Abstract
Research on the FKBP5 gene and FKBP51 protein has more than doubled since the discovery that polymorphisms in this gene could alter treatment outcomes and depressive behavior in humans. This coincided with other data suggesting that the stress hormone axis contributes to the development of numerous mental illnesses. As a result, FKBP51 now lies at the heart of the research of many stress related psychiatric disorders, which has led to advances in the understanding of this protein and its role in humans and in animal models. Specifically, FKBP5−/− mice and a naturally existing overexpression of FKBP5 in 3 genera of new world monkeys have helped understand the effects of FKBP5 in vivo. This review will highlight these finding as well as discuss the current evolutionary need for the FKBP5 gene.
Keywords: FK506-binding protein 51, FKBP5 gene, Heat shock protein 90, glucocorticoid receptor, depression, post-traumatic stress disorder, suicide, polymorphisms, genome-wide association studies, stress, behavior, resilience
Introduction
Mood disorders are characterized by feelings of sadness, frustration, loss, anger, anxiety, fear, or panic that in disease become chronic and interfere with normal life. Research in the last decade has found that dysregulation of steroid hormone receptors can cause mood disorders. The hypothalamus-pituitary-adrenal (HPA) axis, for example, which produces glucocorticoids and releases them into the blood stream, has been linked to depression [1, 2].
Steroid hormones, produced in the periphery by endocrine glands [3], can cross the blood-brain-barrier and bind to steroid hormone receptor complexes that produce changes at the cellular level and global level, such as acting as transcription factors for gene expression upregulation, altering neuronal excitability, and modifying mood and behavior. Steroid hormone receptors are ubiquitously expressed in almost all human tissues including the brain, and are especially abundant in the parts of the brain that control mood and emotion: the hypothalamus, hippocampus, amygdala, and prefrontal cortex [1]. As a result, the brain is susceptible to mood disorders generated by aberrant function of steroid hormone receptors [4].
To this end, researchers have looked for genetic and environmental factors that increase the risk of psychiatric disease. Genome wide association studies for single nucleotide polymorphisms (SNPs) have revealed significant associations between allelic variants of the FKBP5 gene and depression [5, 6], post-traumatic stress disorder (PTSD) [7], bipolar disorder [8], peritraumatic dissociation [9], suicide [10–13], negative personality traits [14], and aggression [15]. An environmental factor found to interact with this gene is stress [16, 17]. The current primary biological role of FKBP51 is thought to be with the protein heterocomplex of steroid hormone receptors within the HPA axis, where it helps regulate receptor sensitivity [18].
Due to the overwhelming evidence that stress and the FKBP5 gene are involved in psychiatric diseases, studying how this gene works is imperative to understanding the mechanisms of mood disorders and finding therapies.
HPA-axis and stress
A general definition for stress is a disruption of homeostasis due to a real or perceived threat to the well-being of the organism [1, 19]. For example, upon the perception of threat, the amygdala immediately activates the autonomic nervous system and the HPA-axis.
The hypothalamic-pituitary connection provides the brain with endocrine function [20]. This system evolved to allow the brain to turn the production of hormones that are made distally in the periphery off and on. These hormones travel through the blood, cross the blood brain barrier and bind to their specific receptors in distinctive brain areas.
The brain uses multiple hormones to trigger the synthesis of cortisol, which is the most abundant stress hormone. The Hypothalamic neurons synapse at the pituitary where they release corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor (CRF) [17]. The pituitary will then respond by producing adrenocorticotropic hormone (ACTH), and releasing it into the blood stream. ACTH will bind to its receptor in the adrenal gland, which is located above the kidneys, and trigger the adrenal cortex to synthesize glucocorticoids, including cortisol, and release them into the blood stream [17].
Glucocorticoids have two main receptors in the brain, the mineralcorticoid receptor (MR) and the glucocorticoid receptor (GR). At low concentrations, the MR is the main active receptor, while at high concentrations the GR is the main active receptor since all MRs are occupied [21, 22].
The MR and GR are cytoplasmic receptors that translocate to the nucleus upon hormone binding [23]. There, they activate as well as suppress transcription of many genes. The system is also self-regulating, such that activation of GR triggers a negative feedback loop that attenuates the axis and consequently stress-hormone production [17]. Thus the rate of feedback inhibition can have dramatic impact on the length of time that the stress hormone system is active. GR is in virtually every tissue of the body, helping to coordinate the body’s response to stress; however the feedback inhibition primarily occurs in the amygdala, hypothalamus, and the pituitary to shut down production. Some systemic functions of GR activation other than those affecting mood, decision-making or memory function, include increased metabolism and inhibition of inflammation [24].
Cellular role and biochemistry of FKBP51 with focus on steroid hormone receptors
FKBP51 (FK506 binding protein 51) is part of the immunophilin family, a superfamily of highly conserved proteins first characterized by their ability to bind to immunosuppressant drugs [25]. The superfamily is divided into two sequence families by the type of immunosuppressant to which it binds. FKBPs are able to bind to FK506 and rapamycin, two immunosuppressants of fungal origin. In addition to their drug-binding capability, some FK506-binding immunophilins are also protein chaperones, with the related but apparently separate ability to isomerize proline residues [25].
FKBP51 (FK-506 Binding Protein 51kDa, p54, FKBP54) was originally identified as a novel FK-506 binding protein capable of peptidyl-prolyl cis-trans isomerization (PPIase) activity [26]. PPIases, like FKBP51, are able to change the conformation of proline residues, a unique amino acid with the capability of existing in cis and trans conformations [25]. Proline cis-trans transitions are important for proper protein folding [25], yet deletion of the N-terminal PPIase domain (FK1) had little effect on FKBP51’s efficacy as a chaperone. Instead, the C-terminus of FKBP51, which includes three highly degenerate 34-amino-acid repeats known as tetratricopeptide repeats (TPRs), has been found to exhibit independent protein-folding activity.
Up until recently, however, FKBP51 was relatively unstudied, particularly because its function in cellular processes was not well known. In fact, it is not understood whether PPIase activity in general is necessary for cell viability. But studies have begun to elucidate its role in the biology of the cell. For example, FKBP51 was recently found to regulate the phosphorylation of the microtubule associating protein tau (MAPT, tau), a protein whose aggregates are a hallmark of Alzheimer’s disease [27]. FKBP51, and other immunophilins, have also been found to interact with steroid hormone receptor heterocomplexes, an interaction mediated by the chaperone heat shock protein 90 (Hsp90) [28], [29].
Three hormone receptor complexes that interact with FKBP51 are the glucocorticoid receptor (GR), progesterone receptor (PR), and androgen receptor (AR). Each, are transcriptionally active steroid hormone receptor complexes whose activation leads to a change in the transcription rate of many genes, one of which is FKBP5. Paradoxically, FKBP51 also inhibits the activation of GR [30] and PR [31]. These receptors thus have a short negative feedback loop built into their activity, while AR, on the other hand, is positively regulated by FKBP51 [32]. Two questions arise from the effect of FKBP51 on GR, PR and AR. Is PPIase activity necessary? Is binding to Hsp90 necessary?
The exact mechanism of action within the steroid hormone receptor heterocomplex has been difficult to piece together, since the importance of PPIase activity of immunophilins is questionable. One study analyzing the relative importance of the PPIase and TPR domains in squirrel monkey FKBP51 rendered each of the protein domains inactive via site-directed mutagenesis and showed that while only the TPR domain was required for FKBP51 to bind to the GR and PR complex with Hsp90, both PPIase and TPR domains were required for the FKBP51 inhibitory effect on GR to be effective [32]. Other studies, however, showed that PPIase activity is not necessary for its inhibitory action on GR [33] but is necessary for its activating action on AR [34]. All three complexes do require Hsp90 for FKBP51 to have an effect, as Hsp90 was found necessary to be bound to FKBP51 for it to have its inhibitory action on GR [32], PR [31], and its activating action on AR [34]. The data indicate that, perhaps due to structural reasons, the physical presence of FKBP51 may be more important than its PPIase enzymatic activity.
SNPs and FKBP51 mechanism of action
Genome-wide association studies (GWAS) have enabled scientists to look at small changes in DNA and the effects of single base pair variations called single nucleotide polymorphisms (SNPs) on gene expression and function. SNPs in FKBP5 have been associated with mood disorders and an increased risk for PTSD, suicide, and overt aggressive behavior. One particular SNP in the FKBP5 gene, rs1360780, was associated with an increased number of depressive episodes [5]. A closer look at the SNP revealed three different polymorphisms possible at the site: TT homozygotes, CT heterozygotes, and CC homozygotes. Association of these SNP variations with episodes of depression and responses to antidepressant treatment showed that individuals with TT homozygosity had more frequent episodes of depression but also a faster recovery from antidepressant therapy. SNP association studies were performed with FKBP51 and other psychiatric disorders, with findings that FKBP51 SNPs are also associated with mood disorders, many of which were formed in conjunction with childhood trauma. Increased risks of developing PTSD [7], suicide [10, 12], and overt aggressive behavior were also associated with certain FKBP51 polymorphisms [15].
Mechanistically, the effect of FKBP5 polymorphisms on mood disorders is elusive. One particular area of study is the HPA axis, as FKBP51 is a known regulator of HPA-axis activity. The HPA-axis has major roles in both PTSD and depression, albeit with opposite phenotypes. Patients with Major Depressive Disorder (MDD) have a hyperactive HPA-axis, where an increased stimulation of adrenal corticotropic hormone (ACTH) and GR desensitization cause high amounts of cortisol to be released and retained in the bloodstream. PTSD patients, on the other hand, have hypersensitive GR and a hypersuppression of cortisol [16]. The SNP rs1360780 is thus of great interest here, as it is associated with increased risks of both PTSD and depression, despite these diseases having different GR sensitivities. For example, the TT genotype may provide mechanistic evidence on how FKBP51 could cause HPA-axis dysregulation, as it was shown that individuals with TT had higher basal levels of FKBP51 and thus very likely a greater inhibition effect on GR activity. However Depressed patients with TT genotypes had greater decreases of ACTH and cortisol levels in response to the DEX/CRH test than depressed patients with CC and TC genotypes. The study did not, however, compare the FKBP51 levels of healthy controls with the TT genotype versus depressed patients with the same genotype, and so it is unclear if FKBP51 levels are higher due to depression or if all people with the genotype exhibit increased levels of FKBP51. Nevertheless healthy individuals with the TT genotype were shown to lack a normalization of cortisol levels after stress as compared to the CC and CT genotypes; TT individuals had higher cortisol levels for a longer period of time [35]. The data from the healthy controls resembled those having high FKBP51 levels, and the data of the depressed patients resembles those having low FKBP51 levels.
How do we make sense of the fact that people with the same genotype had different GR phenotypes depending on the disease state? It may be that healthy individuals only have high levels of FKBP51 in the short term, but the chronic stress state may increase FKBP51 levels in the long term, which could drive adaptive changes in GR activity. However, if FKBP51 protein levels were elevated in this genotype irrespective of disease state, it may be that another risk factor is interacting with FKBP51 to promote depression. In individuals with this genotype with probable PTSD, baseline cortisol activity was not elevated as compared to healthy controls [36], while it was in depressed patients [5]. Thus it is clear that while genotypes do not consistently affect GR in the same way, the risk for mood disorders is clearly elevated by the rs1360780 SNP. This suggests that factors other than just GR must be responsible for the differential disease phenotypes.
FKBP5 animal models
Studies of FKBP51 in animal models have proven helpful in determining endogenous and stress-response levels of FKBP51 expression in different regions of the brain. A study of FKBP5 mRNA levels in the murine brain discovered that under basal conditions, the highest levels occurred in the hippocampus [37], especially the dentate gyrus, and the premammillary nucleus, the motor nuclei of the nervus trigeminus and the nervus facialis. Interestingly, lower levels of FKBP5 mRNA were found in the amygdala and the hypothalamic paraventricular nucleus (PVN), the latter of which is a part of the HPA axis and a stress response mediator. Stressing the mice, however, showed a marked increase in FKBP5 mRNA levels in these two regions. Both moderate and short-term stress and strong and long-lasting stress caused FKBP5 mRNA levels to rise in dose-dependent fashion. Moderate stress was caused through a 4-hour restrain, and a 1-day food deprivation paradigm was used as the long-lasting stressor. Upregulation of FKBP5 mRNA was observed in the central amygdala, the hypothalamic PVN, and the hippocampus after both conditions, with a stronger response after food deprivation in all regions. Injecting the mice with dexamethasone, a corticosteroid analog and GR agonist, also increased the levels of FKBP51 mRNA, supporting the hypothesis that the stress response and FKBP5 mRNA levels are linked to the GR.
However, while all three regions showed increased levels of FKBP5 mRNA, the relative increase differed based on the baseline levels already observed in the mouse brain. The hypothalamic PVN and the central amygdala, which had low endogenous FKBP5 mRNA levels, had average FKBP5 mRNA increases of over 600% from baseline, while the hippocampus, which has a relatively high basal level of FKBP5 mRNA, had average increases of less than 200%. It appears that higher endogenous levels of FKBP5 mRNA caused the GR in those particular brain regions to become less responsive to corticosteroid stimulation. In cases of chronic stress, constant high levels of stress could result in potentially long-term elevated corticosteroid levels, and induce high levels of FKBP5 mRNA expression, in turn causing GR activity to become resistant.
Two additional models have been studied intensively, FKBP5−/− mice and new world monkeys. Three genera of new world monkeys have been discovered to have naturally occurring GR resistance due to excess production of FKBP51 [38]. This begs the question of whether this affects the behavior of the monkeys. An induced model of depression in monkeys exist [39], and extreme social stress causes coronary artery disease [40], presumably due to hypercortisolemia, but it is not clear whether it is FKBP5 dependent. However, it does suggest that the hypercortisolemia seen in humans with depression could be aided by a reduction in the levels of FKBP51.
The function of the HPA axis was studied in the FKBP51−/− mice. The mice displayed a moderate GR hypersensitivity, as they produced less corticosterone (the main murine glucocorticoid) and recovered faster after dexamethasone (DEX) and stress treatment [41]. The DEX/CRH test was compared between FKBP51−/− mice and healthy humans of the rs1360780 SNP. Healthy humans with the TT genotype displayed GR hyposensitivity to dexamethasone while the FKBP51−/− mice displayed GR hypersensitivity, showing that reduction in levels of FKBP51 may be a successful therapy for GR insensitivity.
In terms of protein levels and protein expression, FKBP51−/− mice exposed to chronic social defeat stress produced equal amounts of CRH mRNA as compared to wildtype before and after stress, although levels did increase after stress [42]. This demonstrated that corticosterone levels were not reduced in the FKBP51−/− mice due to the lack of HPA activation, but rather due to the FKBP51-GR negative feedback mechanism that has been characterized so well. It was found, on the other hand, that the lack of FKBP51 alters the levels of GR. In wildtype mice, GR levels decreased during a 1 hr restraint stress experiment, yet in FKBP5−/− mice they increased at 15 minutes then went down slightly, but remained significantly higher than wildtype. It is also possible that FKBP51 may actually be regulating the levels of GR, particularly since Hsp90 is intimately linked to proteasomal degradation [43] and Hsp90 is a part of the GR complex.
Surprisingly, general behavior was not changed in 10–16 week old (young) FKBP5−/− mice. As a result, changes in behavior after stress were studied. Young FKBP5−/− mice showed significantly less time immobile in the forced swim test than wildtype mice after restraint stress [41]. Moreover, FKBP51 is a protein whose expression increases with age [27], and young mice have low basal levels in the hypothalamus and amygdala. It may be that the young mice needed FKBP51 to be expressed after stress to modulate behavior, since basal FKBP51 levels were low. Twenty-two month old mice (old), however, displayed this phenotype in the forced swim test without prior stress [44]. General behavior in the old mice was also unchanged, especially in learning and memory, implying that FKBP51 may have a role limited to the biology of the stress response. But this role may be protective, as stress and its related molecules are known to cause cognitive impairment [45–51].
FKBP51 and neuropsin-mediated effects in the amygdala
Recently, FKBP51 was found to be involved in stress response in the amygdala. FKBP51 was found to be upregulated by NMDA activation and caused anxiety in mice after stress [52]. This effect was dependent on the cleavage of EphB2 by neuropsin, causing EphB2 to disassociate from the NR1 subunit of the NMDA receptor and enhancing NMDA receptor current. Neuropsin is an extracellular serine protease and EphB2 is a receptor tyrosine kinase, and both are heavily expressed in the amygdala and hippocampus, with higher basal expression in the hippocampus, similar to FKBP51. After stress, neuropsin is dramatically upregulated in the amygdala. This data was not shown in the hippocampus; it would be interesting to find out if neuropsin had a similar expression profile to FKBP51 and to see the basal and stress-induced expression of FKBP51 in neuropsin knockout mice. In amygdala neuronal cell cultures, the majority of FKBP51 expression was found to be due to the activity of neuropsin and not by corticosterone, and it may be that FKBP51 has a primary role in stress behavior in a neuropsin dependent mechanism, and that its role with GR is secondary to that. Additionally, despite FKBP51 expression upregulation by NMDA receptor activation, no effects are shown in learning and memory. It may be that a large threshold of NMDA receptor activation is needed to trigger the upregulation of FKBP51, and the large threshold would only be reached during extreme situations like stress, and thus FKBP5 would be activated to promote behavioral signatures of anxiety.
The need of the FKBP51 gene in the brain is unclear, as it seems to be extremely problematic, and its absence does not seem to have negative effects. A posited theory for the development of FKBP51 may be within the fear response. Evolutionary genetics suggest that these genes arose early in development; analysis of phylogenetic relationships among 100 FKBP domains show a clustering pattern that suggests the emergence of the FKBP genes early in eukaryotic evolution [53], and another study suggests that FKBP proteins arose even before the divergence of prokaryotes from eukaryotes [54]. As a result, FKBP5 may have played an important role in the evolution of the stress response. In the past we needed to exhibit a proper response to fear, since the reaction was critical to survival. The problem is, we no longer have the same life or death triggers.
Conclusion
It is clear that FKBP51 is a significant player in the human response to stress. It has a dramatic effect on the biology of steroid hormone receptors, and is upregulated in the amygdala as part of a stress response. SNPs within the FKBP5 gene are associated with mood disorders, but the mechanism of how this happens is not well understood. Surprisingly, the lack of FKBP51 does not appear to have deleterious side effects in mice and its presence causes steroid hormone receptor hypersensitivity. This not only makes FKBP51 an excellent drug target, but it also suggests that FKBP5 may be genetic baggage that no longer provides a competitive advantage for natural selection. As successful adaptation allows for a species to age, genes like FKBP5, which at one point during the evolutionary process were necessary for their survival and propagation, may actually become deleterious to the aging process. Furthermore, societal success no longer follows Darwinian principles. As a result, it may be the role of society to combat these processes; in the same way that bacteria adapt to antibiotic pressure, society may need to develop ways to suppress or even remove these genetic leftovers.
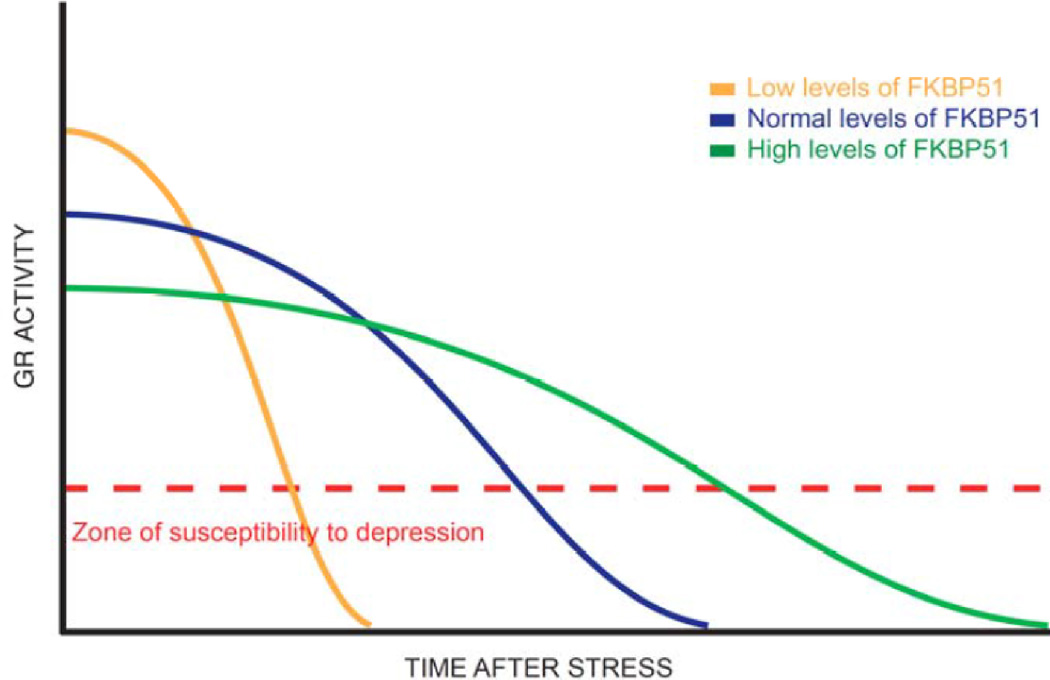
Fig. 1. FKBP5 genotype as a modulator of GR phenotype.
Everyone may be vulnerable to mood disorders involving depression following stress. However, the length of time that an individual remains vulnerable can be dramatically affected by FKBP51 levels due to its ability to decrease GR activity. FKBP51 may widen the length of time after stress that a person is susceptible to stress, while individuals with low FKBP51 levels may be in this state for a shorter period of time. While the magnitude of the acute GR response to stress following an insult may be higher in individuals with low FKBP51 levels, the length of time that an individual with high levels of FKBP51 spends in a susceptible state is longer. As a result, SNP’s in the FKBP5 gene may increase the likelihood of developing psychiatric disorders by altering the levels of FKBP51. This in turn changes the responsiveness of the GR, whose balance appears to be critical for normal function. Thus, in MDD, chronic stress may have a greater effect on those individuals with too much FKBP51.
LIST OF ABBREVIATIONS
- ACTH
Adrenocorticotropic hormone
- AR
adrogen receptor
- CRH/CRF
Corticotropin releasing hormone/factor
- FKBP5
Gene name for FKBP51 protein
- FKBP51
FK506-binding protein 51
- GR
Glucocorticoid receptor
- GWAS
Genome-wide association studies
- HPA-axis
Hypothalamic-pituitary-adrenal-axis
- MDD
Major depressive disorder
- MR
Mineralocorticoid receptor
- NMDA
N-methyl-D-aspartate
- PPIase
Peptydil-prolyl cis-trans Isomerase activity
- PR
Progesterone receptor
- PTSD
Post-traumatic stress disorder
- PVN
Paraventricular nucleus of the hypothalamus
- TPR
Tetratricopeptide repeat
REFERENCES
- 1.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005 Jun;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 2.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000 Nov;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 3.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nature reviews Neuroscience. 2005 Jul;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 4.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998 Jun;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 5.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004 Dec;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 6.Tatro ET, Nguyen TB, Bousman CA, Masliah E, Grant I, Atkinson JH, Everall IP. Correlation of major depressive disorder symptoms with FKBP5 but not FKBP4 expression in human immunodeficiency virus-infected individuals. Journal of neurovirology. 2010 Oct;16(5):399–404. doi: 10.3109/13550284.2010.504248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010 Jul;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, MacKinnon DF, Mondimore FM, Schweizer B, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Molecular psychiatry. 2009 Mar;14(3):261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular psychiatry. 2005 Dec;10(12):1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 10.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010 Jul;35(8):1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supriyanto I, Sasada T, Fukutake M, Asano M, Ueno Y, Nagasaki Y, Shirakawa O, Hishimoto A. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Progress in neuro-psychopharmacology & biological psychiatry. 2011 Jan 15;35(1):252–256. doi: 10.1016/j.pnpbp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of psychiatric research. 2012 Jan;46(1):72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitiello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, DeBar L, Keller M. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. The American journal of psychiatry. 2010 Feb;167(2):190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibuya N, Suzuki A, Sadahiro R, Kamata M, Matsumoto Y, Goto K, Hozumi Y, Otani K. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neuroscience letters. 2010 Nov 26;485(3):194–197. doi: 10.1016/j.neulet.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch MA. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Archives of general psychiatry. 2012 Jan;69(1):62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009 Dec;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009 Jun;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 18.Jaaskelainen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Current opinion in pharmacology. 2011 Aug;11(4):326–331. doi: 10.1016/j.coph.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992 Mar 4;267(9):1244–1252. [PubMed] [Google Scholar]
- 20.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 22.Arriza JL, Simerly RB, Swanson LW, Evans RM. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 23.Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. The European journal of neuroscience. 2001 Aug;14(4):675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000 Feb;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 25.Barik S. Immunophilins: for the love of proteins. Cellular and molecular life sciences : CMLS. 2006 Dec;63(24):2889–2900. doi: 10.1007/s00018-006-6215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiederrecht G, Hung S, Chan HK, Marcy A, Martin M, Calaycay J, Boulton D, Sigal N, Kincaid RL, Siekierka JJ. Characterization of high molecular weight FK-506 binding activities reveals a novel FK-506-binding protein as well as a protein complex. J Biol Chem. 1992 Oct 25;267(30):21753–21760. [PubMed] [Google Scholar]
- 27.Jinwal UK, Koren J, 3rd, Borysov SI, Schmid AB, Abisambra JF, Blair LJ, Johnson AG, Jones JR, Shults CL, O'Leary JC, 3rd, Jin Y, Buchner J, Cox MB, Dickey CA. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Jan 13;30(2):591–599. doi: 10.1523/JNEUROSCI.4815-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996 Dec 13;271(50):32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 29.Davies TH, Ning YM, Sanchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005 Feb 15;44(6):2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 30.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000 Nov;141(11):4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 31.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003 Jun;144(6):2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 32.Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005 Jul;146(7):3194–3201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- 33.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005 Feb 11;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 34.Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. Mar;30(5):1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. The European journal of neuroscience. 2008 Jul;28(2):389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 36.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA : the journal of the American Medical Association. 2008 Mar 19;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharf SH, Liebl C, Binder EB, Schmidt MV, Muller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PloS one. 2011;6(2):e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001 Nov;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 39.Clark LD, Gay PE. Behavioral defeat in squirrel monkeys: an experimental model of reactive depression. Psychological reports. 1980 Dec;47(3 Pt 2):1175–1184. doi: 10.2466/pr0.1980.47.3f.1175. [DOI] [PubMed] [Google Scholar]
- 40.Petticrew M, Davey Smith G. The monkey puzzle: a systematic review of studies of stress, social hierarchies, and heart disease in monkeys. PloS one. 2012;7(3):e27939. doi: 10.1371/journal.pone.0027939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011 Nov 15;70(10):928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB, Schmidt MV. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012 Jan;62(1):332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Ballatore C, Brunden KR, Piscitelli F, James MJ, Crowe A, Yao Y, Hyde E, Trojanowski JQ, Lee VM, Smith AB., 3rd Discovery of brain-penetrant, orally bioavailable aminothienopyridazine inhibitors of tau aggregation. Journal of medicinal chemistry. 2010 May 13;53(9):3739–3747. doi: 10.1021/jm100138f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Leary JC, 3rd, Dharia S, Blair LJ, Brady S, Johnson AG, Peters M, Cheung-Flynn J, Cox MB, de Erausquin G, Weeber EJ, Jinwal UK, Dickey CA. A New Anti-Depressive Strategy for the Elderly: Ablation of FKBP5/FKBP51. PloS one. 2011;6(9):e24840. doi: 10.1371/journal.pone.0024840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004 Dec;5(12):917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 46.de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394(6695):787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 47.Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16(7):571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- 48.Diamond DM, Park CR, Campbell AM, Woodson JC. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus. 2005;15(8):1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- 49.Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14(3):281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- 50.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 51.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992 Oct;2(4):421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 52.Attwood BK, Bourgognon JM, Patel S, Mucha M, Schiavon E, Skrzypiec AE, Young KW, Shiosaka S, Korostynski M, Piechota M, Przewlocki R, Pawlak R. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011 Apr 20; doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson CE, Gao J, Rooney AP, Davis EC. Genomic organization of mouse and human 65 kDa FK506-binding protein genes and evolution of the FKBP multigene family. Genomics. 2002 Jun;79(6):881–889. doi: 10.1006/geno.2002.6777. [DOI] [PubMed] [Google Scholar]
- 54.Trandinh CC, Pao GM, Saier MH., Jr Structural and evolutionary relationships among the immunophilins: two ubiquitous families of peptidyl-prolyl cis-trans isomerases. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992 Dec;6(15):3410–3420. doi: 10.1096/fasebj.6.15.1464374. [DOI] [PubMed] [Google Scholar]