Abstract
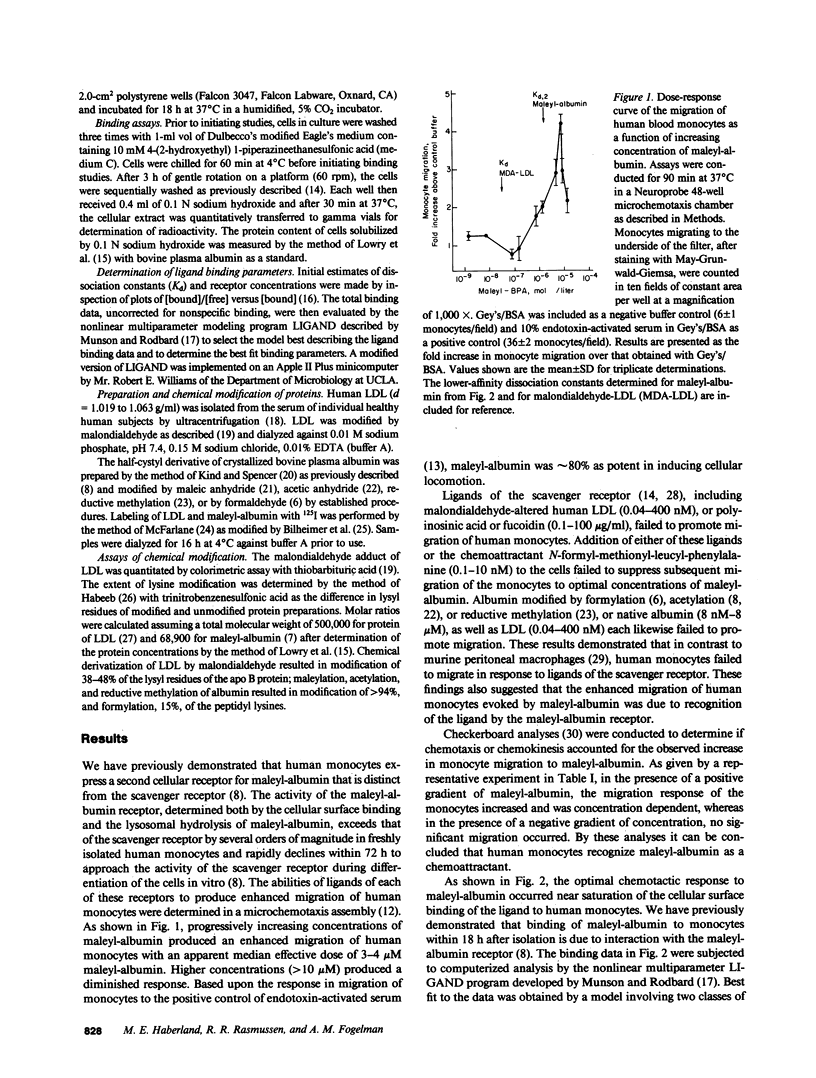
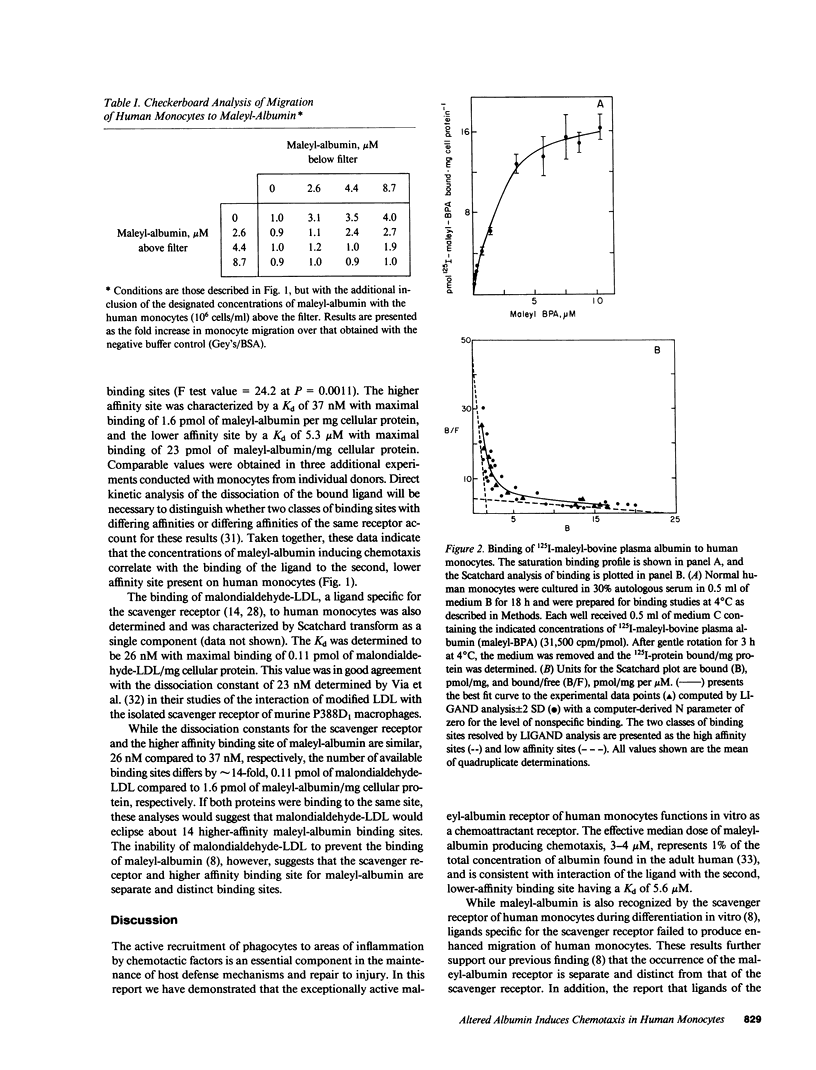
We demonstrate here that the exceptionally active maleyl-albumin receptor of human monocytes functions in vitro as a chemoattractant receptor. Chemotaxis of human monocytes occurs at an effective median dose of 3-4 microM maleyl-albumin, a concentration representing 1% of the total albumin in the adult human. Computerized analyses by LIGAND of the saturable binding of maleyl-albumin to human monocytes reveal two classes of binding sites, described by dissociation constants of 37 nM and 5.3 microM with maximal binding of 1.6 and 23 pmol maleyl-albumin/mg cellular protein, respectively. Chemotaxis of human monocytes thus occurs at concentrations of maleyl-albumin promoting binding to the lower-affinity sites. We propose that conformational isomers of albumin that are chemotactic may form in vivo and that albumin, in addition to receptor-independent plasma transport functions, may also play an important role in the receptor-mediated recruitment and accumulation of phagocytic cells at sites of inflammation and injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Schambelan M., Matulich D. T., Spindler B. J., Taylor A. A., Bartter F. C. Aldosterone receptors and the evaluation of plasma mineralocorticoid activity in normal and hypertensive states. J Clin Invest. 1976 Sep;58(3):579–589. doi: 10.1172/JCI108504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Seager J., Popják G. Abnormal induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in leukocytes from subjects with heterozygous familial hypercholesterolemia. J Biol Chem. 1975 Mar 25;250(6):2045–2055. [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M. Scavenger receptor-mediated recognition of maleyl bovine plasma albumin and the demaleylated protein in human monocyte macrophages. Proc Natl Acad Sci U S A. 1985 May;82(9):2693–2697. doi: 10.1073/pnas.82.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Haberland M. E., Rasmussen R. R., Olch C. L., Fogelman A. M. Two distinct receptors account for recognition of maleyl-albumin in human monocytes during differentiation in vitro. J Clin Invest. 1986 Mar;77(3):681–689. doi: 10.1172/JCI112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Takata K., Maeda H., Morino Y. Scavenger function of sinusoidal liver cells. Acetylated low-density lipoprotein is endocytosed via a route distinct from formaldehyde-treated serum albumin. J Biol Chem. 1985 Jan 10;260(1):53–56. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- King T. P., Spencer M. Structural studies and organic ligand-binding properties of bovine plasma albumin. J Biol Chem. 1970 Nov 25;245(22):6134–6148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Endothelial cell-derived chemotactic activity for mouse peritoneal macrophages and the effects of modified forms of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5949–5953. doi: 10.1073/pnas.82.17.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Raymond W. Monocyte chemotactic activity induced by intravitreal endotoxin. Invest Ophthalmol Vis Sci. 1985 Sep;26(9):1267–1273. [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., McInroy R. J., McKay S., McCartney A. C., Arbuthnott J. P. Effects of staphylococcal products on locomotion and chemotaxis of human blood neutrophils and monocytes. J Med Microbiol. 1976 Nov;9(4):433–439. doi: 10.1099/00222615-9-4-433. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Garlick R. L., Bunn H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984 Mar 25;259(6):3812–3817. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol. 1984;2:257–281. doi: 10.1146/annurev.iy.02.040184.001353. [DOI] [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Molecular weight and hydrodynamic properties of apolipoprotein B in guanidine hydrochloride and sodium dodecyl sulfate solutions. J Biol Chem. 1979 Mar 10;254(5):1639–1643. [PubMed] [Google Scholar]
- Via D. P., Dresel H. A., Cheng S. L., Gotto A. M., Jr Murine macrophage tumors are a source of a 260,000-dalton acetyl-low density lipoprotein receptor. J Biol Chem. 1985 Jun 25;260(12):7379–7386. [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



