Abstract
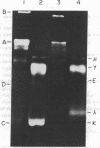
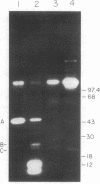
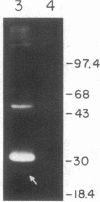
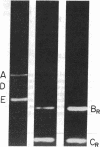
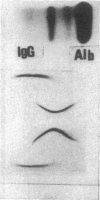
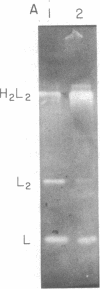
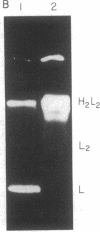
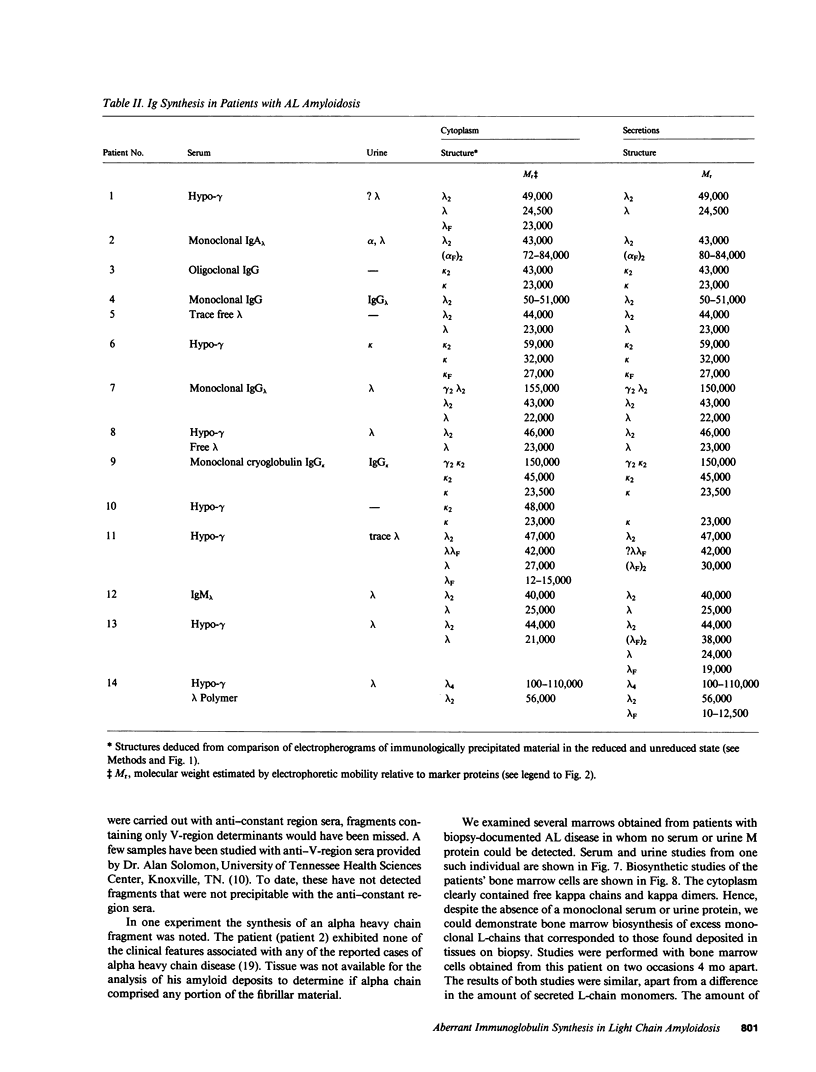
Bone marrow cells obtained from 14 patients with light chain amyloid (AL) deposition were examined by biosynthetic labeling techniques. These analyses identified free monoclonal light chain (L-chain) synthesis even in those patients whose serum or urine contained no M protein or free L-chains or only an intact M protein. The experiments also identified a subset of patients whose plasma cells synthesized polypeptides bearing constant region antigenic determinants that migrated more rapidly than intact L-chains on polyacrylamide gels. Since most AL fibrils contain L-chain fragments rather than intact L-chains, these studies suggested that the genesis of the fibril components may reflect aberrant synthesis, proteolytic processing, or both. We also noted that in some individuals the pattern of Ig synthesis normalized after several courses of cytotoxic therapy. Thus, we could use bone marrow Ig synthesis as a sensitive biochemical parameter for monitoring therapy. Finally, the presence of aberrant synthetic products in these clones raised questions about their origin with respect to the normal processes of transcription, translation, and posttranslational modification in Ig-producing cells.
Full text
PDF


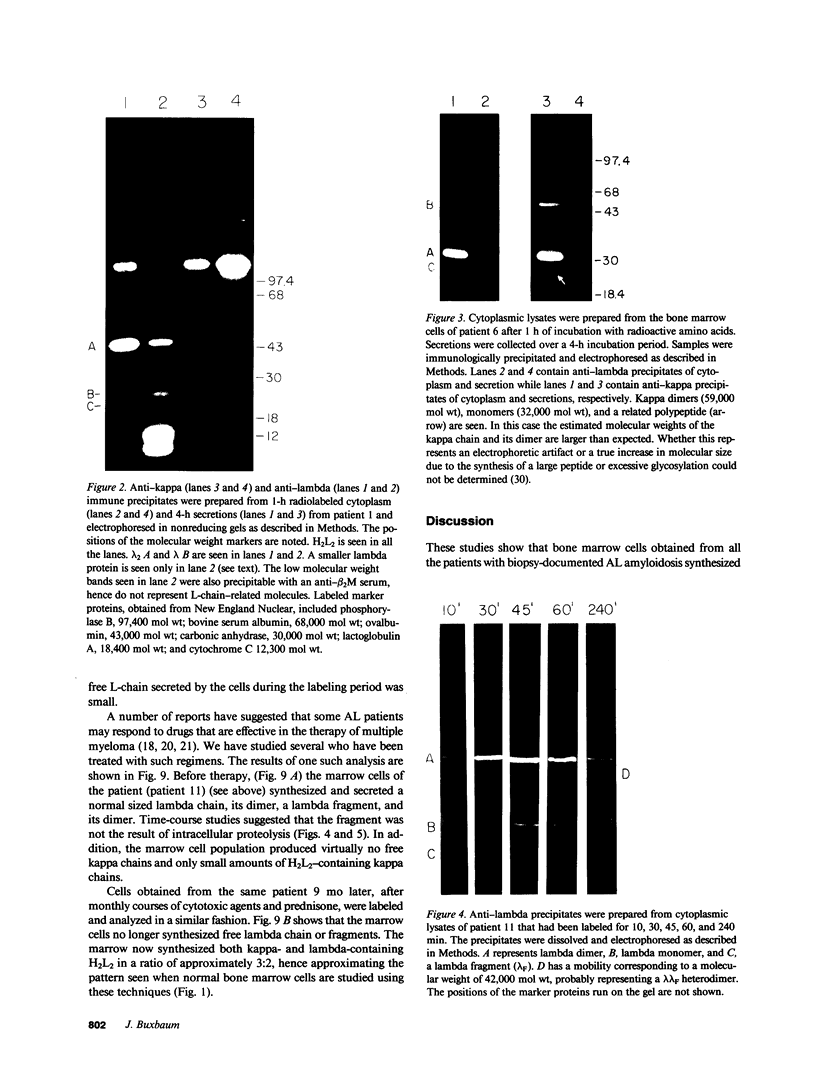
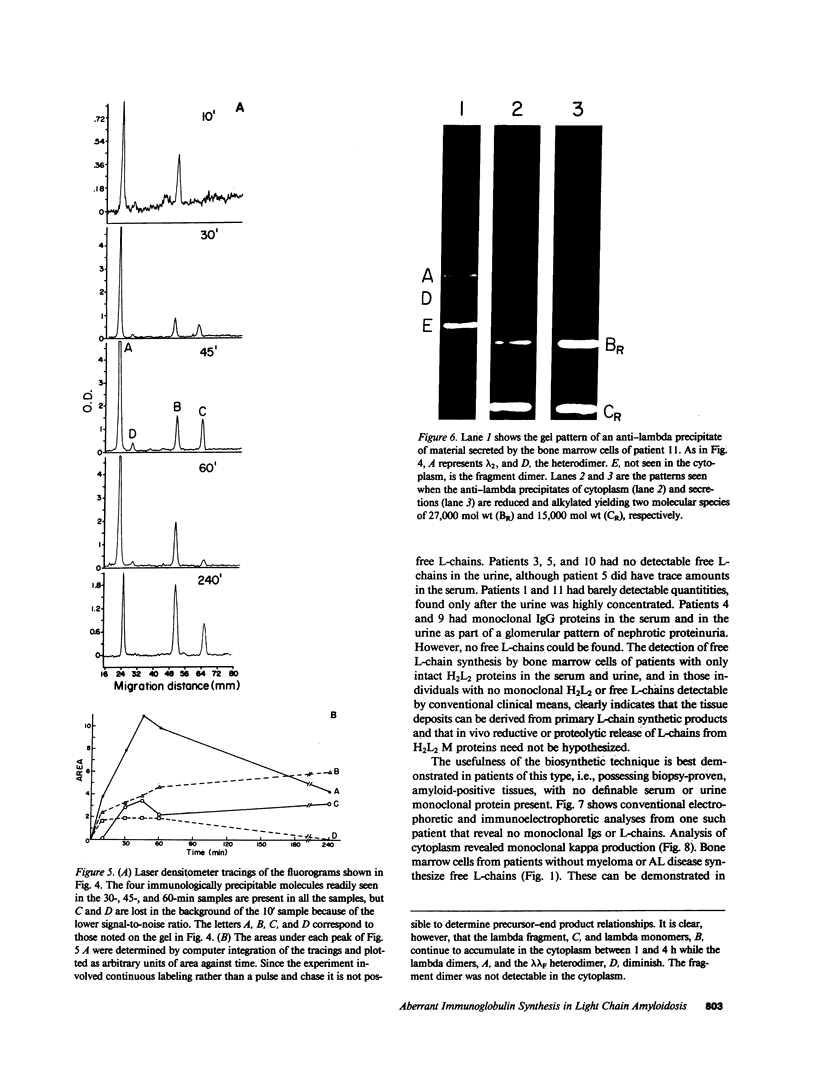
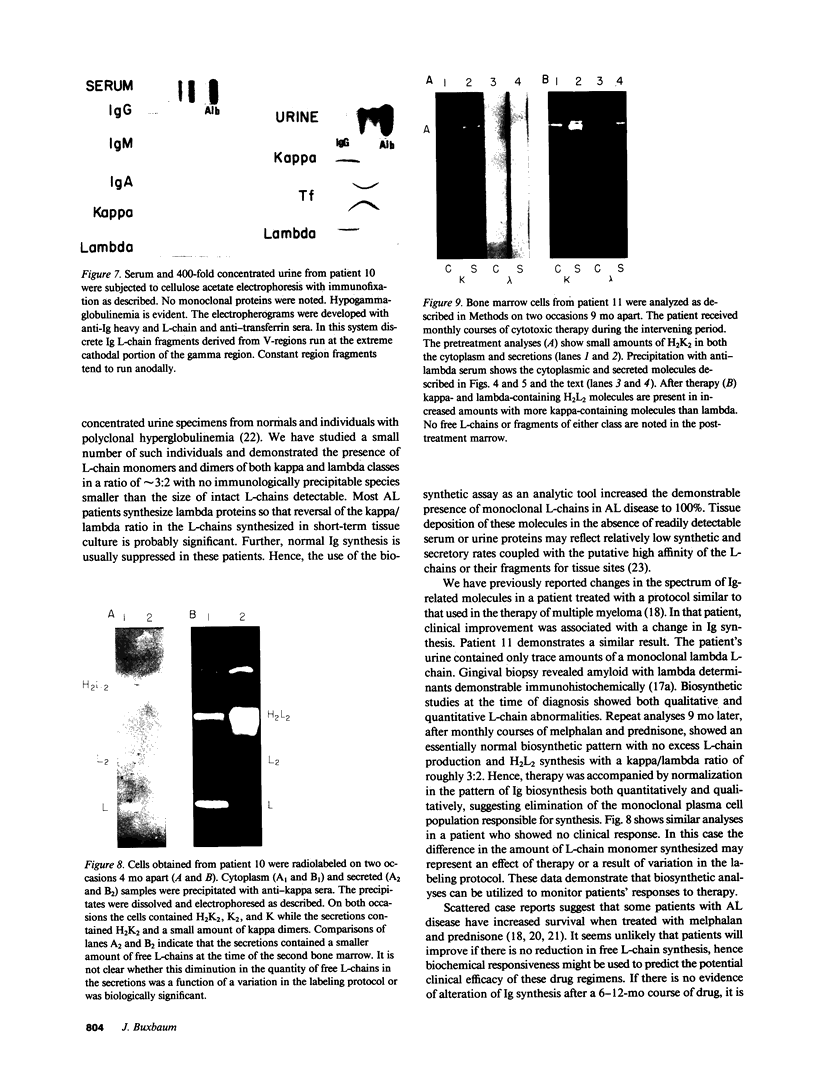


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argon Y., Burrone O. R., Milstein C. Molecular characterization of a nonsecreting myeloma mutant. Eur J Immunol. 1983 Apr;13(4):301–305. doi: 10.1002/eji.1830130406. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Pillot J., Liacopoulos P. Human myeloma light chains with increased molecular weight: high frequency among lambda chains. Mol Immunol. 1983 Apr;20(4):397–407. doi: 10.1016/0161-5890(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. N., Hurley M. E., Chuba J., Spiro T. Amyloidosis of the AL type. Clinical, morphologic and biochemical aspects of the response to therapy with alkylating agents and prednisone. Am J Med. 1979 Nov;67(5):867–878. doi: 10.1016/0002-9343(79)90747-2. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Lessin L. S., Hallal J., Burkholder P. Resolution of primary amyloidosis during chemotherapy. Studies in a patient with nephrotic syndrome. Ann Intern Med. 1975 Apr;82(4):466–473. doi: 10.7326/0003-4819-82-4-466. [DOI] [PubMed] [Google Scholar]
- Gallo G. R., Feiner H. D., Chuba J. V., Beneck D., Marion P., Cohen D. H. Characterization of tissue amyloid by immunofluorescence microscopy. Clin Immunol Immunopathol. 1986 Jun;39(3):479–490. doi: 10.1016/0090-1229(86)90175-3. [DOI] [PubMed] [Google Scholar]
- Gallo G. R., Feiner H. D., Katz L. A., Feldman G. M., Correa E. B., Chuba J. V., Buxbaum J. N. Nodular glomerulopathy associated with nonamyloidotic kappa light chain deposits and excess immunoglobulin light chain synthesis. Am J Pathol. 1980 Jun;99(3):621–644. [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Blumenkrantz N., Danielsen L. Characterization of an amyloid fibril protein from localized amyloidosis of the skin as lambda immunoglobulin light chains of variable subgroup I (A lambda I). Clin Exp Immunol. 1981 Jul;45(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Osserman E. F. Patterns of amyloidosis and their association with plasma-cell dyscrasia, monoclonal immunoglobulins and Bence-Jones proteins. N Engl J Med. 1974 Feb 28;290(9):473–477. doi: 10.1056/NEJM197402282900902. [DOI] [PubMed] [Google Scholar]
- Jones N. F., Hilton P. J., Tighe J. R., Hobbs J. R. Treatment of "primary" renal amyloidosis with melphalan. Lancet. 1972 Sep 23;2(7778):616–619. doi: 10.1016/s0140-6736(72)93014-0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kyle R. A., Greipp P. R. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983 Oct;58(10):665–683. [PubMed] [Google Scholar]
- Lian J. B., Skinner M., Benson M. D., Cohen A. S. Fractionation of primary amyloid fibrils. Characterization and chemical interaction of the subunits. Biochim Biophys Acta. 1977 Mar 28;491(1):167–176. doi: 10.1016/0005-2795(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Natvig J. B., Westermark P., Sletten K., Husby G., Michaelsen T. Further structural and antigenic studies of light-chain amyloid proteins. Scand J Immunol. 1981 Jul;14(1):89–94. doi: 10.1111/j.1365-3083.1981.tb00187.x. [DOI] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Picken M. M., Gallo G., Buxbaum J., Frangione B. Characterization of renal amyloid derived from the variable region of the lambda light chain subgroup II. Am J Pathol. 1986 Jul;124(1):82–87. [PMC free article] [PubMed] [Google Scholar]
- Pras M., Frangione B., Franklin E. C., Gafni J. Idiopathic AL-kiv amyloidosis presenting as giant hepatomegaly. Isr J Med Sci. 1982 Aug;18(8):866–869. [PubMed] [Google Scholar]
- Preud'homme J. L., Morel-Maroger L., Brouet J. C., Cerf M., Mignon F., Guglielmi P., Seligmann M. Synthesis of abnormal immunoglobulins in lymphoplasmacytic disorders with visceral light chain deposition. Am J Med. 1980 Nov;69(5):703–710. doi: 10.1016/0002-9343(80)90421-0. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Whitley E. J., Jr, Paul C., Davidson J. N. Amino acid sequence of a kappa Bence Jones protein from a case of primary amyloidosis. Biochemistry. 1973 Sep 11;12(19):3763–3780. doi: 10.1021/bi00743a028. [DOI] [PubMed] [Google Scholar]
- Reimer R. R., Hoover R., Fraumeni J. F., Jr, Young R. C. Acute leukemia after alkylating-agent therapy of ovarian cancer. N Engl J Med. 1977 Jul 28;297(4):177–181. doi: 10.1056/NEJM197707282970402. [DOI] [PubMed] [Google Scholar]
- Rosner F., Grünwald H. Multiple myeloma terminating in acute leukemia. Report of 12 cases and review of the literature. Am J Med. 1974 Dec;57(6):927–939. doi: 10.1016/0002-9343(74)90171-5. [DOI] [PubMed] [Google Scholar]
- Seligmann M. Immunochemical, clical, and pathological features of alpha-chain disease. Arch Intern Med. 1975 Jan;135(1):78–82. [PubMed] [Google Scholar]
- Solomon A., Frangione B., Franklin E. C. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982 Aug;70(2):453–460. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Killander J., Grey H. M., Kunkel H. G. Low-molecular-weight proteins related to Bence Jones proteins in multiple myeloma. Science. 1966 Mar 11;151(3715):1237–1239. doi: 10.1126/science.151.3715.1237. [DOI] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Abel C. A., Fishkin B. G., Grey H. M. Localization of the carbohydrate within the variable region of light and heavy chains of human gamma g myeloma proteins. Biochemistry. 1970 Oct 13;9(21):4217–4223. doi: 10.1021/bi00823a025. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Page D. L., Kimura S., Isobe T., Osserman E. F., Glenner G. G. Structural identity of Bence Jones and amyloid fibril proteins in a patient with plasma cell dyscrasia and amyloidosis. J Clin Invest. 1973 May;52(5):1276–1281. doi: 10.1172/JCI107295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella G., Coelho I. M. Unexpected mobility of human lambda chains in sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Immunochemistry. 1974 Mar;11(3):157–160. doi: 10.1016/0019-2791(74)90213-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse C., Abraham G., Vaughan J. The relationship between L-chain synthesis and gamma-globulin production. J Clin Invest. 1973 May;52(5):1067–1077. doi: 10.1172/JCI107272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Pinnell S. R., Bratt G. T. Low molecular weight L-chain components related to Bence-Jones proteins. J Lab Clin Med. 1966 Jul;68(1):81–89. [PubMed] [Google Scholar]