Abstract
Monkey B virus (Macacine herpesvirus 1; BV) is endemic in macaques. BV (a BSL4 agent) is the primary zoonotic concern for persons working with macaques in research, and human BV infections frequently are fatal. We assessed the use of a BSL2 baboon herpesvirus (Papiine herpesvirus 1; HVP2) for predicting the drug sensitivity of BV by comparing the sensitivity of the 2 viruses to 12 antiherpetic drugs. Plaque reduction assays showed that 4 drugs (HBPG, BVdU, PFA, and BrdU) were ineffective against both viruses. Of the 8 effective drugs, both viruses were most sensitive to TFT, whereas sensitivity to the remaining 7 drugs varied between BV and HVP2 as well as between strains of HVP2. In addition, the efficacy of 5 drugs (ACV, PCV, GCV, CDV, and EDU) was tested by using a murine model. ACV and EDU were completely ineffective against both HVP2 and BV, and high doses of PCV only delayed death by a few days. GCV and CDV both protected mice against death, and CDV also prevented the development of neurologic symptoms. When the initiation of drug therapy was delayed until after virus gained access to the CNS, both GCV and CDV were ineffective. The similarity of the drug sensitivities of HVP2 and BV in both models validates the use of HVP2 as a BSL2 level model that can be used to predict drug sensitivity of BV. The greater efficacy of CDV relative to GCV suggests the potential for use of CDV in the treatment of zoonotic BV infections.
Abbreviations: ACV, acyclovir; AraA, 9-β-D-arabinofuranosyl-adenine; BrdU, 5-bromo-2′-deoxyuridine; BV, monkey B virus; BVdU, (E)-5-(2-bromovinyl)-2′-deoxyuridine; CDV, cidofovir; EDU, 5-ethyl-2′-deoxyuridine; GCV, ganciclovir; HBPG, 9-(4-hydroxybutyl)-N2-phenylguanine; HSV, herpes simplex virus; HVP2, Herpesvirus papio 2; IUdR, 5-iodo-2’-deoxyuridine; PCV, penciclovir; PFA, phosphonoformic acid; TFT, trifluorothymidine
Macacine herpesvirus 1 (monkey B virus; BV) is an alpha-herpesvirus of macaque monkeys and is closely related to human herpes simplex virus (HSV) types 1 and 2.8,11,27 Although BV primarily causes asymptomatic or mild, self-limiting disease in healthy macaques, the virus is extremely neurovirulent when transmitted via bites or scratches to other nonmacaque primate species, including humans. Although human infections are not common, approximately 80% of untreated patients die of BV infection, and survivors frequently continue to suffer from neurologic sequelae. As a consequence of its lethality in humans, BV is classified as a BSL4 pathogen3 and is the single most serious zoonotic concern for veterinary and research personnel who work with macaques. The increasing popularity of ecotourism to monkey temples in Southeast Asia, where tourists and wild, BV-infected macaque populations come into direct contact, represents another potential concern for zoonotic BV infections.9,12,13,21
The antiviral drugs recommended for use in treating BV infections all were originally developed for treatment of HSV infections.4,18 Because the genes encoding the enzymes targeted by these drugs are conserved between these viruses, BV is sensitive to many of these anti-HSV drugs. However, compared with HSV, BV is less sensitive to these drugs.2,10,14 Although more effective drugs are needed for the treatment of BV infections, the biohazardous nature of and facility requirements associated with studying a BSL4 agent severely limit research on BV. A potential solution to this problem is using a closely related virus whose biologic and molecular properties are very similar to those of BV as a surrogate or model system in which preliminary research can be conducted safely, leaving only confirmative testing to be done with infectious BV.
Baboons carry an alpha-herpesvirus (Papiine herpesvirus 2; HVP2) that is biologically and genetically very similar to BV and HSV.7,8,15,26 In mice, most HVP2 isolates are extremely neurovirulent and closely reflect the pathogenesis of BV in mice20,23 At the antigenic level, HVP2 and BV are so similar that HVP2 has found use as an alternative antigen for diagnostic BV serology.17,25,28 Despite the virus's similarity to BV, HVP2 infections have never been reported in humans. Consequently, HVP2 is rated as a BSL2 pathogen and, as such, HVP2 can be used under BSL2/ABSL2 containment. This study was conducted to assess the potential use of HVP2 as a surrogate model system for predicting the sensitivity of BV to antiviral drugs.
Materials and Methods
Viruses and cells.
African green monkey kidney (Vero) cells were obtained from the Oklahoma Animal Disease Diagnostic Laboratory and were propagated in DMEM supplemented with 5% FBS and 2 mM glutamine. After infection, Vero cell cultures were maintained in DMEM supplemented with 2% FBS and 2 mM glutamine. Vero cells were used for all experiments and to prepare and titrate viral stocks. The E90-136 strain of BV isolated from a cynomolgus macaque16,24 and HVP2 strains OU1-76 and X3135,6,15 were used in this study. BV and HVP2 strain OU1-76 were passed fewer than 10 times in cell culture; the passage history of HVP2 strain X313 is unknown. All work with infectious BV was performed under biocontainment conditions approved by the Oklahoma State University Institutional Biosafety Committee and the US Centers for Disease Control and Prevention.
Drugs.
Analytical grade reagents were used in all experiments. Drugs evaluated were acyclovir (ACV), penciclovir (PCV), ganciclovir (GCV), cidofovir (CDV), 5-iodo-2′-deoxyuridine (IUdR), 5-trifluoromethyl-2′-deoxyuridine (TFT), (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVdU), 5-bromo-2′-deoxyuridine (BrdU), 5-ethyl-2′-deoxyuridine (EDU), 9-(4-hydroxylbutyl)-N2-phenylguanine (HBPG), arabosinyladenine (AraA), and foscarnet (PFA). Drugs were purchased from LKT Laboratories (PCV; St Paul, MN), Sigma Chemical Company (ACV, AraA, BrdU, BVdU, EDU, GCV, and IUdR; St Louis, MO), and Gemini Biologicals (CDV, GCV; West Sacramento, CA) or were synthesized by GLSynthesis (EDU, HBPG, and TFT; Worchester, MA). Stock solutions of all drugs were prepared in DMSO, and dilutions for in vitro testing were made in sterile DMEM. For in vivo testing, drugs were dissolved in sterile acidified water and brought to pH neutrality by the addition of sterile 10× PBS.
Plaque reduction assay.
Details of plaque assays have been described previously.10 Briefly, Vero cell monolayers in 6-well plates were infected with 100 PFU of virus, and virus adsorbed for 45 min at 37 °C. Duplicate wells then were overlaid with medium containing 1% methylcellulose and antiviral drug. Wells with no drug were included in all assays as negative controls. At 48 to 60 h after infection, plaques were counted. Although the initial antiviral effect of some drugs was evident as a decrease in plaque size, plaque size was not considered in determining drug efficacy.
Mouse model.
All animal experiments were reviewed and approved by the Oklahoma State University IACUC, and mice were maintained in AAALAC-accredited facilities. The mouse model used for BV and HVP2 has been described previously.22,23 Briefly, the left flank of female (weight, 10 to 12 g) Balb/c mice was shaved, lightly scarified to disrupt the integrity of the epidermis (without breaking the skin or drawing blood) by scratching the skin with a 22-gauge needle in a 6 × 6 checkerboard pattern, and 1 × 105 PFU of virus (approximately 10 LD50) in 10 μL was applied and then rubbed in using the side of a micropipet tip. Drugs were diluted in sterile PBS and administered by intraperitoneal injection (250 μL) every 12 h for a total of 7 d. Groups of 8 to 10 mice were used in all experiments. Because some mice receiving drug treatment survived with neurologic symptoms, acetaminophen was included in drinking water (2 mg/mL) in all experiments. Mice euthanized later than 10 d after infection were bled and their serum tested by ELISA for antiviral IgG to confirm infection.16
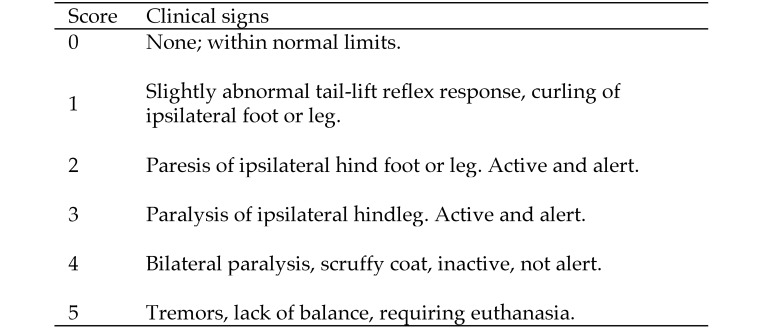
In addition to using mortality as a measure of protection, neurologic disease was assessed. A scoring system based on the highly reproducible progression of neurologic symptoms in untreated infected mice was developed (Figure 1). The clinical signs and disease progression were the same for BV and HVP2, although some variation in the timing of disease progression was evident among different virus strains. The initial sign of infection was an abnormal reflex in abduction of the hindlegs when lifted by the tail, with marked flexion of the foot ipsilateral to site of infection. This condition rapidly evolved into paresis of the ipsilateral foot, followed by spastic then flaccid paralysis of the leg. Mice progressively became immobile with a decrease in body temperature, developed bilateral paralysis of the hindlegs, developed tremors or exhibited loss of balance control, and were euthanized. Some mice receiving drugs survived at various points along this disease progression, and survivors infected with the X313 strain of HVP2 sometimes demonstrated additional symptoms including bloating (due to intestinal ileus), incontinence, and urinary retention.20 Although skin lesions were often evident, their presence was not used in the scoring system because not all mice developed lesions, lesions were inconsistent in severity, some mice regrew hair very quickly making detection of lesions problematic, and lesions appeared both soon after infection (as a result of primary infection), with new lesions occurring a few days later when virus traveled retrograde from the dorsal root ganglia back to the skin.
Figure 1.
Scoring system for HVP2- and BV-infected mice.
Statistical analyses.
EC50 values for plaque reduction assays and ED50 values for in vivo drug studies were calculated by using the Hill model (Kinetica version 5.0, Thermo Fisher Scientific, Waltham, MA). All in vitro tests were done at least 3 times for each drug.
Results
Comparative in vitro drug sensitivity of HVP2 and BV.
To determine whether the sensitivity of HVP2 to various drugs is similar to that of BV, we assessed the sensitivity of 2 different strains of HVP2 and the E90-136 strain of BV to 12 drugs by using a plaque assay (Table 1). Four drugs (HBPG, BVdU, BrdU, and PFA) had very poor activity against HVP2 and BV, and reliable EC50 values could not be calculated. Although very toxic, TFT was the most effective drug against both BV and HVP2, with EC50 values of 0.7 to 1.3 µg/mL. Both ACV and IUdR showed considerable variation in EC50 values among the 3 viruses. Whereas PCV, GCV, CDV, and AraA all showed some activity against all 3 viruses, virus sensitivity varied (2- to 8-fold differences in EC50 values). BV was somewhat more sensitive to GCV and PCV than was HVP2. Overall, these results indicate that HVP2 and BV exhibit similar in vitro sensitivity to the 12 drugs tested.
Table 1.
Comparison of EC50values (Mean ± 1 SD) for antiviral drugs against BV and HVP2
| EC50 value (μg/mL) for |
||||||||||||
| ACV | AraA | BUdR | BVdU | CDV | EDU | GCV | HBPG | IUdR | PCV | PFA | TFT | |
| BV (E90-136) | 11.0 ± 7.2 | 3.9 ± 0.1 | >200 | >200 | 13.6 ± 2.8 | 5.7 ± 3.4 | 3.5 ± 2.5 | >200 | 2.3 ± 2.2 | 3.1 ± 2.2 | >200 | 1.3 ± 0.3 |
| HVP2 (OU1-76) | 39.9 ± 13.4 | 14.3 ± 3.7 | >200 | >200 | 8.4 ± 3.4 | 12.8 ± 5.0 | 10.9 ± 2.4 | >200 | 41.9 ± 12.4 | 16.7 ± 5.7 | >200 | 0.7 ± 0.2 |
| HVP2 (X313) | 25.8 ± 3.4 | 5.0 ± 0.6 | >200 | >200 | 14.0 ± 7.4 | 2.7 ± 2.0 | 25.0 ± 13.4 | >200 | 9.3 ± 2.8 | 16.2 ± 1.5 | >200 | 1.2 ± 0.7 |
Comparison of drug efficacy in vivo.
Drug efficacy as determined by in vitro testing does not always reflect the efficacy obtained in vivo. To further assess the predictive accuracy of HVP2 for BV drug sensitivity, we used a mouse model to compare the efficacy of several drugs in vivo. For initial assessment of drug efficacy, antiviral drugs were administered prophylactically to mice over 7 d beginning 1 d prior to infection, thus providing maximal opportunity for drug efficacy. Because ACV, PCV, and GCV are currently recommended for treatment of zoonotic BV infections,4,18 these 3 drugs were tested. We also tested EDU and CDV because they exhibited efficacy similar to those of ACV, PCV, and GCV in the in vitro assay.
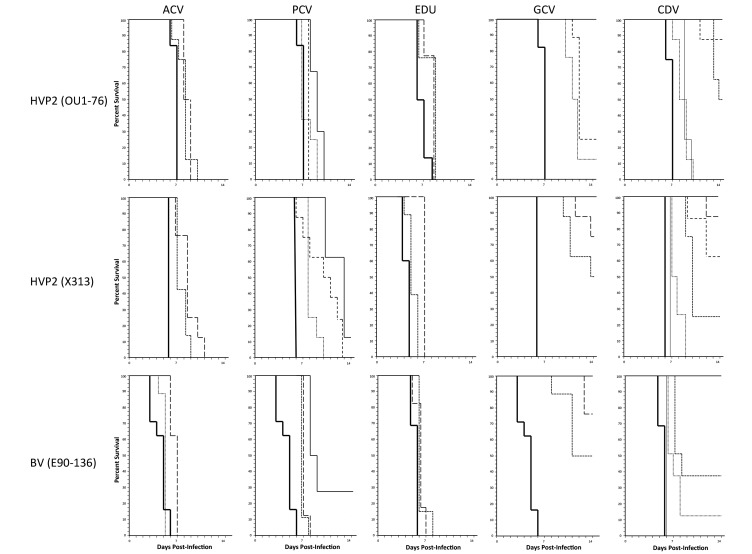
Despite having antiviral activity in vitro, ACV and EDU were completely ineffective against both HVP2 and BV in the in vivo model (Figure 2). All mice dosed with either of these drugs developed neurologic symptoms that progressed in severity to lethality along a time course similar to that of untreated (no-drug) control mice. At high doses, PCV delayed the development of neurologic symptoms and death in HVP2- and BV-infected mice. However, even at the highest doses tested, PCV was ineffective in protecting mice from the development of neurologic symptoms or lethality due to HVP2 or BV.
Figure 2.
Comparative in vivo efficacy of drugs against HVP2 and BV. Groups of mice were infected with virus on day 0 and observed for neurologic symptoms through day 14 after infection. Drugs were administered for 7 d, beginning 1 d prior to infection. Untreated (no drug) control groups are indicated by a heavy solid line. Drug and doses (mg/kg daily) tested were ACV (150, dashed line; 100, dotted line); PCV (200, solid line; 100, dashed line; 50, dotted line); EDU (400, dashed line; 200, dotted line); GCV (100, solid line; 50 dashed line; 25, dotted line); and CDV (200, 100, and 50: solid lines; 25, large dashed line; 12.5, medium dashed line; 6.25, small dashed line; 3.1, dotted line; 1.6, thin solid line).
Although PCV and GCV had similar EC50 values in vitro, GCV—unlike PCV—was effective in vivo against both HVP2 and BV (Figure 2). GCV doses of 200 or 100 mg/kg daily provided complete protection against lethal infection by BV and HVP2 strain OU1-76, with progressively lower doses providing increasingly less protection. Although these doses protected against lethality, all mice infected with BV or HVP2 strain OU1-76 still developed neurologic symptoms consistent with infection. Information from the manufacturer of GCV (Pfizer) lists an LD50 in mice of 1 g/kg IP, making it extremely unlikely that the clinical signs were due to drug toxicity.
In repeated experiments, HVP2 strain X313 gave slightly different results from those of the other viruses. Like mice infected with BV or OU1-76, X313-infected mice treated with GCV doses of 25 and 12.5 mg/kg daily and most mice treated with 50 mg/kg daily died 7 to 9 d after infection after developing typical signs of neurologic involvement. Also like mice infected with BV or OU1-76, X313-infected mice given GCV at 200 or 100 mg/kg daily developed mild or no neurologic symptoms involving the ipsilateral hindlimb until 10 d after infection. However, after 10 d, some mice infected with X313, even those receiving the highest GCV dose, developed severe urinary retention and marked dilation of the cecum and colon due to inflammatory destruction of the intramural and myenteric ganglia, respectively. These mice exhibited progressively severe intestinal and urinary bladder distension and inactivity and died or were euthanized as a result. At necropsy, very segmentally restricted and chronic spinal cord lesions limited to the entry zone of the dorsal root were evident; the severity of these spinal cord lesions was consistent with nongut-related neurologic symptoms. According to their location, lesions in gastrointestinal neurons resulted from centrifugal spread of virus from the CNS.
Like GCV dosage, CDV doses of 200, 100, 50, and 25 mg/kg daily provided complete protection against lethal HVP2 and BV infection (Figure 2). Some mice treated with 12.5 mg/kg CDV daily developed neurologic symptoms that progressed to fatality, and CDV doses less than 12.5 mg/kg daily were not completely protective; all mice developed neurologic disease, and most progressed to lethality. Also of note, mice infected with HVP2 X313 did not exhibit delayed development of the debilitating urinary retention and constipation that occurred in GCV-treated mice.
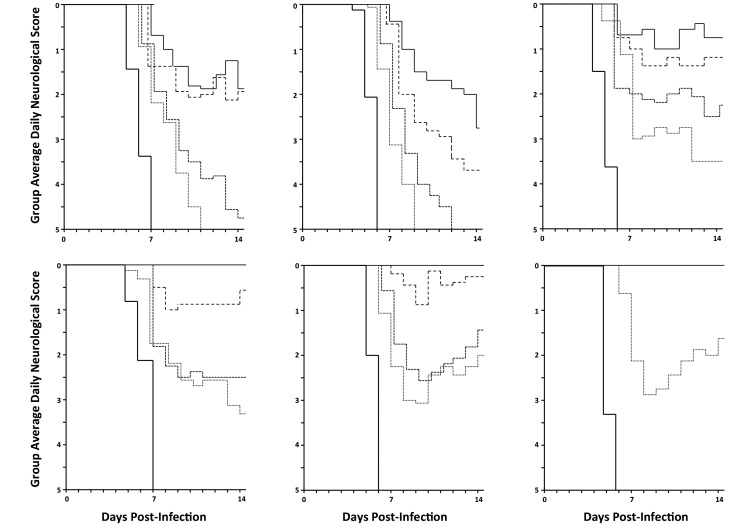
Although high GCV doses provided complete protection against lethality, all infected (BV or HVP2) mice developed neurologic symptoms up to and including paralysis of the ipsilateral hindlimb (Figure 3). In addition, all GCV-treated survivors developed antiviral IgG serum antibodies, indicating that although GCV is protective, it does not immediately or completely stop the infection and so allows the development of an adaptive immune response to the virus. In contrast to GCV-treated mice, many mice treated with higher doses of CDV developed much milder or no neurologic symptoms or skin lesions. Information from the manufacturer of CDV (Roche) an LD50 of 40 mg/kg intraperitoneally, a concentration well above the highest doses used in our experiments, making the occurrence of any drug toxicity unlikely. Consistent with these results, most mice in the higher CDV dose groups remained serologically negative at the end of the experiment (87% to 100% in the 50-, 100-, and 200-mg/kg dose groups with all 3 viruses).
Figure 3.
Neurologic symptoms in mice treated with GCV compared with CDV. Mice were infected with the virus indicated at top, treated with GCV or CDV as described in Figure 1, and scored for neurologic symptoms. GCV doses (mg/kg daily) shown are 200 (solid line), 100 (large dashed line), 50 (small dashed line), and 25 (dotted line). CDV doses (mg/kg daily) were 100 (solid line), 50 (large dashed line), 25 (small dashed line), and 12.5 (dotted line). No-drug controls are indicated by heavy solid lines.
ACV, PCV, and EDU were ineffective, so in vivo ED50 values could not be calculated for these 3 drugs. For GCV and CDV, ED50 values (mg/kg daily) were calculated based on protection against lethal infection (Table 2). CDV ED50 values for the 2 strains of HVP2 and BV were very similar (5.9 to 6.5 mg/kg daily). Much greater variation in the efficacy of GCV occurred among the 3 viruses, as evidenced by the differences in the ED50 values for the 3 viruses. GCV was effective against both OU1-76 and BV, although somewhat more effective against BV. The GCV ED50 for HVP2 strain X313 was higher than those for the other viruses, because mice died at later times of enteric or urinary involvement even at the highest doses.
Table 2.
Efficacy of antiviral drugs in a mouse model of HVP 2 infection
| ED50 (μg/mL) for lethality |
ED50 (μg/mL) for paralysis |
|||||
| BV | OU1-76 | X313 | BV | OU1-76 | X313 | |
| ACV | >150 | >150 | >150 | >150 | >150 | >150 |
| EDU | >400 | >400 | >400 | >400 | >400 | >400 |
| PCV | >200 | >200 | >200 | >200 | >200 | >200 |
| GCV | 27.3 | 51.7 | 102.7 | 100 | 94.9 | 102.7 |
| CDV | 5.9 | 6.5 | 6.1 | 14.7 | 13.6 | 9.9 |
In human BV cases, prevention of even mild neurologic involvement is important. To address this need, another ED50 value was calculated for the GCV and CDV doses that protected against severe neurologic involvement as defined by paralysis of the ipsilateral hindleg (a score of 3). Again, paralysis ED50 values for CDV were similar among the 3 viruses and were approximately twice that for protection against death. Unlike lethality ED50 values, GCV ED50 values for paralysis did not differ markedly among the 3 viruses.
Effect of delaying drug therapy.
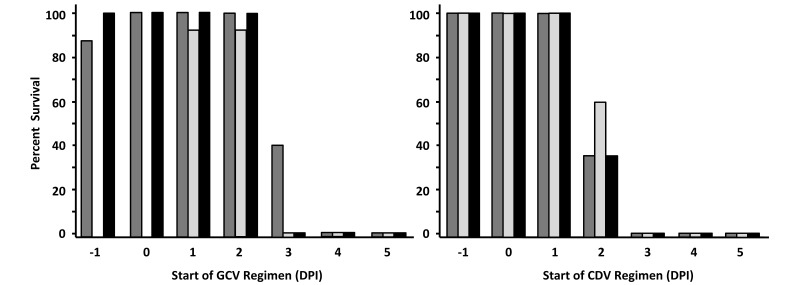
Patients with zoonotic BV infection start drug therapy sometime after infection, not the day before infection as used in our previous experiments. To assess the efficacy of GCV and CDV when initiation of therapy is delayed until after infection, the effect of starting therapy at various times after infection was investigated. The lowest dose of drug that provided 100% protection against lethal infection (100 mg/kg daily for GCV, 25 mg/kg daily for CDV) was used for these experiments, and results are shown in Figure 4. Mice infected with either HVP2 or BV showed a definite temporal transition from complete to no protection with increasing delay in the initiation of drug therapy. In control mice, the temporal progression of infection (from inoculation to severe neurologic signs warranting euthanasia) is typically 6 to 7 d for BV strain E90-136 and HVP2 strain X313 and 7 to 8 d for HVP2 strain OU1-76. Consistent with these slight temporal variations in lethality for the 3 viruses, the maximal time that the start of therapy could be delayed yet remain protective varied slightly for the 3 viruses. Drug therapy for BV or X313 infection was only protective when initiated on or before day 2. Protection against lethality after infection with OU1-76 was effective when initiated on day 2 in one experiment and day 3 in another. These results were the same for both GCV and CDV.
Figure 4.
Effect of delaying initiation of GCV and CDV treatment regimens. Mice were infected with virus (HVP2 OU1-76, dark gray; HVP2 X313, light gray; BV, black) and treated with the minimal 100% protective dose of GCV (100 mg/kg daily) or CDV (25 mg/kg daily). Mice received a 7-d treatment regimen, with groups starting treatment on the indicated day after infection (samples were not collected on days –1 and 0) from HVP2 X313-infected mice treated with GCV.
The abrupt temporal transition from protective to ineffective suggested that some event occurred at this transition point and had a major effect on the efficacy of drug therapy. Because both GCV and CDV are relatively polar, weak bases, neither drug would be expected to partition effectively into the CNS. This situation raised the possibility that once the virus had invaded the CNS, drug therapy may become less effective. To address this possibility, mice infected with HVP2 were euthanized every 24 h between 0 and 5 d after infection. Ipsilateral dorsal root ganglia, lumbar spinal cord, and skin from the site of inoculation (strain X313 only) were collected and assayed for the presence of infectious virus (Table 3). Virus was first detected in dorsal root ganglia at 2 (X313) or 3 (OU1-76) days after infection, a finding that is consistent with the slightly longer progression of OU1-76 infection. In both cases, virus was detected in the spinal cord 24 h after its detection in dorsal root ganglia. The lack of efficacy thus correlated with the presence of HVP2 in the spinal cord, indicating that once the virus has invaded the CNS, initiating drug therapy is ineffective.
Table 3.
Temporal progression of HVP2 invasion of neural tissue
| Time (d) after infection |
||||||||||||||||||
| 0 |
1 |
2 |
3 |
4 |
5 |
|||||||||||||
| Virus | S | D | C | S | D | C | S | D | C | S | D | C | S | D | C | S | D | C |
| OU1-76 (n = 4) | ND | 0 | 0 | ND | 0 | 0 | ND | 0 | 0 | ND | 4 | 0 | ND | 4 | 4 | ND | 4 | 4 |
| X313 (n = 3) | 3 | 0 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
D, ipsilateral dorsal root ganglia; ND, not done; C, lumbar spinal cord; S, skin
Discussion
We performed these studies to assess the accuracy of HVP2 drug sensitivity as a potential predictive model for BV drug sensitivity. If HVP2 can serve as a predictive model for BV, its use would allow preliminary screening of many drugs under BSL2/ABSL2 containment rather than the restrictive BSL4/ABSL4 measures recommended by the Centers for Disease Control and Prevention for work with BV.3 We used 2 neurovirulent strains of HVP2 to assess the potential role of strain variation in drug sensitivity. The 2 HVP2 strains did display some variation (>2-fold differences in EC50 values) in sensitivity to some drugs (GCV, IUdR, and AraA), but EC50 values were very similar between HVP2 strains for most drugs. Part of this variation may have been due to the time period over which these experiments were conducted (approximately 5 y) or in some cases acquisition of drugs from multiple sources. However, similar variation in the sensitivity of different BV strains to several drugs has been reported.10,14 HVP2 and BV were resistant to the same 4 drugs and were most sensitive to TFT, with sensitivity to all other drugs falling between these extremes. Despite variations in EC50 values among individual drugs, overall HVP2 and BV do have similar in vitro drug sensitivity profiles.
Similarly, in vivo drug testing using a mouse model also demonstrated comparable sensitivity of BV and HVP2, with 4/5 drugs showing little difference in ED50 values for HVP2 and BV. The X313 strain of HVP2 appeared to be less sensitive to GCV in vivo than was HVP2 strain OU1-76 since the virus spread from the CNS to gut neurons after the 7 d course of GCV treatment was over. Despite these differences, overall the drugs tested had quite similar efficacy against HVP2 and BV. From this we conclude that HVP2 drug sensitivity can serve as an accurate predictor of BV drug sensitivity in both in vitro and in vivo assays.
Current recommendations for the treatment of persons potentially exposed to BV include ACV and valacyclovir, a prodrug form of ACV.4,18 Whereas very effective against HSV, ACV is considerably less effective against BV according to in vitro sensitivity testing.10,29 These treatment recommendations are based in part on studies of rabbits, which were inoculated by subcutaneous injection of BV.1,2 These studies found ACV to be effective in that survival after potentially lethal infection was significantly greater in treated than in control rabbits.1 However, protection required intravenous administration of 200 mg/kg ACV every 6 h at for 14 d, with delayed disease appearing when ACV treatment was stopped after 9 d PI. Therefore, although ACV provided partial protection in this rabbit model, the drug was not highly effective, and the complete lack of efficacy of ACV in the mouse model is consistent with these results.
The murine model that we describe here involved drug administration by intraperitoneal injection for 7 d, beginning 1 d before infection and ceasing on day 6. With the exception of mice infected with HVP2 strain X313 and treated with GCV, this 7-d dosing regimen clearly identified drugs that were effective compared with those that were not. For all drugs, doses that were not protective resulted in death of all mice within a 3- to 5-d time span, whereas mouse deaths occurred over a longer time span for doses that were partially protective. Whereas mice treated with PCV showed delayed development of neurologic disease and death relative to that in untreated controls, mice treated with ACV or EDU died on time courses that were only slightly longer than the termination of drug dosing. This pattern suggests that these drugs had little inhibitory effect on the virus in vivo; consequently, prolonged drug administration likely would not improve survival.
Mice infected with HVP2 strain X313 and treated with GCV continued to develop symptoms and die until termination of the experiment at 21 d after infection. Perhaps administering GCV for longer than 7 d would have resulted in greater efficacy, as occurred in previous experiments with ACV in rabbits.1 The X313 strain of HVP2 is more neuroaggressive than is strain OU1-76 as evidenced by the more rapid death of untreated X313-infected controls, the eventual development of urinary retention and intestinal ileus as X313 invaded mural ganglia, and its slightly lower LD50.19 In the delayed treatment initiation experiment, X313-infected groups that began therapy on days 1 and 2 (and ended on days 8 and 9) after infection failed to develop this neurologic gastrointestinal involvement, again suggesting that treatment with GCV for longer than 7 d would be more effective. Even though prolonged administration of PCV may be effective, clearly both GCV and CDV are much more effective than is PCV in protecting against BV and HVP2 infection. Given the apparent high lethality of BV in humans, the differential efficacy of the nucleoside analogs against disease progression is a very important factor to know.
Humans experiencing BV exposure typically begin drug therapy within 24 h of an exposure incident, and GCV is recommended if neurologic symptoms are evident. If, as in mice (both in vitro and in vivo, this study) and in rabbits,2 ACV is not very effective against BV in humans, initial treatment of patients with ACV may not effectively inhibit virus replication but rather would allow the virus to replicate at the site of infection and so gain access to the nervous system. In addition, such primary treatment with ACV would delay initiation of therapy with a more effective drug like GCV. In experiments designed to examine the effect of delaying the onset of drug therapy, it was evident that if sufficient time elapsed before drug treatment was started that the virus was able to invade the CNS, at which point even an effective drug like GCV was unable to protect mice from lethality. This scenario is consistent with the poor prognosis for BV patients once neurologic symptoms become evident and suggests that immediate initiation of treatment with GCV may be warranted (at least in high-risk exposure incidents) despite the necessity of administering the drug in hospital due to its significant toxicity.
Currently the most effective drug used for treatment of human BV infections is GCV. Although CDV has shown efficacy against BV in vitro,10 its use for treating human BV infections has not been reported. Several observations from our current mouse model suggest that CDV may actually be more effective in vivo against BV than is GCV. At the higher CDV doses tested (200 to 100 mg/kg daily), infected mice did not develop any clinical signs of neurologic involvement or skin lesions at the site of inoculation. In addition, most of these mice failed to develop antiviral IgG titers. Because seronegative survivors only rarely occur when mice are inoculated with 105 PFU of virus via skin scarification (<5% in greater than 800 mice infected to date), the high incidence of seronegative survivors in CDV-treated mice suggests that CDV completely suppresses viral replication early in infection such that not only do mice survive infection but an adaptive immune response is also not stimulated. Taken together, these observations suggest that viral replication in the skin at the site of infection was inhibited sufficiently effectively to prevent the stimulation of an adaptive immune response to the virus. In contrast, although higher doses of GCV did protect against lethal HVP2 and BV infection, all mice developed significant clinical signs of neurologic involvement and a strong antiviral IgG response. Furthermore, mice infected with HVP2 strain X313 and treated with GCV developed delayed neurologic involvement of the gut, while those infected with X313 infected but treated with CDV did not. This pattern again indicates that whereas both GCV and CDV effectively suppressed viral replication, only CDV completely eradicated the infection. The potential effectiveness of CDV for treatment of zoonotic BV infections may be somewhat offset by its greater toxicity (relative to GCV) in humans. CDV doses used to treat cytomegalovirus infections (intravenous) are approximately 15-fold lower (mg/kg daily) than for GCV. However, CDV doses 10-fold lower than the protective dose of GCV were still protective in the mouse BV model. Given these findings, additional testing of CDV and consideration of its potential use in treating zoonotic BV infections clearly are warranted.
Acknowledgments
This work was supported in part by grants from the Dolphin Trust, the Elizabeth R Griffin Research Foundation, the ACLAM Foundation, and NIH (grants P40 OD010431 and P40 OD010988). KAM was supported by NIH training grant T38 OD011186, and LAB was supported by fellowships from the US Department of Homeland Security 2009-ST-104-000025 and the OSU Center for Veterinary Health Sciences.
References
- 1.Boulter EA, Thornton B, Bauer DJ, Bye A. 1980. Successful treatment of experimental B virus (Herpesvirus simiae) infection with acyclovir. BMJ 280:681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulter EA, Zwartouw HT, Thornton B. 1981. Postexposure immunoprophylaxis against B virus (Herpesvirus simiae) infection. Br Med J (Clin Res Ed) 283:1495–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention and National Institutes of Health 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Washington (DC): Department of Health and Human Services. [Google Scholar]
- 4.Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine herpesvirus 1). Clin Infect Dis 35:1191–1203. [DOI] [PubMed] [Google Scholar]
- 5.Eberle R, Black DH, Lehenbauer TW, White GL. 1998. Shedding and transmission of baboon Herpesvirus papio 2 (HVP2) in a breeding colony. Lab Anim Sci 48:23–28. [PubMed] [Google Scholar]
- 6.Eberle R, Black DH, Lipper S, Hilliard JK. 1995. Herpesvirus papio 2, an SA8-like alphaherpesvirus of baboons. Arch Virol 140:529–545. [DOI] [PubMed] [Google Scholar]
- 7.Eberle R, Blas-Machado U, Wolf R, White G. 2008. Microbiology of captive baboons, p 111–138. In: VandeBerg J, Williams-Blangero S, Tardif S, The baboon in biomedical research. New York (NY): Springer. [Google Scholar]
- 8.Elmore D, Eberle R. 2008. Monkey B virus (Cercopithecine herpesvirus 1). Comp Med 58:11–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Engel GA, Jones-Engel L, Schillaci MA, Suaryana KG, Putra A, Fuentes A, Henkel R. 2002. Human exposure to herpesvirus B-seropositive macaques, Bali, Indonesia. Emerg Infect Dis 8:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Focher F, Lossani A, Verri A, Spadari S, Maioli A, Gambino JJ, Wright GE, Eberle R, Black DH, Medveczky P, Medveczky M, Shugar D. 2007. Sensitivity of monkey B virus (Cercopithecine herpesvirus 1) to antiviral drugs: role of thymidine kinase in antiviral activities of substrate analogs and acyclonucleosides. Antimicrob Agents Chemother 51:2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huff JL, Barry PA. 2003. B virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg Infect Dis 9:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones-Engel L, Engel GA, Heidrich J, Chalise M, Poudel N, Viscidi R, Barry PA, Allan JS, Grant R, Kyes R. 2006. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg Infect Dis 12:900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones-Engel L, Engel GA, Schillaci MA, Babo R, Froehlich J. 2001. Detection of antibodies to selected human pathogens among wild and pet macaques (Macaca tonkeana) in Sulawesi, Indonesia. Am J Primatol 54:171–178. [DOI] [PubMed] [Google Scholar]
- 14.Krug PW, Schinazi RF, Hilliard JK. 2010. Inhibition of B virus (Macacine herpesvirus 1) by conventional and experimental antiviral compounds. Antimicrob Agents Chemother 54:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin JL, Hilliard JK, Lipper SL, Butler TM, Goodwin WJ. 1988. A naturally occurring epizootic of simian agent 8 in the baboon. Lab Anim Sci 38:394–397. [PubMed] [Google Scholar]
- 16.Ohsawa K, Black D, Ohsawa M, Eberle R. 2014. Genome sequence of a pathogenic isolate of Macacine herpesvirus 1 (monkey B virus). Arch Virol Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsawa K, Lehenbauer TW, Eberle R. 1999. Herpesvirus papio 2: a safer and sensitive alternative for serodiagnosis of B virus infection in macaque monkeys. Lab Anim Sci 49:605–616. [PubMed] [Google Scholar]
- 18.Reme T, Jentsch KD, Steinmann J, Kenner S, Straile U, Buse E, Sauerbrei A, Kaup FJ. 2009. Recommendation for postexposure prophylaxis after potential exposure to herpes B virus in Germany. J Occup Med Toxicol 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchey JW, Ealey KA, Payton M, Eberle R. 2002. Comparative pathology of infections with baboon and African green monkey alphaherpesviruses in mice. J Comp Pathol 127:150–161. [DOI] [PubMed] [Google Scholar]
- 20.Ritchey JW, Payton ME, Eberle R. 2005. Clinicopathological characterization of monkey B virus (Cercopithecine herpesvirus 1) infection in mice. J Comp Pathol 132:202–217. [DOI] [PubMed] [Google Scholar]
- 21.Ritz N, Curtis N, Buttery J, Babl FE. 2009. Monkey bites in travelers: should we think of herpes B virus? Pediatr Emerg Care 25:529–531. [DOI] [PubMed] [Google Scholar]
- 22.Rogers KM, Deatheridge M, Breshears MA, Chapman S, Black D, Ritchey JW, Payton M, Eberle R. 2009. Type I IFN response to papiine herpesvirus 2 (Herpesvirus papio 2; HVP2) determines neuropathogenicity in mice. Virology 386:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers KM, Ritchey JW, Payton M, Black DH, Eberle R. 2006. Neuropathogenesis of Herpesvirus papio 2 in mice parallels Cercopithecine herpesvirus 1 (B virus) infections in humans. J Gen Virol 87:267–276. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA, Daniel MD, Lee-Parritz D, King NW, Ringler DJ. 1993. Disseminated B virus infection in a cynomolgus monkey. Lab Anim Sci 43:545–550. [PubMed] [Google Scholar]
- 25.Tanaka S, Mannen K, Sato H. 2004. Use of herpesvirus papio 2 as an alternative antigen in immunoblotting assay for B virus diagnosis. J Vet Med Sci 66:529–532. [DOI] [PubMed] [Google Scholar]
- 26.Tyler SD, Severini A. 2006. The complete genome sequence of Herpesvirus papio 2 (Cercopithecine herpesvirus 16) shows evidence of recombination events among various progenitor herpesviruses. J Virol 80:1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigler BJ. 1992. Biology of B virus in macaque and human hosts: a review. Clin Infect Dis 14:555–567. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto H, Ohsawa K, Walz SE, Mitchen JL, Watanabe Y, Eberle R, Origasa H, Sato H. 2005. Validation of an enzyme-linked immunosorbent assay kit using Herpesvirus papio 2 (HVP2) antigen for detection of Herpesvirus simiae (B virus) infection in rhesus monkeys. Comp Med 55:244–248. [PubMed] [Google Scholar]
- 29.Zwartouw HT, Humphreys CR, Collins P. 1989. Oral chemotherapy of fatal B virus (Herpesvirus simiae) infection. Antiviral Res 11:275–283. [DOI] [PubMed] [Google Scholar]