerase gene. Cancer Res 58, 622–625 (1998). [PubMed] [Google Scholar]
- Newman D. J. & Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981-2010. J Nat Prod 75, 311–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L. A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr Med Chem 10, 155–171 (2003). [DOI] [PubMed] [Google Scholar]
- Morgan A. G. & McAdam W. A. Glycyrrhiza glabra. (Monograph). Altern Med Rev 10, 230–237 (2005). [PubMed] [Google Scholar]
- Asl M. N. & Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res 22, 709–724 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanam S., Xu L., Ramaswamy K. & Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep 20, 1387–1392 (2008). [PubMed] [Google Scholar]
- Tripathi M., Singh B. K. & Kakkar P. Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes. Food Chem Toxicol 47, 339–347 (2009). [DOI] [PubMed] [Google Scholar]
- Kim K. J., Choi J. S., Kim K. W. & Jeong J. W. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother Res 27, 841–846 (2013). [DOI] [PubMed] [Google Scholar]
- Baltina L. A. et al. Synthesis and antiviral activity of 18α-glycyrrhizic acid and its esters. Pharm Chem J 44, 299–302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A. V. et al. 18α-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol 39, 635–640 (2011). [DOI] [PubMed] [Google Scholar]
- Qu Y. et al. 18α-Glycyrrhizin induces apoptosis and suppresses activation of rat hepatic stellate cells. Med Sci Monit 18, BR24–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. A. et al. Synthesis, molecular docking and biological evaluation of glycyrrhizin analogs as anticancer agents targeting EGFR. Molecules 19, 6368–6381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman A. J., Gooding A. R. & Cech T. R. Tetrahymena telomerase protein p65 induces conformational changes throughout telomerase RNA (TER) and rescues telomerase reverse transcriptase and TER assembly mutants. Mol. Cell. Biol. 30, 4965–4976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama B. M., Loper J., Najarro K. & Stone M. D. The C-terminal domain of Tetrahymena thermophila telomerase holoenzyme protein p65 induces multiple structural changes in telomerase RNA. RNA 18, 653–660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information