Abstract
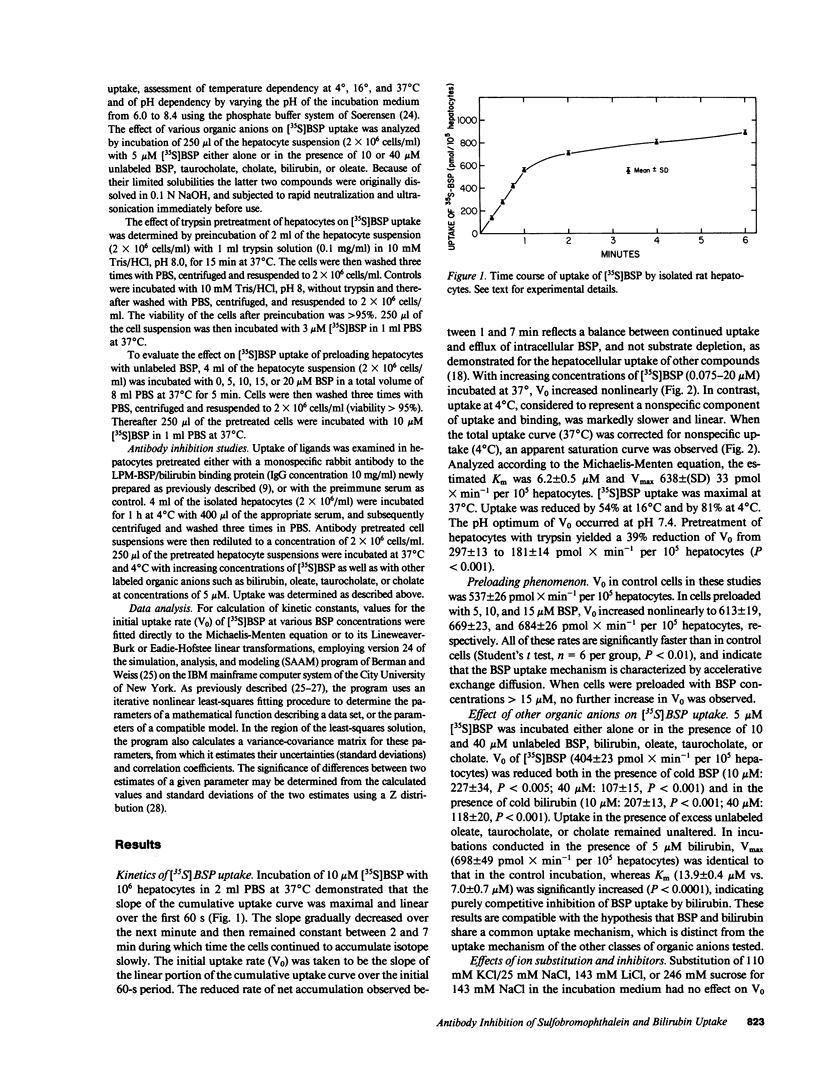
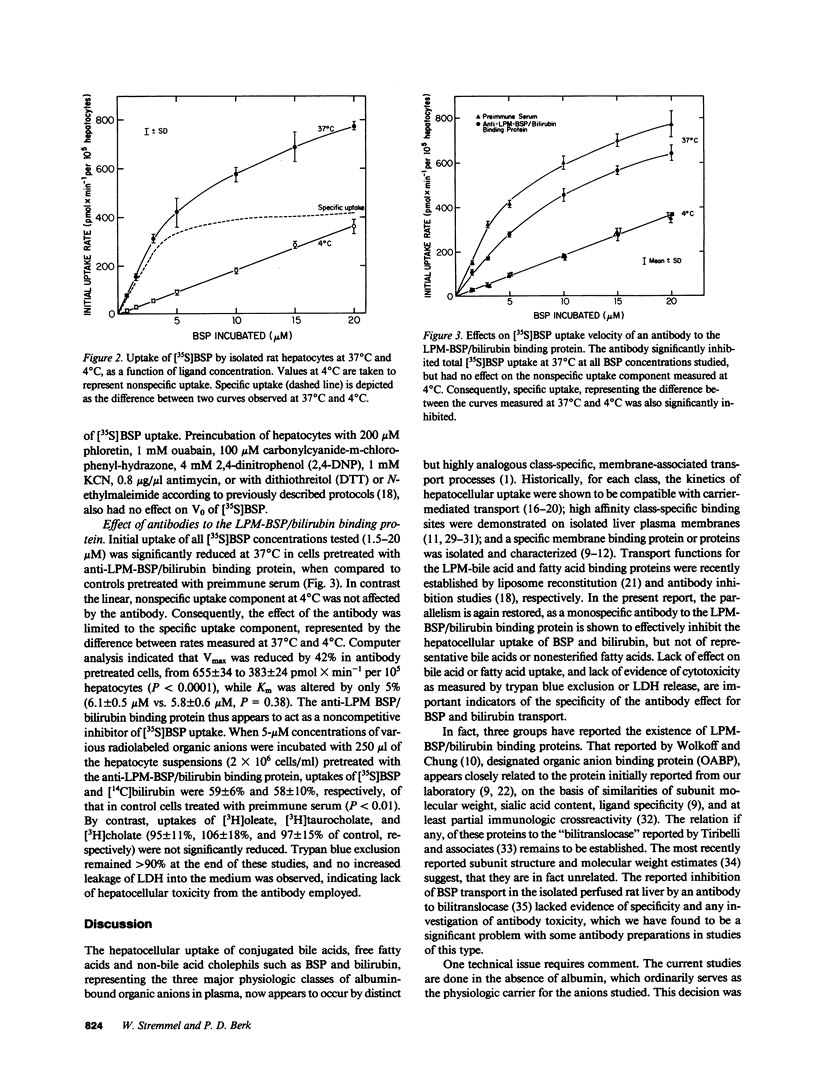
To clarify sulfobromophthalein (BSP) and bilirubin uptake mechanisms, isolated rat hepatocytes were incubated with [35S]BSP. The initial uptake velocity (V0), determined from the first, linear portion of the cumulative uptake curve, was saturable (Michaelis constant [Km] = 6.2 +/- 0.5 microM; Vmax = 638 +/- 33 pmol X min-1 per 10(5) hepatocytes), maximal at 37 degrees C and pH 7.4, and competitively inhibited by bilirubin, but not by taurocholate, cholate, or oleate. Preloading with unlabeled BSP led to trans-stimulation of V0. Sodium substitution or pretreatment of hepatocytes with ouabain or metabolic inhibitors had no effect on V0; trypsin reduced V0 by 39% (P less than 0.001). A rabbit antiserum to the rat liver plasma membrane (LPM)-BSP/bilirubin binding protein selectively reduced V0 of 5 microM [35S]BSP and [14C]bilirubin by 41 and 42%, respectively (P less than 0.01); uptakes of [3H]oleate, [3H]cholate and [3H]taurocholate were not affected. Hence, the LPM-BSP/bilirubin binding protein plays a role in the carrier-mediated uptake of BSP and bilirubin by hepatocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accatino L., Simon F. R. Identification and characterization of a bile acid receptor in isolated liver surface membranes. J Clin Invest. 1976 Feb;57(2):496–508. doi: 10.1172/JCI108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M., WEISS M. F., SHAHN E. Some formal approaches to the analysis of kinetic data in terms of linear compartmental systems. Biophys J. 1962 May;2:289–316. doi: 10.1016/s0006-3495(62)86856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. J., Bradley S. E. Binding of sulfobromophthalein (BSP) sodium by plasma albumin. Its role in hepatic BSP extraction. J Clin Invest. 1966 Feb;45(2):281–287. doi: 10.1172/JCI105341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Levy D. Characterization of the anion transport system in hepatocyte plasma membranes. J Biol Chem. 1980 Apr 10;255(7):2637–2640. [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin-mediated transport of rose bengal by perfused rat liver. Kinetics of the reaction at the cell surface. J Clin Invest. 1983 Nov;72(5):1764–1771. doi: 10.1172/JCI111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A., Snell M., Shurmantine W. O. Effect of albumin binding on the hepatic transport of rose bengal: surface-mediated dissociation of limited capacity. J Pharmacol Exp Ther. 1982 Nov;223(2):342–347. [PubMed] [Google Scholar]
- Kramer W., Buscher H. P., Gerok W., Kurz G. Bile salt binding to serum components. Taurocholate incorporation into high-density lipoprotein revealed by photoaffinity labelling. Eur J Biochem. 1979 Dec;102(1):1–9. doi: 10.1111/j.1432-1033.1979.tb06257.x. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunazzi G., Tiribelli C., Gazzin B., Sottocasa G. Further studies on bilitranslocase, a plasma membrane protein involved in hepatic organic anion uptake. Biochim Biophys Acta. 1982 Feb 23;685(2):117–122. doi: 10.1016/0005-2736(82)90087-6. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Kane J. P. Fatty acid binding protein. Isolation from rat liver, characterization, and immunochemical quantification. J Biol Chem. 1982 Jul 10;257(13):7872–7878. [PubMed] [Google Scholar]
- Paumgartner G., Reichen J. Kinetics of hepatic uptake of unconjugated bilirubin. Clin Sci Mol Med. 1976 Aug;51(2):169–176. doi: 10.1042/cs0510169. [DOI] [PubMed] [Google Scholar]
- Reichen J., Berk P. D. Isolation of an organic anion binding protein from rat liver plasma membrane fractions by affinity chromatography. Biochem Biophys Res Commun. 1979 Nov 28;91(2):484–489. doi: 10.1016/0006-291x(79)91547-x. [DOI] [PubMed] [Google Scholar]
- Reichen J., Blitzer B. L., Berk P. D. Binding of unconjugated and conjugated sulfobromophthalein to rat liver plasma membrane fractions in vitro. Biochim Biophys Acta. 1981 Jan 8;640(1):298–312. doi: 10.1016/0005-2736(81)90554-x. [DOI] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Kinetics of taurocholate uptake by the perfused rat liver. Gastroenterology. 1975 Jan;68(1):132–136. [PubMed] [Google Scholar]
- Scharschmidt B. F., Stephens J. E. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):986–990. doi: 10.1073/pnas.78.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Baldini G., Sandri G., Lunazzi G., Tiribelli C. Reconstitution in vitro of sulfobromophthalein transport by bilitranslocase. Biochim Biophys Acta. 1982 Feb 23;685(2):123–128. doi: 10.1016/0005-2736(82)90088-8. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Gerber M. A., Glezerov V., Thung S. N., Kochwa S., Berk P. D. Physicochemical and immunohistological studies of a sulfobromophthalein- and bilirubin-binding protein from rat liver plasma membranes. J Clin Invest. 1983 Jun;71(6):1796–1805. doi: 10.1172/JCI110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Kochwa S., Berk P. D. Studies of oleate binding to rat liver plasma membranes. Biochem Biophys Res Commun. 1983 Apr 15;112(1):88–95. doi: 10.1016/0006-291x(83)91801-6. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Yamada T., Kaplowitz N. Newly identified bile acid binders in rat liver cytosol. Purification and comparison with glutathione S-transferases. J Biol Chem. 1983 Mar 25;258(6):3602–3607. [PubMed] [Google Scholar]
- Tiribelli C., Lunazzi G., Luciani M., Panfili E., Gazzin B., Liut G., Sandri G., Sottocasa G. Isolation of a sulfobromophthalein-binding protein from hepatocyte plasma membrane. Biochim Biophys Acta. 1978 Jan 25;532(1):105–112. doi: 10.1016/0005-2795(78)90453-1. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A. Dissociation from albumin: a potentially rate-limiting step in the clearance of substances by the liver. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1563–1567. doi: 10.1073/pnas.82.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Sosiak A., Greenblatt H. C., Van Renswoude J., Stockert R. J. Immunological studies of an organic anion-binding protein isolated from rat liver cell plasma membrane. J Clin Invest. 1985 Aug;76(2):454–459. doi: 10.1172/JCI111993. [DOI] [PMC free article] [PubMed] [Google Scholar]



