Abstract
Over several years, 0% to 5% of adolescent animals in a captive colony of common marmosets (Callithrix jacchus) showed severely bended arms and legs over several years. The animals showed no pain, discomfort, or altered behavior but were unable to stretch their distal limbs to their full extent. To characterize the lesion morphologically, the bones of 4 affected marmosets were compared macroscopically and radiographically with those of 6 unaffected animals. The deformities were characterized by mid- to distal diaphyseal bending and pronounced shortening of long bones. The morphology and density of other bones including the skull and vertebrae were unaffected. Although vitamin D values were low in a fifth affected marmoset during 10 to 16 mo of age, lesions associated with rickets were not observed. To our knowledge, this report is the first to describe a micromelic dysplasia-like syndrome comprising severe, idiopathic bending and shortening of long bones in a colony of marmosets.
Abbreviations: AL, actual length; PTH, parathyroid hormone, SL, shortest length
Common marmosets (Callithrix jacchus) are widely used as laboratory animals in biomedical research, where they serve as models for several human diseases such as Parkinson disease,2,7 multiple sclerosis,17 tardive dyskinesia,13 stroke29 and osteopenia and osteoporosis.9,23 These animals also are used in a variety of infection, vaccination,8,31 and toxicologic studies.32 In many of these applications, the evaluation of the locomotor system of the experimental animals is crucial.9 Common marmosets are known to have a few ‘background’ pathologies, which in some instances makes them excellent models for similar human diseases, but in other instances could put a strain on breeding programs and experimental animal selection.16 For example, common marmosets have a relative end-organ resistance for 1,25-dihydroxy vitamin D3, which requires them to have very high blood levels of vitamin D3. Marmosets that receive inadequate amounts of this vitamin are prone to development of vitamin D-dependent rickets (type II) when they are young or osteomalacia later in life.4,16,24,30 The clinical syndrome associated with vitamin D, calcium, or phosphorus deficiency is termed ‘metabolic bone disease’ and manifests itself as very fragile bones, due to a decreased mineral density, that easily fracture.10,16,24 Due to the availability of well-balanced commercial diets, supplements and housing improvements, metabolic bone disease has become a rare spontaneous disease among captive marmosets.16,10 The present report describes the skeletons of 4 common marmosets that, despite optimal care, presented with severe, progressive bending and shortening of the limbs. Serum 25-hydroxy vitamin D, parathyroid hormone (PTH), and blood biochemistry values of another affected animal were compared with those of nonaffected marmosets.
Materials and Methods
Animals and housing.
The common marmosets lived in breeding groups consisting of 2 parents and their offspring. The entire breeding colony averages 150 to 200 animals annually, with a male:female ratio of 1:1. On average, 32 new babies are born each year. The animals in this study were housed in cages with access to an inside and outside enclosure and later in experimental facilities in which they were housed as sibling pairs in an indoor cage. All marmosets were fed commercial monkey pellets (Ssniff, Soest, Germany) ad libitum, supplemented with vitamin D droplets (Davitamon, Omega Pharma, Rotterdam, The Netherlands) and limited amounts of Arabic gum, fresh fruit, and live insects. Water was provided ad libitum in drinking bottles.
A veterinarian observed the animals daily, and a complete physical examination was performed on a yearly basis.
Study design.
In a first study, 4 affected (A1 through A4) and 6 nonaffected (C1 through C6) marmosets were used for radiographic and morphologic analysis of the skeleton. The age, sex, and relationship of the animals are summarized in Table 1. As part of an end-stage experimental procedure, the marmosets were euthanized by intracardiac injection of an overdose of pentobarbiturate (200 mg/kg; Euthasol 20%, AST Farma BV, Oudewater, The Netherlands) after a deep sedation with alfaxalone (16 mg/kg). The experiments were not related to or could not have elicited musculoskeletal pathologies. The euthanized animals were collected during a period of 6 mo and frozen until further processing of the skeleton.
Table 1.
Sex, relationship and age at sampling of affected (A) and nonaffected (C) marmosets
| Marmoset | Sex | Relationship | Sampling age |
| Morphologic study | |||
| A1 | M | son of C6 | 3 y |
| A2 | F | daughter of C6 | 3 y |
| A3 | F | 5 y | |
| A4 | M | 5 y | |
| C1 | F | 2 y | |
| C2 | F | 6 y | |
| C3 | F | 3 y | |
| C4 | M | 2 y | |
| C5 | F | 2 y | |
| C6 | F | mother of A1 and A2 | 6 y |
| Blood analysis | |||
| A5 | M | twin of C8 | 10–16 mo |
| C7 | M | 10–16 mo | |
| C8 | F | twin of A5 | 10–16 mo |
| C9 | M | 10–16 mo | |
| C10 | M | twin of C11 | 10–16 mo |
| C11 | M | twin of C10 | 10–16 mo |
| C12 | M | 10–16 mo | |
| C13 | M | 10–16 mo | |
In a second study, blood samples were collected every other month from one affected (A5) and 7 nonaffected (C7 through C13) animals between the ages of 10 to 16 mo. The marmosets were derived from the same breeding colony as those of the first study (Table 1). All animals were radiographed at each blood sampling to ensure the absence or presence of bone deformities. The study was approved by the Ethical Committee of the Biomedical Primate Research Centre.
Preparation of skeletons.
The skeletons were prepared as described previously.3 Briefly, the carcasses arrived frozen and were thawed before manual removal of skin, muscles, and organs. The remaining soft tissues were digested by dermestid beetles. Before complete disintegration of the skeletons, they were treated with a solution containing approximately 10% hydrogen peroxide (Univar Benelux NV, Brussels, Belgium) to bleach the bones and macerate the tissue remnants. Finally, the skeletons were degreased with methylene chloride (Univar Benelux NV).
Radiography.
The macerated skeletons underwent digital radiographic imaging (40 kV, 5 mAs; Film–focus distance, 100 cm) to assess bone densities and measure the actual lengths (AL) of the bones.
Bone measurements.
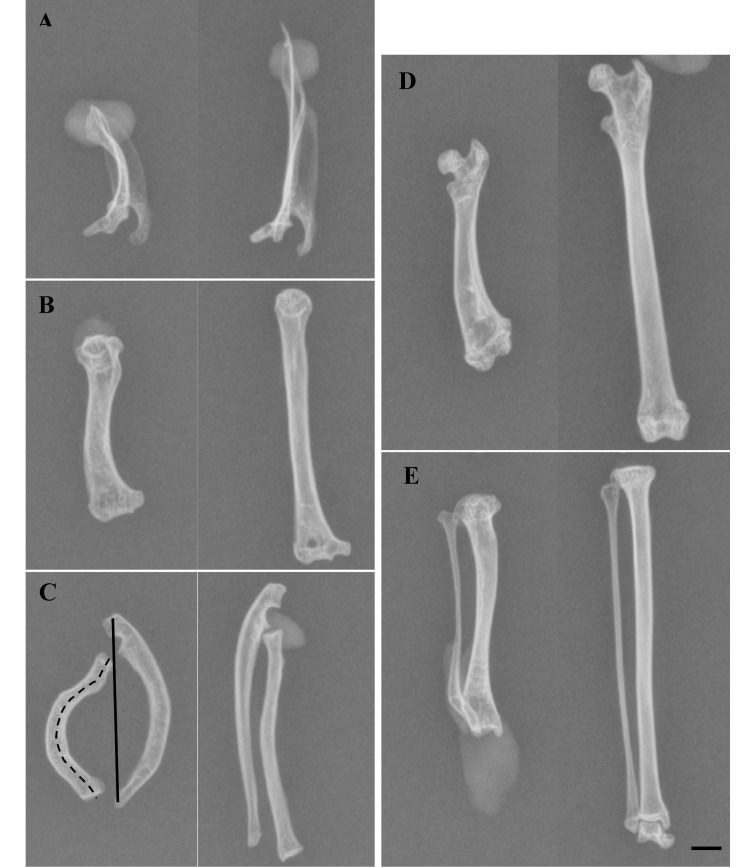
Eight scapulae, humeri, radii, and ulnae; 7 tibiae and fibulae; and 2 femurs of affected animals and 12 scapulae, humeri, radii, ulnae, femurs, tibiae, and fibulae of control animals were used for bone measurements. Some bones of affected animals were processed for histology and therefore not included in the measurements. To quantify the degree of bending in the bones, the ratio between the shortest length (SL) and AL was determined. The SL (Figure 1 C, solid line) of a bone was defined as the straight line between the most proximal and the most distal point of the bone. The AL (Figure 1 C, dotted line) was defined by the distance between those same points but followed the curvature of the bone. For the scapula, the spina scapulae was measured instead of the most proximal and most distal points because the spina scapulae followed the curvature of the bended bones. SL was measured by using a digital caliper held vertically on the macerated bone.3 AL was measured from the radiographs by using ImageJ 1.43r software (National Institutes of Health, Bethesda, MD).
Figure 1.
Radiographs of the macerated scapulae and long bones of an affected and a nonaffected marmoset. On the left are a (A) scapula, (B) humerus, (C) radius and ulna, (D) femur, and (E) tibia and fibula of affected marmoset A1. The corresponding bones of nonaffected marmoset C1 are on the right. Shadows on the pictures are from modeling clay used to fix the bones. All images are craniocaudal views, except for the radius and ulna (C), which are shown in a lateromedial direction. The different bones of each animal originally were all on the same radiograph but were reorganized for clarity. In panel C, the solid line shows how the shortest length (SL) was measured and the dashed line shows how the actual length (AL) was measured. Scale bar, 5 mm.
Histology.
Samples of left and right femoral condyles of marmosets A2, A3, and A4 were fixed for 24 h in a buffered 4% formaldehyde solution, rinsed with tap water for 8 h, and dehydrated at 4 °C by use of an ethanol gradient over a 48-h period. Thereafter, the samples were defatted in xylene for 60 h at 4 °C and embedded in a cold-curing resin at 0 °C. Slides (10-μm sections) were prepared by using a heavy-duty microtome and were stained with hematoxylin and eosin.
Blood biochemistry, vitamin D, and PTH measurements.
Serum 25-hydroxy vitamin D at the ages of 10, 12, 14, and 16 mo and PTH at the ages of 12 and 14 mo were measured by using a chemiluminescence microparticle immunoassay (SSDZ Medical Lab, Delft, The Netherlands). Biochemical values of blood collected at the age of 12 mo were evaluated by using a Cobas Integra 400 Plus (F Hoffmann–La Roche, Basel, Switzerland).
Statistical analysis.
The SL, AL, and SL:AL ratio of affected and nonaffected animals were statistically compared by using a nonparametric Mann–Whitney test. Prism software (version 5.0c, GraphPad Software, San Diego, California) was used to perform the calculations. P values less than 0.05 were considered statistically significant.
Results
Physical examination.
Yearly physical examinations and daily visual inspections revealed an average of 0% to 5% (0 to 3 new cases each year, based on an average of 32 births a year) of common marmosets that showed progressive bending of the long bones. In addition, thoracic and pelvic limbs were interpreted as being shorter when compared with the limbs of other marmosets in the colony. In one animal, the abnormalities were first noted at the age of 6 mo, whereas the other marmosets were 10 mo old. Affected animals showed no pain, discomfort, or altered behavior. However, on physical examination, their lower limbs could not be stretched to their full extent. Once adulthood was reached, the condition did not seem to progress any further. In none of the animals included in this study, nor in any other animals of the colony, were fractures diagnosed.
Macroscopic and radiographic description of the lesions.
The degree of bone deformations varied in severity between animals and between left and right limbs, but the lesions were very similar qualitatively.
The scapulae of affected marmosets showed mild to moderate outward (lateral) bending (Figure 1 A). The distal parts of the diaphyses of the humeri were mildly bent laterally, resulting in a slight S-shaped appearance of the bone (Figure 1 B). The humeri were moderately widened, especially at the distal epiphysis. The radius was mildly to severely bent in a more or less cranial direction at mid- to distal diaphysis (Figure 1 C). The ulna was deformed in a similar way as was the radius but in the opposite direction, thus creating a widened space between the antebrachial bones (Figure 1 C). The distal epiphysis of the femur was slightly widened and bent laterally (Figure 1 D). The mid-diaphyses of the tibia and fibula were bent caudally in 3 out of 4 deformed animals (Figure 1 E). In animal A3, both bones seemed to be relatively normal in shape but were shorter than in the controls. In all the abnormal bones, the bone cortex was thickened on the bent side. No signs of other skeletal abnormalities were noted in the affected marmosets.
All macroscopic lesions were confirmed on radiographs. Most of the bones showed at least some evidence of osteoarthrosis, expressed radiographically as subchondral cysts, subchondral sclerosis, and irregular outlines of the subchondral bone. No difference in cortical bone thickness or density was seen on comparison with nonaffected bones.
Bone measurements and statistical analysis.
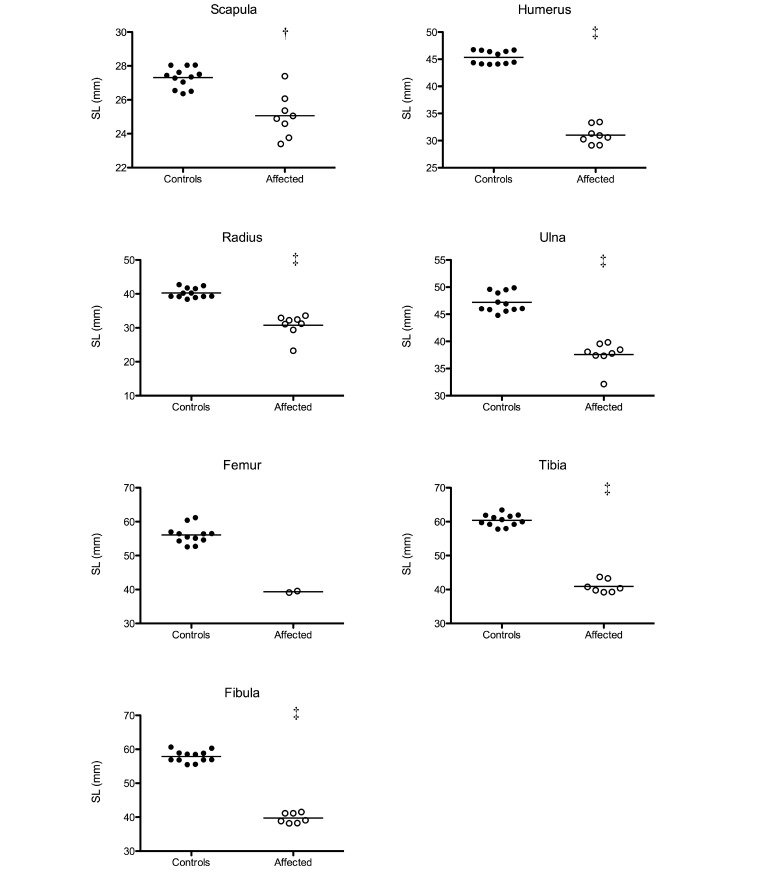
The SL of the bones of affected and control animals are shown in Figure 2. The SL of the scapulae ranged between 23.4 to 27.4 mm for affected animals compared with 26.4 to 28.0 mm for control animals. The SL of humeri ranged between 29.1 to 33.4 mm (affected) and 44.1 to 46.8 mm (controls). The SL of respectively the radii and ulnae ranged between 23.3 to 33.6 mm (affected) and 38.4 to 42.7 mm (controls) and 32.1 to 39.8 mm (affected) and 44.8 to 49.9 mm (controls). Femur SL ranged between 39.1 to 39.6 mm (affected) and 52.6 to 61.2 mm (controls). Tibia and fibula SL ranged respectively between 39.2 to 43.7 mm (affected) and 57.8 to 63.4 mm (controls) and 38.2 to 41.5 mm (affected) and 55.5 to 60.7 mm (controls). The mean SL of all bones, except the femur, was significantly (P < 0.05) shorter in affected compared with nonaffected animals. Femoral lengths could not be analyzed statistically due to the low number of femurs from affected marmosets. However, the SL of the femurs was clearly shorter in affected than in control animals.
Figure 2.
The shortest lengths (SL) of the scapula, humerus, radius, ulna, femur, tibia, and fibula of affected marmosets and controls were measured and compared by using the nonparametric Mann–Whitney test (†, P < 0.01; ‡, P < 0.001).
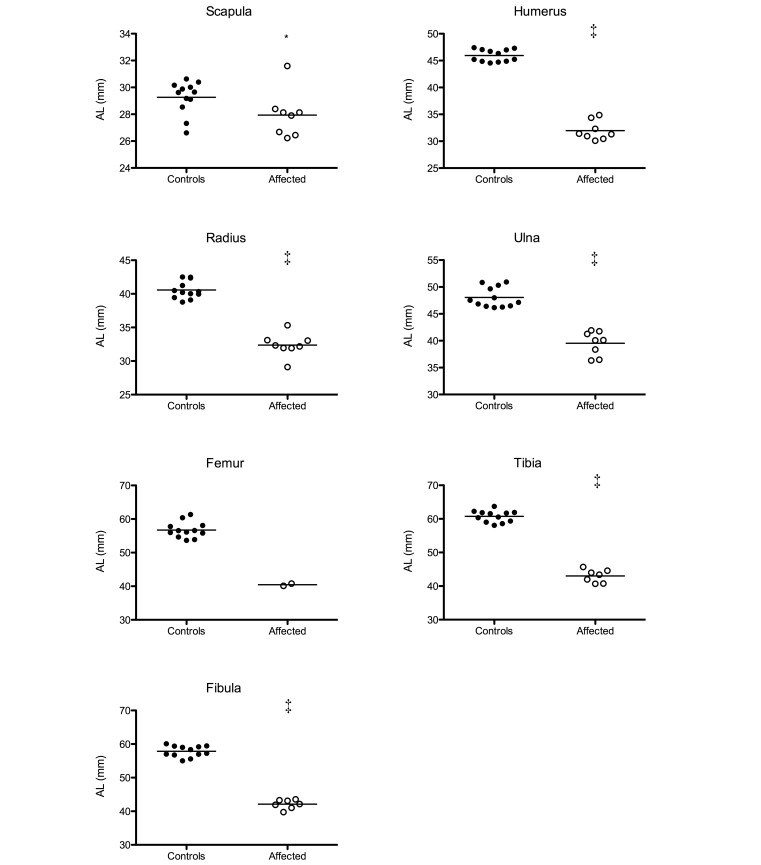
The AL of the bones of affected and control animals are shown in Figure 3. The AL of the scapulae ranged between 26.2 to 31.6 mm for affected animals and 26.6 to 30.6 mm for controls. Humerus AL ranged between 30.1 to 34.8 mm (affected) and 44.5 to 47.4 mm (controls). Radius and ulna AL respectively ranged between 29.1 to 35.3 mm (affected) and 38.8 to 42.5 mm (controls) and between 36.3 to 41.9 mm (affected) and 46.2 to 50.9 mm (controls). The AL of affected femurs ranged between 40.1 to 40.8 mm (affected) and 53.7 to 61.4 mm (controls). Tibia and fibula AL were respectively 40.7 to 44.0 mm (affected) and 58.1 to 63.7 mm (controls) and 39.7 to 43.6 mm (affected) and 55.0 to 59.5 mm (controls). The mean AL of affected bones was significantly (P < 0.05) shorter than the AL of nonaffected bones, except for the femurs, for which significance could not be determined due to the few femurs available. However, both affected femurs were clearly shorter than control femurs.
Figure 3.
The actual lengths (AL) of the scapula, humerus, radius, ulna, femur, tibia, and fibula of affected marmosets and controls were measured and compared by using the nonparametric Mann–Whitney test (*, P < 0.05; ‡, P < 0.001).
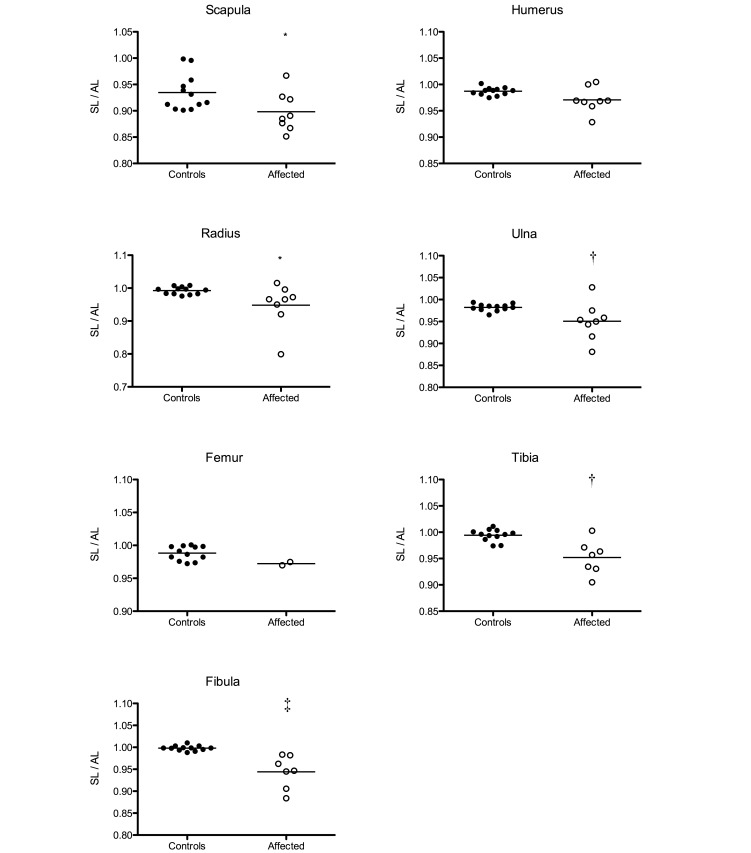
The SL:AL ratio (Figure 4) was calculated to obtain an objective parameter that represented the degree of curvature in each bone. The SL:AL ratio of the scapulae ranged between 0.851 and 0.967 for affected animals and between 0.901 and 0.998 for controls. Humerus ratios ranged between 0.928 and 1.005 (affected) and 0.975 and 1.002 (controls). Radius and ulna SL:AL ratios were 0.799 to 1.015 (affected) and 0.975 to 1.008 (controls) for the radii and 0.881 to 1.028 (affected) and 0.965 to 0.994 (controls) for the ulnae. SL:AL ratios for the femur ranged between 0.970 and 0.975 for affected marmosets and 0.972 and 1.001 for control marmosets. Tibia and fibula SL:AL ratios respectively ranged between 0.905 and 1.003 (affected) and 0.974 and 1.011 (controls) and were 0.884 to 0.983 (affected) and 0.988 to 1.010 (controls). All bones, except the humerus and femur (for which no statistical analysis was performed), showed a significantly lower SL:AL ratio in affected animals as compared with nonaffected animals.
Figure 4.
The ratios of the shortest length (SL) to the actual length (AL) of the scapula, humerus, radius, ulna, femur, tibia, and fibula of affected and control animals were determined and compared by using the nonparametrical Mann–Whitney test (*, P < 0.05; †, P < 0.01; ‡, P < 0.001).
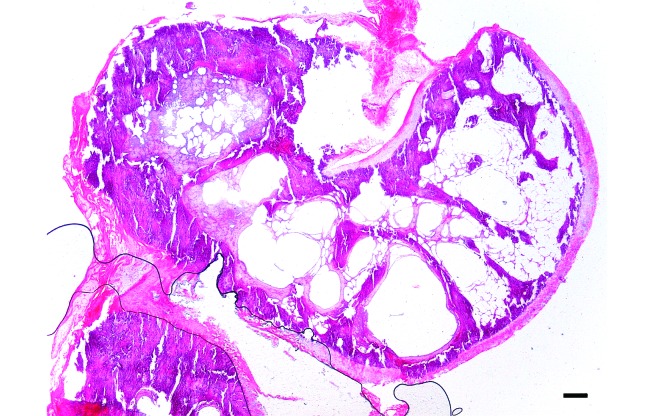
Histology.
All cancellous bone was homogeneously calcified. In 4 condyles, subchondral bone cysts were present (Figure 5). They ranged from 50 to 350 µm in diameter, were lined by a thin fibrous capsule and were optically empty. One cyst was surrounded by hemosiderin-laden macrophages. In 2 samples, focal retention of epiphyseal cartilage replaced by collagen was observed. In 3 samples, articular cartilage showed focal degeneration associated with chondrone formation and fibrocartilaginous tissue.
Figure 5.
Sagittal section of femoral condyl of marmoset A2, demonstrating homogeneous calcification of cancelous bone and subchondral bone cysts. Bar, 200 µm.
Blood analysis.
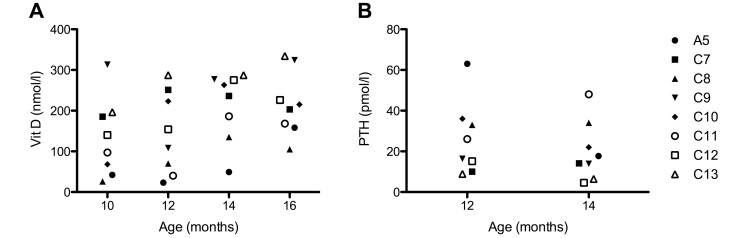
Results of clinical chemistry (Table 2), 25-hydroxy vitamin D, and PTH analyses (Figure 6) of all animals fell within reference ranges,14 except for sodium values, which were decreased in affected and nonaffected animals. Serum 25-hydroxy vitamin D levels (Figure 6A) of the affected marmoset (A5) was low in samples collected at 10, 12, and 14 mo of age compared with nonaffected animals but increased at the age of 16 mo. PTH levels of A5 were high at 12 mo but moderate at 14 mo (Figure 6B).
Table 2.
Blood biochemistry values of 1 affected (A5) and 7 control (C7–C13) common marmosets
| A5 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | Referencea | |
| Albumin, g/L | 41.60 | 40.66 | 47.86 | 45.88 | 42.44 | 41.80 | 46.10 | 45.40 | 10.77-57.88 |
| ALP, U/L | 178.40 | 136.76 | 202.10 | 215.40 | 252.05 | 204.20 | 227.30 | 266.30 | 44.00-426.25 |
| ALT, U/L | 11.50 | 8.56 | 16.32 | 7.55 | 17.68 | 18.20 | 9.50 | 7.70 | 0.79-45.33 |
| AST, U/L | 258.40 | 251.34 | 194.68 | 162.80 | 242.55 | 471.90 | 139.70 | 187.60 | 51.24-316.21 |
| Bilirubin, μmol/L | 1.50 | 1.76 | 1.82 | 1.60 | 1.38 | 1.90 | 1.50 | 1.30 | 0.03-4.24 |
| Ca, mmol/L | 2.54 | 2.54 | 2.54 | 2.47 | 2.38 | 2.59 | 2.52 | 2.49 | 1.78-2.96 |
| Cholesterol, mmol/L | 3.97 | 4.10 | 3.88 | 3.78 | 3.92 | 4.14 | 3.03 | 3.69 | 2.28-5.09 |
| Cl, mmol/L | 108.25 | 106.02 | 107.98 | 109.33 | 108.45 | 109.20 | 107.16 | 106.24 | 101.85-119.90 |
| Creatinine, μmol/L | 46.60 | 51.40 | 44.25 | 40.00 | 39.00 | 49.25 | 45.67 | 40.50 | 14.00-48.28 |
| GGT, U/L | 0.83 | 2.00 | 9.32 | 3.90 | 2.57 | 1.53 | 0.83 | 1.03 | 0.24-13.50 |
| Glucose, mmol/L | 5.14 | 6.77 | 4.54 | 5.06 | 5.46 | 3.44 | 8.27 | 6.72 | 4.01-11.89 |
| Fe, μmol/L | 22.52 | 24.35 | 24.93 | 25.87 | 23.59 | 31.94 | 20.94 | 21.70 | 17.63-39.48 |
| K, mmol/L | 2.81 | 2.78 | 3.46 | 2.78 | 2.64 | 3.40 | 3.18 | 2.74 | 2.30-4.65 |
| LDH, U/L | 283.33 | 377.20 | 442.40 | 318.50 | 531.50 | 483.00 | 295.40 | 362.40 | 134.77-1119.69 |
| Mg, mmol/L | 0.99 | 1.02 | 0.93 | 0.92 | 0.98 | 0.94 | 0.99 | 0.92 | 0.60-1.03 |
| Na, mmol/L | 149.07 | 153.78 | 149.70 | 151.08 | 148.75 | 151.66 | 147.90 | 148.42 | 155.50-174.00 |
| P, mmol/L | 2.08 | 2.34 | 2.31 | 2.01 | 2.38 | 2.43 | 2.35 | 2.16 | 0.47-2.43 |
| Total protein, g/L | 55.17 | 53.60 | 62.96 | 59.25 | 57.88 | 55.76 | 62.04 | 60.56 | 41.10-74.8 |
From reference 11.
Figure 6.
(A) Serum vitamin D and (B) PTH levels of 1 affected (A5) and 7 nonaffected (C7 through C13) marmosets between the ages of 10 and 16 mo.
Discussion
This report is the first description of idiopathic bending of the scapula and long bones in a colony of common marmosets (Callithrix jacchus). Morphologic analysis of macerated bones showed bending of the scapula, humerus, radius, ulna, tibia, and fibula at the mid- to distal diaphyseal region. To morphologically characterize the bone deformities, we compared the SL, AL and SL:AL ratio of affected bones with bones from nonaffected marmosets of the same colony. These measurements revealed that affected bones were not only bent but also were shortened. Although conditions that alter the long bones of primates are rare, a similar case has been reported in a rhesus macaque (Macaca mulatta).9 In the cited case,9 skeletal abnormalities were first noted around 8 mo of age, and no signs of discomfort or behavioral changes were observed. The macaque showed asymmetric bending of the metaphyseal regions of the humerus, radius, ulna, tibia, fibula, and femur (to a lesser extent). However, no apparent shortening of bones was present. In human medicine, micromelic dysplasia without or with minimal involvement of facial deformities is rarely reported.1
To characterize the underlying pathology of the bone deformities, it was important to determine whether bones were shortened as well as bent. Bending of appendicular bones can be caused by growth disorders such as skeletal dysplasias, which usually are also characterized by short stature.16 When bended bones are present without shortening, differential diagnoses should include all diseases that cause (temporary) weakening of the bones. In these cases, the bowing could be physiologic and self-correcting, as has been described in young children, or pathologic,4,8 but both etiologies are secondary to muscle traction and weight distribution on the skeleton. Because both bending and shortening of long bones characterized the deformities in our marmosets, a primary bone growth disorder is most likely. Such bone dysplasias typically are related to disturbance in growth, and therefore the growth plates should be examined. However we were unable to do so in the present study because all growth plates were already closed. In fact, the observed histologic changes could all be sequelae to the malformation, except for the focal retention of epiphyseal cartilage.
Skeletal dysplasias are less well characterized in veterinary medicine than in human medicine. Skeletal dysplasias of long bones can be categorized as diaphyseal, epiphyseal, or metaphyseal, depending on the location of the main abnormality.27 Alternatively, they can be categorized as rhizomelic, mesomelic, or micromelic when there is shortening of the proximal limb bones (humerus, femur), distal limb bones (radius, ulna, tibia, fibula), or all long bones, respectively.22 According to this terminology, the lesions we note in the present study resemble those of micromelic dysplasia. In addition, the lesions are radiographically similar to those of mesomelic dysplasia caused by mutations in the SHOX gene in humans.15 However, the condition in humans is accompanied by high-arched palates, short necks, micrognathia, and abnormal auricle development.17 A human fluorescence in situ hybridization test on blood of one affected marmoset failed to demonstrate the SHOX genetic mutation, although it is unclear whether this assay is applicable to marmosets (data not shown).
Because skeletal dysplasias in humans are often related to a genetic defect,18 the lesions we observed in this colony of marmosets (short stature with bowing of long bones) without other clinical abnormalities might be attributed to an underlying genetic defect. However, differential diagnoses for short, bended appendicular bones should include all primary growth disorders caused by metabolic disorders as well as toxic insults. Morphologic analysis of 4 affected marmosets did not reveal vitamin D deficiency-related lesions, such as rickets or osteomalacia. However, these findings don't exclude a possible role of vitamin D. Therefore, we determined the vitamin D, PTH, and Ca levels in the serum of one affected marmoset at different ages and compared these data with those from nonaffected marmosets in the colony. Interestingly, vitamin D levels were decreased in the affected animal, whereas calcium levels were within the normal range. Similar findings were found in the nonaffected twin, which was reared under the same conditions. These low vitamin D levels were unexpected because all of the animals received adequate amounts of vitamin D in their diet and had all-day access to outdoor, sun-exposed enclosures. Generally, vitamin D deficiency due to poor nutrition and lack of sunlight is characterized by low levels of 25-hydroxy vitamin D3 and is of key importance in New World primates.6,12,20 However, both conditions result in a rachitic or osteomalacic phenotype,6,20 of which typical clinical and radiographic features were lacking in the affected animals in our study. Nevertheless, hypovitaminosis D as a complicating causal factor cannot completely be ruled out, because New World nonhuman primates are well known to have innate end-organ resistance, which may impair some metabolic pathways.26 Indeed, some human and veterinary bone dysplasias are associated with hypovitaminosis D19,28 or at least show improvement after vitamin D supplementation,12 thus suggesting that suboptimal levels of vitamin D may aggravate the clinical outcome of some genetic and congenital conditions.
Other toxic or metabolic disorders that affect extracellular matrix formation include fluoride toxicity,29 vitamin A toxicity 5,22 and vitamin C deficiency,21,27 but all of these seem unlikely because none of the marmosets showed any clinical abnormalities other than bone deformities.
Blood reference values for vitamin D and PTH in common marmosets have not yet been determined but are known for black-tuffed marmosets (Callithrix penicillata).26 The authors of that study26 examined 3 juvenile and 12 adult, free-living animals, so extrapolation of their results to captive animals belonging to another species is tentative. In addition, serum parameters vary widely in captive black-tuffed marmosets,25 further complicating the extrapolation of those values. Therefore, we included 7 age-matched nonaffected marmosets. Intriguingly, we noted wide variations in vitamin D and PTH levels in common marmosets as well, demonstrating the need for larger scale examination.
In conclusion, a clinical syndrome causing bending and shortening of the long bones of the appendicular skeleton is present in a laboratory colony of common marmosets. This newly described micromelic dysplasia-like syndrome should be examined further for the underlying (genetic) cause and its possible association with vitamin D metabolism.
Acknowledgment
We thank Professor Dr Paul Simoens for critical reading of the manuscript.
References
- 1.Al-Gazali LI, Bakir M, Hamid Z, Nath D, Haas D. 2001. Micromelic dwarfism—humerus, femur and tibia type. Clin Dysmorphol 10:24–28. [DOI] [PubMed] [Google Scholar]
- 2.Allen JM, Cross AJ, Yeats JC, Ghatei MA, McGregor GP, Close SP, Pay S, Marriott AS, Tyers MB, Crow TJ, Bloom SR. 1986. Neuropeptides and dopamine in the marmoset. Effect of treatment with 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP): an animal model for Parkinson's disease? Brain 109:143–157. [DOI] [PubMed] [Google Scholar]
- 3.Casteleyn C, Bakker J, Breugelmans S, Kondova I, Saunders J, Langermans JA, Cornillie P, Van den Broeck W, Van Loo D, Van Hoorebeke L, Bosseler L, Chiers K, Decostere A. 2012. Anatomical description and morphometry of the skeleton of the common marmoset (Callithrix jacchus). Lab Anim 46:152–163. [DOI] [PubMed] [Google Scholar]
- 4.Chun RF, Chen H, Boldrick L, Sweet C, Adams JS. 2001. Cloning, sequencing, and functional characterization of the vitamin D receptor in vitamin D-resistant New World primates. Am J Primatol 54:107–118. [DOI] [PubMed] [Google Scholar]
- 5.Clark L. 1970. The effect of excess vitamin A on longbone growth in kittens. J Comp Pathol 80:625–634. [DOI] [PubMed] [Google Scholar]
- 6.Dittmer KE, Thompson KG. 2011. Vitamin D metabolism and rickets in domestic animals: a review. Vet Pathol 48:389–407. [DOI] [PubMed] [Google Scholar]
- 7.Eslamboli A. 2005. Marmoset monkey models of Parkinson's disease: which model, when, and why? Brain Res Bull 68:140–149. [DOI] [PubMed] [Google Scholar]
- 8.Greenough TC, Carville A, Coderre J, Somasundaran M, Sullivan JL, Luzuriaga K, Mansfield K. 2005. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol 167:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grohmann J, Kuehnel F, Buchwald U, Koeller G, Habla C, Einspanier A. 2012. Analysis of the bone metabolism by quantitative computer tomography and clinical chemistry in a primate model (Callithrix jacchus). J Med Primatol 41:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Hatt JM, Sainsbury AW. 1998. Unusual case of metabolic bone disease in a common marmoset (Callithrix jacchus). Vet Rec 143:78–80. [DOI] [PubMed] [Google Scholar]
- 11.Hopper K, Morales P, Garcia A, Wagner J. 2010. Camptomelia in a rhesus macaque (Macaca mulatta). J Am Assoc Lab Anim Sci 49:863–867. [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SH, Shahid R, Mian AA, Sardar R, Anjum MA. 2010. Effect of the level of cholecalciferol supplementation of broiler diets on the performance and tibial dyschondroplasia. J Anim Physiol Anim Nutr (Berl) 94:584–593. [DOI] [PubMed] [Google Scholar]
- 13.Klintenberg R, Gunne L, Andren PE. 2002. Tardive dyskinesia model in the common marmoset. Mov Disord 17:360–365. [DOI] [PubMed] [Google Scholar]
- 14.Kuehnel F, Grohmann J, Buchwald U, Koeller G, Teupser D, Einspanier A. 2012. Parameters of haematology, clinical chemistry, and lipid metabolism in the common marmoset and alterations under stress conditions. J Med Primatol 41:241–250. [DOI] [PubMed] [Google Scholar]
- 15.Leka SK, Kitsiou-Tzeli S, Kalpini-Mavrou A, Kanavakis E. 2006. Short stature and dysmorphology associated with defects in the SHOX gene. Hormones 5:107–118. [DOI] [PubMed] [Google Scholar]
- 16.Ludlage E, Mansfield K. 2003. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med 53:369–382. [PubMed] [Google Scholar]
- 17.Mancardi G, Hart B, Roccatagliata L, Brok H, Giunti D, Bontrop R, Massacesi L, Capello E, Uccelli A. 2001. Demyelination and axonal damage in a nonhuman primate model of multiple sclerosis. J Neurol Sci 184:41–49. [DOI] [PubMed] [Google Scholar]
- 18.Parnell SE, Wall C, Weinberger E. 2013. Interactive digital atlas of skeletal surveys for common skeletal dysplasias. Pediatr Radiol 43:803–813. [DOI] [PubMed] [Google Scholar]
- 19.Petramala L, Giustini S, Zinnamosca L, Marinelli C, Colangelo L, Cilenti G, Formicuccia MC, D'Erasmo E, Calvieri S, Letizia C. 2012. Bone mineral metabolism in patients with neurofibromatosis type 1 (von Recklingausen disease). Arch Dermatol Res 304:325–331. [DOI] [PubMed] [Google Scholar]
- 20.Pritzker KPH, Kessler MJ. 2012. Arthritis, muscle, adipose tissue, and bone diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T. Nonhuman primates in biomedical research. Amsterdam (the Netherlands): Elsevier. [Google Scholar]
- 21.Ratterree MS, Didier PJ, Blanchard JL, Clarke MR, Schaeffer D. 1990. Vitamin C deficiency in captive nonhuman primates fed commercial primate diet. Lab Anim Sci 40:165–168. [PubMed] [Google Scholar]
- 22.Rothenberg AB, Berdon WE, Woodard JC, Cowles RA. 2007. Hypervitaminosis A-induced premature closure of epiphyses (physeal obliteration) in humans and calves (hyena disease): a historical review of the human and veterinary literature. Pediatr Radiol 37:1264–1267. [DOI] [PubMed] [Google Scholar]
- 23.Seidlova-Wuttke D, Schlumbohm C, Jarry H, Dullin C, Wuttke W. 2008. Orchidectomized (orx) marmoset (Callithrix jacchus) as a model to study the development of osteopenia–osteoporosis. Am J Primatol 70:294–300. [DOI] [PubMed] [Google Scholar]
- 24.Shinki T, Shiina Y, Takahashi N, Tanioka Y, Koizumi H, Suda T. 1983. Extremely high circulating levels of 1α,25-dihydroxyvitamin D3 in the marmoset, a new world monkey. Biochem Biophys Res Commun 114:452–457. [DOI] [PubMed] [Google Scholar]
- 25.Teixeira DS, Castro LCG, Nobrega YKM, Almeida RC, Gandolfi L, Pratesi R. 2010. 25-Hydroxy-vitamin D levels among Callithrix penicillata primate species raised in captivity. J Med Primatol 39:77–82. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira DS, Nobrega YKM, Valencia CEU, Gandolfi L, Pratesi R, Castro LCG. 2012. Evaluation of 25-hydroxy-vitamin D and parathyroid hormone in Callithrix penicillata primates living in their natural habitat in Brazil. J Med Primatol 41:364–371. [DOI] [PubMed] [Google Scholar]
- 27.Thompson K. 2007. Bones and joints. In: Maxie MG. Jubb, Kennedy, and Palmer's pathology of domestic animals. Philadelphia (PA): Elsevier. [Google Scholar]
- 28.Toumba M, Neocleous V, Shammas C, Anastasiadou V, Allgrove J, Phylactou LA, Skordis N. 2013. A family with Camurati-Engelman disease: the role of the missense p.R218C mutation in TGFbeta1 in bones and endocrine glands. J Pediatr Endocrinol Metab 26:1189–1195. [DOI] [PubMed] [Google Scholar]
- 29.Virley D, Hadingham SJ, Roberts JC, Farnfield B, Elliott H, Whelan G, Golder J, David C, Parsons AA, Hunter AJ. 2004. A new primate model of focal stroke: endothelin-1-induced middle cerebral artery occlusion and reperfusion in the common marmoset. J Cereb Blood Flow Metab 24:24–41. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi A, Kohno Y, Yamazaki T, Takahashi N, Shinki T, Horiuchi N, Suda T, Koizumi H, Tanioka Y, Yoshiki S. 1986. Bone in the marmoset: a resemblance to vitamin D-dependent rickets, type II. Calcif Tissue Int 39:22–27. [DOI] [PubMed] [Google Scholar]
- 31.Yokota T, Iijima S, Kubodera T, Ishii K, Katakai Y, Ageyama N, Chen Y, Lee YJ, Unno T, Nishina K, Iwasaki Y, Maki N, Mizusawa H, Akari H. 2007. Efficient regulation of viral replication by siRNA in a non-human primate surrogate model for hepatitis C. Biochem Biophys Res Commun 361:294–300. [DOI] [PubMed] [Google Scholar]
- 32.Zuhlke U, Weinbauer G. 2003. The common marmoset (Callithrix jacchus) as a model in toxicology. Toxicol Pathol 31 Suppl:123–127. [DOI] [PubMed] [Google Scholar]