Abstract
All-trans retinoic acid (RA) is a potent inducer of regeneration. Because the liver is the principal site for storage and bioactivation of vitamin A, the current study examines the effect of RA in mouse hepatocyte proliferation and liver regeneration. Mice that received a single dose of RA (25 μg/g) by oral gavage developed hepatomegaly with increased number of Ki67-positive cells and induced expression of cell cycle genes in the liver. DNA binding data revealed that RA receptors retinoic acid receptor β (RARβ) and retinoid x receptor α (RXRα) bound to cell cycle genes Cdk1, Cdk2, Cyclin B, Cyclin E, and Cdc25a in mice with and without RA treatment. In addition, RA treatment induced novel binding of RARβ/RXRα to Cdk1, Cdk2, Cyclin D, and Cdk6 genes. All RARβ/RXRα binding sites contained AGGTCA-like motifs. RA treatment also promoted liver regeneration after partial hepatectomy (PH). RA signaling was implicated in normal liver regeneration as the mRNA levels of RARβ, Aldh1a2, Crabp1, and Crbp1 were all induced 1.5 days after PH during the active phase of hepatocyte proliferation. RA treatment prior to PH resulted in early up-regulation of RARβ, Aldh1a2, Crabp1, and Crbp1, which was accompanied by an early induction of cell cycle genes. Western blotting for RARβ, c-myc, Cyclin D, E, and A further supported the early induction of retinoid signal and cell proliferation by RA treatment. Taken together, our data suggest that RA may regulate cell cycle progression and accelerates liver regeneration. Such effect is associated with an early induction of RA signaling, which includes increased expression of the receptor, binding proteins, and processing enzyme for retinoids.
Keywords: retinoic acid receptor, nuclear receptor, hepatocyte, partial hepatectomy, proliferation
1. Introduction
All-trans retinoic acid (RA) has long been recognized as a regeneration-inducing derivative of vitamin A. In amphibians, RA causes “super-regeneration” of naturally regenerative organs, while in mammals, RA induces regeneration of organs that do not normally regenerate, such as the adult mammalian lung (1–3). Interestingly, the liver is the only mammalian organ with highly regenerative properties, making it a prime target for accelerated regrowth through RA. As the principal site for storage and bioactivation of vitamin A in the body, the liver experiences constant RA exposure. Lecithin:retinol acyltransferase-deficient mice, completely lacking in hepatic retinoid stores, have reduced RA levels and impaired liver regeneration in response to partial hepatectomy (PH). Thus, hepatic retinoid storage is required for normal liver regeneration (4).
Despite the essential role of endogenous RA on liver regeneration, there lacks a consensus on the effects of exogenous RA on hepatocyte proliferation in vivo, since previous studies have yielded conflicting results (5–8). Early in vitro studies reported that RA is a potent inhibitor of DNA synthesis in primary rat hepatocytes (9). Similarly, when administered after PH, RA disrupted rat hepatocyte proliferation in vivo by repressing early response genes and increasing the activity of transglutaminase and ornithinedecarboxylase (10–14). In contrast to these findings, other studies have shown that RA enhances hepatocyte proliferation and survival. RA augmented TNFα-stimulated mouse hepatocyte DNA synthesis in vitro (15). The direct mitogenic effect of RA has also been demonstrated in normal hepatocyte proliferation of both mice and rats (16, 17). Post-operative administration of RA or synthetic retinoid NIK33 enhanced hepatocyte proliferation in the regenerating rat liver (18).
It is problematic that the existing literature has such contradictory findings since RA is considered a chemotherapeutic agent against certain types of cancer. Despite its anti-proliferative and differentiation effects against malignant cells, it is necessary to acknowledge the role of RA in proliferation and regeneration as well as the clinical implications. Although RA is often used to treat patients with acute promyelocytic leukemia (APL), major complications can manifest in the form of hepatomegaly and hepatotoxicity (19). The conflicting results from previous studies may be attributable to differences in dosage, route of administration, and animal species. Another important factor to consider is the timing of exogenous RA administration. In the previous studies, RA was administered after surgical resection of the liver (10–14, 18). The hepatoprotective role of RA preconditioning has recently been proposed in an ischemic/reperfusion injury model (20), but there is no evidence in the literature that clearly documents the effects of RA treatment prior to PH-induced liver regeneration. Therefore, this study aims to clarify the role of RA in the regenerating liver by demonstrating, for the first time, the effects of RA administration prior to PH.
In this study, RA pretreatment is shown to accelerate PH-induced liver regeneration in mice by increasing the expression of genes encoding Cyclin-Cdk complexes. Moreover, the data indicate that RA pretreatment promoted hepatocyte proliferation in the regenerating mouse liver by modulating cell cycle progression through direct binding by retinoic acid receptor β (RARβ) and retinoid x receptor α (RXRα). Together, these findings emphasize the potential utility of employing retinoids to facilitate liver regrowth following injury.
2. Materials and Methods
2.1 Mice, partial hepatectomy, and sample preparation
Wild type (WT) male mice (3–5 months old) were housed in steel micro-isolator cages (4 mice per cage) at 22°C with a 12-hr/12-hr light/dark cycle. Food and water were provided ad libitum throughout the entire study. RA (25 μg/g) (Sigma-Aldrich Corp., St. Louis, MO) or vehicle control (carboxymethyl cellulose) (Sigma-Aldrich Corp., St. Louis, MO) was administered by oral gavage 48 hours prior to surgery. Standard two-thirds liver resection was performed using the procedure previously described (21–23). Sham-operated mice were included as controls. Surgeries were performed between 9:00 to 11:00 AM. Mice were killed at indicated time points and their liver and body weight recorded at the time of death were used to calculate liver-to-body weight ratio. The data presented were calculated from the mean of three to five mice per time point. Liver tissues were collected and snap frozen in liquid nitrogen and stored at −80°C. A section of each liver was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin for histological analysis. All animal experiments were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee (IACUC) at the University of California, Davis.
2.2 Ki67 immunostaining
To assess hepatocyte proliferation, immunostaining was performed with anti-Ki67 antibody (NeoMarkers, Fremont, CA). The number of Ki67-labeled nuclei was counted in at least 15 microscopic fields (20X) for each liver section.
2.3 Chromatin immunoprecipitation followed by sequencing (ChIP-Seq)
ChIP analyses were performed as described previously (5). Briefly, chromatin lysate was precleared before incubation with a ChIP-quality anti-RARβ antibody (Santa Cruz, CA). Anti-IgG (Santa Cruz, CA) and anti-RNA Polymerase II antibodies (Millipore, MA) were used as negative and positive controls, respectively. Target sequences were aligned to the mouse genome based on USC genome browser with Bowtie 0.12.7 (24) followed by peak-calling using Model-based Analysis of ChIP-Seq (MACS) 1.4.1 (25). The called peaks were annotated against the database (GRCm38/mm10) by Peak Analyzer (26). Motif Analysis of Large DNA Datasets (MEME-ChIP) was used to identify potential motifs for target binding.
2.4 Quantification of hepatic mRNA
Hepatic RNA isolated by TRIzol (Invitrogen, Carlsbad, CA) was reverse transcribed to generate cDNA followed by amplification using the ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Hepatic mRNA levels were normalized to the mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers were designed using Primer3 Input Software (v0.4.0) and sequences are available upon request.
2.5 Western blot
Liver protein (40 μg) was electrophoresed on SDS-polyacrylamide gels under reducing conditions. Proteins from the gels were transferred to the polyvinylidene fluoride membranes. Anti-Cyclin A, Cyclin D, Cyclin E (Cell Signaling Technology, MA), c-myc, RARβ, and β-Actin (Santa Cruz, CA) antibodies were used for detection of proteins. Protein expression levels were quantified using ImageJ.
2.6 Statistical Analysis
Data are given as mean ± SD. Statistical analysis was performed using Student’s t test or one-way analysis of variance. Statistical significance was defined by p < 0.05.
3. Result
3.1 RA induces hepatocellular proliferation in mice
WT mice received oral gavage with RA or vehicle 48 hours prior to a sham operation. Liver-to-body weight ratio was significantly higher in RA-treated versus control mice 1 to 7 days after sham operation (Fig. 1A). The RA-induced increase in liver mass correlated with greater hepatocellular proliferation, as shown by increased numbers of Ki67-positive hepatocytes at studied time points (Fig. 1B, C). Together, results from the sham-operated RA treatment group indicate that the administration of RA promotes hepatomegaly and cell proliferation in the normal mouse liver.
Figure 1. Increased hepatocyte proliferation in RA-treated mouse liver.
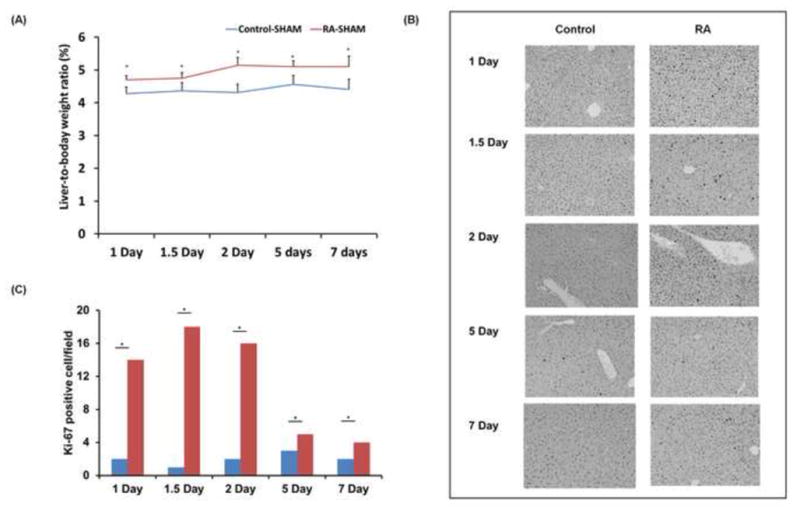
WT mice were treated with RA or vehicle by oral gavage 48 hours prior to a sham operation. (A) Mice were sacrificed and weighed 1, 1.5, 2, 5 and 7 days after surgery to calculate differences in liver to body weight ratio between the groups. (B) Representative photomicrographs of Ki67 immunohistochemical staining of liver sections from WT mice with and without RA treatment at indicated time points (n≥4). (C) Ki67-positive cells in the livers of WT mice with and without RA pre-treatment after sham operation. The number of proliferating hepatocytes was determined by counting Ki67-positive hepatocytes in at least 15 microscopic fields (20X) per liver sample. Liver sections from all mice were used for analyses. Means ± SD are graphed with * indicating p < 0.05.
3.2 RA enhances RARβ/RXRα binding to cell cycle genes
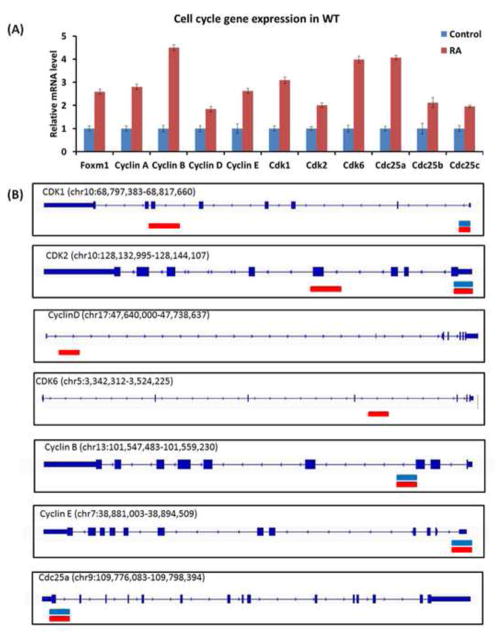
RARβ is a RA-inducible receptor that has been shown to be up-regulated during the early phase of liver regeneration (27). RARβ forms a heterodimer with RXRα to bind target genes and regulate their transcription. RA-induced hepatomegaly and hepatocyte proliferation were studied on a genomic level by analyzing RARβ and RXRα common DNA binding sites in livers derived from WT mice with or without RA treatment (5, 28). The results suggest that RARβ/RXRα binding mediates the effect of RA on cell cycle regulation. In the vehicle-treated group, 34 out of 129 cell cycle genes obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG) cell cycle pathway database were bound by RARβ/RXRα heterodimer. In the RA-treated group, there was a significant increase in both the number of cell cycle genes (50 vs. 34) that were bound by RARβ/RXRα as well as the number of peaks (66 vs. 41). These findings are consistent with the observation that RA treatment enhanced cell proliferation. Cell cycle regulatory genes were modulated by RA treatment in a manner supportive of accelerated cell cycle progression. From the KEGG pathway database, the expression of 18 cell cycle genes was studied and 11 of them demonstrated significantly higher mRNA levels following RA treatment (Fig. 2A). Of these 11 genes, five (Cyclin-dependent kinase 1 (Cdk1), Cdk2, Cyclin B, Cyclin E, Cell division cycle 25a (Cdc25a)) shared common RARβ/RXRα binding in both control and RA-treated groups. Additional RARβ/RXRα binding was noted in the Cdk1 and Cdk2 genes after RA treatment. RARβ/RXRα binding was absent in the Cyclin D and Cdk6 genes in the control group, but was induced in the RA treatment group (Fig. 2B). All aforementioned RARβ/RXRα binding regions contained AGGTCA-like motifs as hormone response elements. However, RARβ/RXRα binding was not found in forkhead box M1 (Foxm1), Cyclin A, Cdc25b, and Cdc25c genes even though RA treatment induced their mRNA levels (Fig. 2A).
Figure 2. Differential RARβ/RXRα binding in cell cycle genes after RA treatment.
(A) Quantitative RT-PCR analysis of a panel of cell cycle genes derived from the KEGG pathway database. From a selection of 18 cell cycle genes, 11 showed significantly increased expression following RA treatment. (B) The effects of RA were studied on a genomic level by analyzing RARβ and RXRα common DNA binding sites with AGGTCA-like motifs from mouse livers with or without RA treatment. Of the 11 cell cycle genes that were up-regulated after RA treatment, five genes (Cdk1, Cdk2, Cyclin B, Cyclin E, Cdc25a) had common RARβ/RXRα binding in both the control and RA treatment groups, as demonstrated by the concurrent blue (control) and red (RA treatment) bars. Novel RARβ/RXRα binding was observed on Cdk1 and Cdk2 genes after RA treatment. Unique RARβ/RXRα binding was found on Cyclin D and Cdk6 genes in the RA-treated group.
3.3 RA treatment accelerated liver regeneration in response to PH
Based on the RA treatment experiments in sham-operated mice, the proliferative effect exerted by exogenous RA in the regenerating liver was studied. WT mice were treated with RA or vehicle control 48 hours prior to the surgery. In agreement with findings from sham-operated groups, RA administration increased the liver-to-body weight ratio in regenerating livers 1 to 5 days after PH (Fig. 3A). In addition, the increase in liver-to-body weight ratio was accompanied by greater hepatocyte proliferation, as demonstrated by the higher number of Ki67-positive cells in RA-treated mouse livers (Fig. 3B, C). Thus, these results indicate that RA administration prior to hepatic resection accelerated regeneration in the mouse liver.
Figure 3. RA treatment accelerated hepatocyte proliferation in regenerating mouse liver.
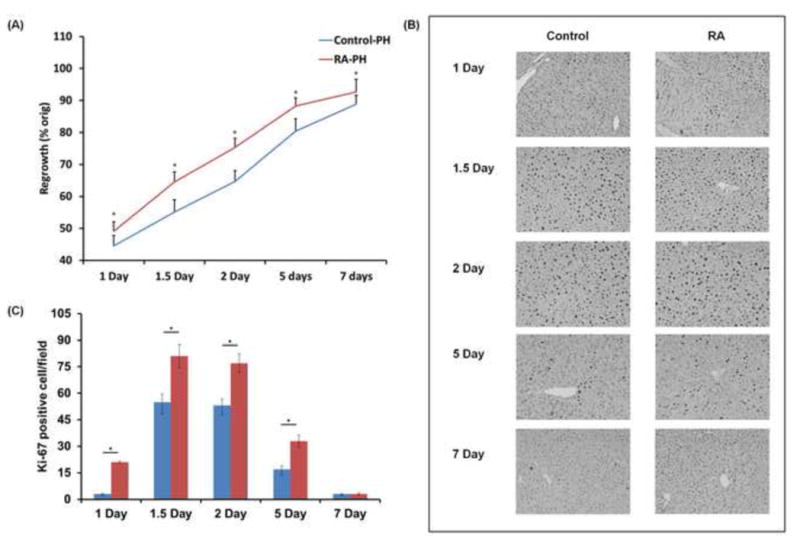
WT mice were treated with RA or vehicle by oral gavage 48 hours prior to partial hepatectomy. (A) Mice were sacrificed and weighed at 1, 1.5, 2, 5 and 7 days after surgery to calculate differences in liver to body weight ratio between treatment groups. (B) Representative photomicrographs of Ki67 immunohistochemical staining of liver sections from wild type mice with and without RA treatment at indicated time points (n≥4). (C) Ki67-positive cells in the livers of wild type mice with and without RA pre-treatment after PH. The number of proliferating hepatocytes was determined by counting Ki67-positive hepatocytes in at least 15microscope fields (20X) per liver sample. Liver sections from all mice were used for analyses. Means ± SD are graphed with * indicating p < 0.05.
3.4 Regenerating livers in RA-treated mice displayed early induction of RA signaling
Because RA signaling has been implicated in liver regeneration, the expression of genes involved in the RA signaling pathway including RARβ, Aldehyde dehydrogenase family 1 member A2 (Aldh1a2), Cellular retinoic acid binding protein 1 (Crabp1), and Cellular retinol binding protein 1 (Crbp1) were studied in regenerating livers from control and RA-treated mice (29). RARβ is a RA receptor whose expression is inducible by RA (7) while Aldh1a2 is responsible for producing RA (29). Crbp1 and Crabp1 are binding proteins for retinoids with Crabp1 also being a RA target gene (30). The expression of all four genes was transiently induced 1.5 days after PH in control mice. The coordinated induction of all four genes at the same time point suggests the importance of RA signaling in hepatocyte proliferation, which peaked 1.5–2 days after PH (Fig. 4). RA treatment prior to the surgery resulted in early induction of RARβ, Aldh1a2, Crbp1, and Crabp1 1 day after PH. Western blotting showed elevated RARβ protein levels resulting from RA treatment 1 day after PH (Fig. 4E). Moreover, Crbp1 induction was sustained for up to 5 days following surgery. The accelerated liver regeneration in RA-treated mice was accompanied by early activation of RA signaling via increased expression of the receptor, binding proteins, and oxidation enzyme for retinoids.
Figure 4. Early induction of RA signaling in RA-treated regenerating mouse livers.
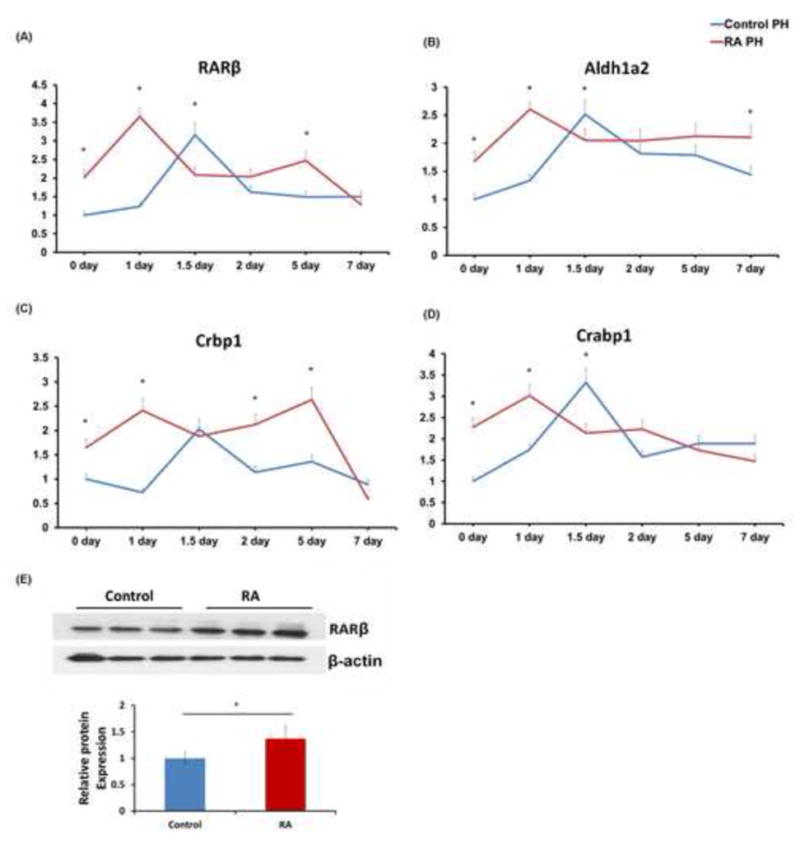
(A–D) RARβ, Aldh1a2, Crbp1, and Crabp1 are genes encoding the RA receptor, retinoid oxidation enzyme, and binding proteins, respectively. mRNA levels of these genes were quantified at indicated time points after PH in control and RA treatment groups. The expression of all four genes was transiently induced 1.5 days after PH in the control treatment group. The introduction of RA treatment 48 hours prior to surgery led to an earlier induction of these RA signaling genes. (E) Western blotting analysis of RARβ protein levels in regenerating liver 1 day after PH from control and RA treatment groups. Means ± SD are graphed with * indicating p < 0.05.
3.5 RA modulates cell cycle gene expression during liver regeneration
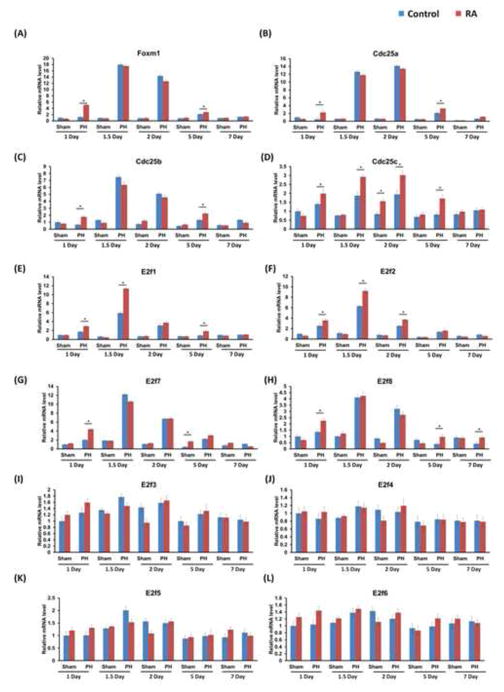
To gain further insights into the effect of RA on cell cycle progression, the expression of cell cycle genes in regenerating mouse livers was studied. Foxm1 and its target genes Cdc25a, b and c as well as the E2f family have been implicated in regulating Cyclin/cdk activity at key cell cycle checkpoints (31). RA treatment facilitated the induction of Foxm1, Cdc25a, Cdc25b, Cdc25c, and E2f transcription factor 1 (E2f1), E2f2, E2f7, E2f8 mRNA levels as early as 1 day after PH, peaking 1.5 days after PH when the hepatocytes are actively proliferating (Fig. 5A–H). RA treatment did not alter the expression of E2f3, 4, 5, and 6 (Fig. 5I–L). In accordance with Ki67 staining data, Proliferating Cell Nuclear Antigen (Pcna) mRNA levels were markedly higher in RA-treated than the control group 1 to 5 days after PH (Fig. 6A). Quantification of cyclins and cdks expression revealed that in the vehicle control mice, hepatic induction of these key cell cycle genes is observed 1.5 days after PH, a well-established indicator of peak hepatocyte proliferation in regenerating mouse liver (32). In RA-treated mice, hepatic expression of cyclins and cdks was significantly elevated 1 day after PH compared to control (Fig. 6B–H). Consistently, western blotting showed higher protein levels of c-myc, Cyclin D, Cyclin E, and Cyclin A 1 day after PH in RA-treated group relative to the control group (Fig. 7). Taken together, RA treatment accelerated the expression of a panel of cell cycle genes in the regenerating mouse liver.
Figure 5. RA modulated the expression of cell cycle regulators during liver regeneration.
WT mice with or without RA treatment received PH and were killed at indicated time points. The expression of cell cycle regulating genes was studied. These genes include transcriptional regulators (A) Foxm1, (B–D) Cdc25a, b, c, and (E–H) the E2f family of cell cycle regulators. The peaks in gene expression between 1.5 and 2 days after PH in control mice correspond with the peak in hepatocyte proliferation in regenerating livers. RA treatment facilitated early induction of these cell cycle genes 1 day after PH compared with the control group. (I–L) E2f3-6 did not demonstrate a significant change in gene expression levels with RA treatment. Means ± SD are graphed with * indicating p < 0.05.
Figure 6. RA up-regulated cell cycle gene expression during liver regeneration.
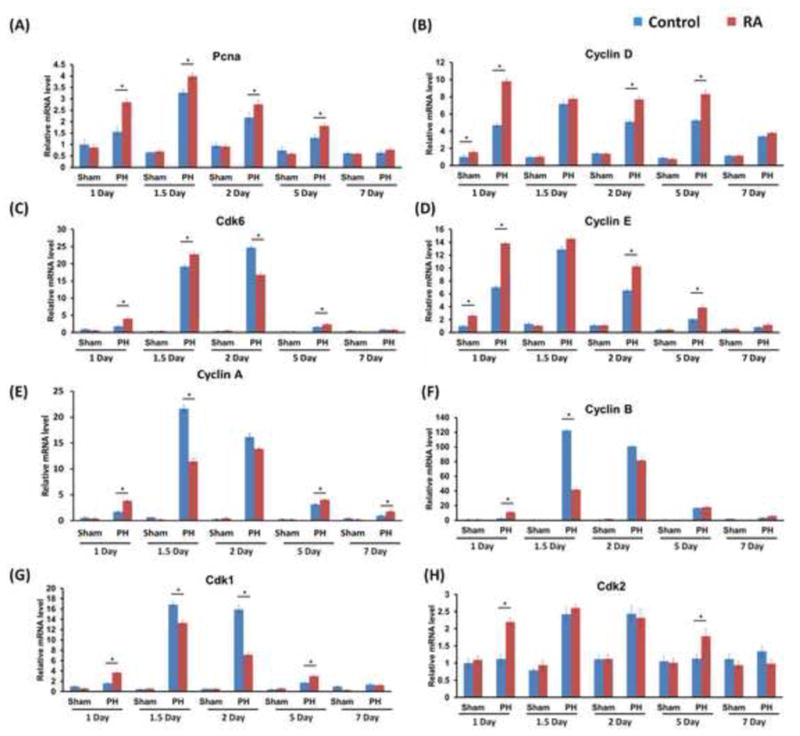
WT mice with or without RA treatment received PH and were killed at indicated time points. The expression of cell cycle genes was studied. (A) mRNA levels of Pcna, an indicator of DNA synthesis, were consistently higher in the RA treatment group compared to the control group from 1 to 5 days after PH. (B–G) mRNA levels of Cyclins and Cdks were measured by quantitative RT-PCR. In the control group, characteristic induction of these key cell cycle genes is observed between 1.5–2 days after PH. RA treatment led to an earlier induction of cyclins and cdks 1 day after PH compared to control. Means ± SD are graphed with * indicating p < 0.05.
Figure 7. Effect of RA on cell cycle protein expression during liver regeneration.
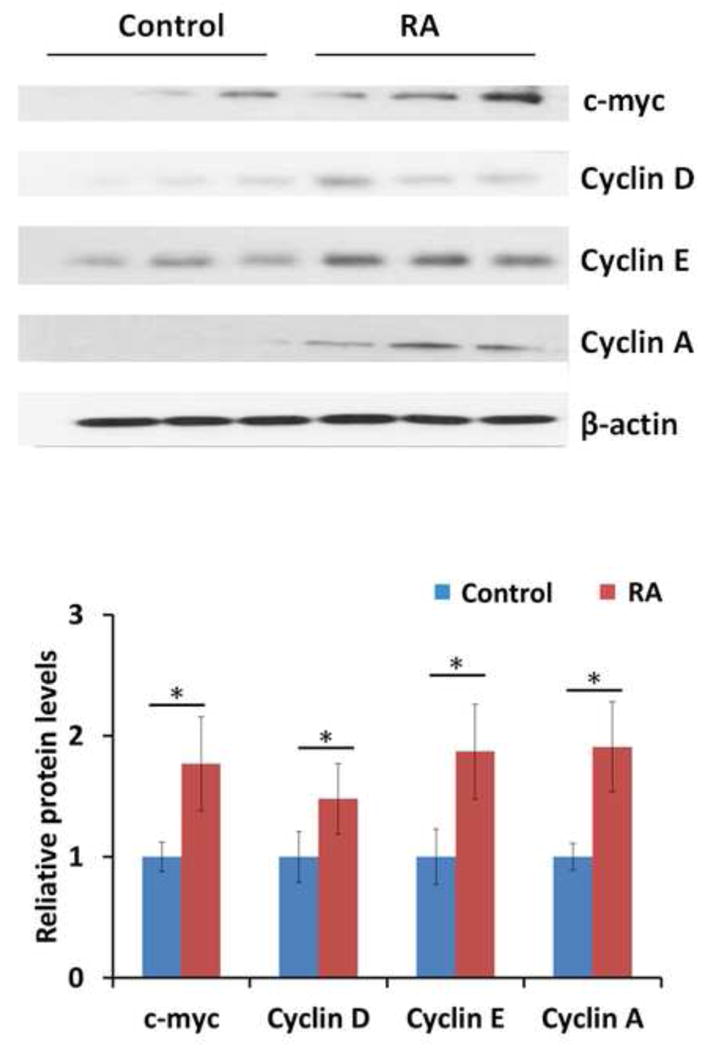
WT mice with or without RA treatment received PH and were killed 1 day after surgery. Western blots showed hepatic protein levels of c-myc, Cyclin D, Cyclin E, and Cyclin A in both groups. The protein levels were quantified by densitometry. Values for individual mouse were normalized to β-actin levels and were expressed relative to untreated mice. RA treatment led to higher hepatic induction of c-myc, Cyclin D, Cyclin E, and Cyclin A 1 day after PH compared to control. Means ± SD are graphed with * indicating p < 0.05.
4. Discussion
RA is well known for its roles in differentiation, anti-proliferation, and regeneration. The complex and pleiotropic effects of RA have yielded conflicting findings on its many functions. Our data showed that oral gavage of RA increased liver size and hepatocyte proliferation. This mitogenic effect was also observed in the regenerating liver when mice were treated with RA prior to PH. Furthermore, new insights into the role of RA and its receptor RARβ in vivo were obtained. Although RARβ is generally considered as a tumor suppressor gene, this study demonstrated that hepatic RARβ binding is also implicated in cell cycle signaling. RA treatment accelerates liver cell proliferation and these effects are facilitated by early induction of cell cycle genes, which were accompanied by early activation of RA-mediated signaling.
To determine the molecular basis by which RA promotes hepatocellular proliferation, RARβ/RXRα binding to cell cycle genes was analyzed before and after RA treatment. Within the identified RARβ/RXRα binding sites that contain an AGGTCA-like motif, additional transcription factor binding motifs for SP1, GABPA, and FOXA2 were detected (data not shown). Of particular interest is SP1, which has previously been shown to interact with RARβ and whose binding sites have been identified in genes associated with growth and cell cycle regulation as well as RA signaling. For instance, RA-induced expression of the retinol binding protein gene is regulated by a SP1-RARβ/RXRα complex (33). Moreover, RA can regulate SP1 phosphorylation (34), and SP1 down-regulation leads cell cycle arrest in human cancer cells (35). Specifically, SP1 modulates the activity of checkpoint proteins p21 and p27 in human hepatoma cells (36). Therefore, it appears that the action of RA-RARβ in regulating the cell cycle could be exerted through SP1 or other transcriptional factors. Further study will be necessary to determine whether these additional transcription factors indeed contribute to enhanced hepatocyte proliferation by interacting with RARβ and transcriptionally activating cell cycle genes.
Liver regeneration is a complex process regulated by many signals from the hepatic environment. Different signaling pathways will lead to the activation of transcription factors that either stimulate hepatocyte proliferation or promote cell survival to facilitate liver regrowth (21, 23, 37, 38). The presented data suggest that one such pathway is the RA signaling pathway itself. Our findings indicate that RA signaling is highly orchestrated in the regenerating liver. This coordinated up-regulation of RARβ, ALDH1A2, CRBP1, and CRABP1 peaked earlier in the RA-treated group than the control group, suggesting the important role of RA signaling in promoting and even accelerating hepatocyte proliferation. Since vitamin A is stored in the liver, it is likely that endogenous RA is mobilized in regenerating liver to up-regulate its direct target genes including CRABP1 and RARβ and their proliferative downstream pathways.
Accelerated regeneration was also associated with elevated expression of cell cycle regulators and increased hepatocyte DNA replication and mitosis. RA-mediated activation of Foxm1 facilitates a coordinated induction of several common target genes through RARβ binding. Foxm1 also regulates Cdc25, Cyclin E/Cdk2, and Cyclin B/Cdk1, making it a critical gatekeeper of G1/S and G2/M cell cycle transitions, as well as mitotic spindle assembly (39). The E2f transcription factor family plays an important role in regulating cellular proliferation by activating a panel of genes involved in progression through the G1 phase as well as DNA replication (23). The E2f family includes transcription activators and repressors. Activators such as E2f1-3 promote cell cycle progression, while repressors (E2f4-8) inhibit cell cycle. E2f7-8 can inhibit the action of E2f1 via a negative feedback loop (40). Cyclins and Cdks are E2f transcriptional targets (41). RA treatment increased the induction of Foxm1, E2f1, and E2f2 expression. Furthermore, RA treatment elevated the expression of Cyclin D-Cdk 4/6, Cyclin E-Cdk2, and Cyclin A-Cdk2 kinase complexes suggesting increased hepatocellular G1/S phase entry activities, all of which are associated with enhanced hepatocyte DNA replication. Finally, RA treatment induced up-regulation in Cyclin B-Cdk1 complex and Cdc25s mRNA levels in regenerating livers, which are indicative of mitotic entry.
Our findings run contrary to the hallmark anti-proliferative characteristics of RA, which has been used as a chemotherapeutic treatment against certain forms of cancer (42–44). Among the RAR isoforms (α, β, γ), RARβ is the predominant receptor mediating the inhibitory effects of RA on cancer cell proliferation and is considered as a tumor suppressor gene (45, 46). However, the application of RA and other retinoids does not always demonstrate efficacy in preventing or treating cancer (47–54). On the contrary, RA and other retinoids can promote rather than inhibit cancer cell survival and growth as well as increase the incidence of certain cancers in mouse models and human subjects (46, 47, 49, 53). Among the most notable examples are large scale trials on the incidence of lung cancer that found no benefit and even potentially adverse effects of RA or retinoid supplementation (55, 56). Similarly, retinoid chemoprevention did not have a significant effect on lung cancer patients (47). Furthermore, the presence of RARβ actually promotes the growth of mammary carcinoma in the stromal cell compartment (57). The conflicting data on the proliferative effect of RA underscores the need to exercise caution when evaluating RA as a chemotherapeutic agent. Nevertheless, this does not discount the potentially beneficial applications of RA, particular in the context of liver regeneration. The accelerated liver regeneration previously demonstrated sheds additional light on a promising approach to protect the liver during transplants or when challenged with acute or chronic injury. Because efficient liver regeneration is vital for improving surgical outcomes, the pivotal role of RA in enhancing hepatocyte proliferation makes it a strong candidate for reducing post-operative hepatic failure.
In summary, RARβ is implicated as a key component in RA-induced stimulation of cell cycle signaling pathways that promote hepatocyte proliferation. We anticipate that future studies will employ a RARβ liver-specific knockout mouse model as well as use receptor specific ligands to further characterize the role of hepatic RARβ in cell cycle progression. A better mechanistic understanding of the proliferative effects exerted by RA may lead to the development of therapeutics to promote liver regeneration in the clinical setting.
Acknowledgments
The authors thank Ms. Lisa Teixeira and Mr. Thinh Chau for editing the manuscript. This study is supported by grants funded by National Institutes of Health CA53596 and DK092100.
Abbreviations
- Aldh1a2
aldehyde dehydrogenase family 1 member A2
- Cdk1
Cyclin-dependent kinase 1
- Cdc25
cell division cycle 25
- ChIP-seq
chromatin immunoprecipitation followed by next generation sequencing
- Crabp1
cellular retinoic acid binding protein 1
- Crbp1
cellular retinol binding protein 1
- E2f
E2f transcription factor
- Foxm1
forkhead box M1
- KEGG
Kyoto encyclopedia of Genes
- Pcna
proliferating cell nuclear antigen
- PH
partial hepatectomy
- qRT-PCR
real-time quantitative reverse transcription PCR
- RA
all-trans retinoic acid
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- WT
wild type
Footnotes
Authors’ Contributions
Hui-Xin Liu: Performed experiments, analyzed data, generated figures and prepared manuscript.
Irene Ly: Assisted in experiments and prepared manuscript.
Ying Hu: Assisted in experiments and analyzed data.
Yu-Jui Yvonne Wan: Generated idea and supervised overall performance of the project.
Disclosure of potential Conflicts of Interest
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hui-Xin Liu, Email: huixinliu2012@gmail.com.
Irene Ly, Email: Irene.ly@ucdmc.ucdavis.edu.
Ying Hu, Email: yinghu0821@gmail.com.
Yu-Jui Yvonne Wan, Email: yjywan@ucdavis.edu.
References
- 1.Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–5. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- 2.White JA, Boffa MB, Jones B, Petkovich M. A zebrafish retinoic acid receptor expressed in the regenerating caudal fin. Development. 1994;120:1861–72. doi: 10.1242/dev.120.7.1861. [DOI] [PubMed] [Google Scholar]
- 3.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Developmental dynamics: an official publication of the American Association of Anatomists. 2003;226:237–44. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- 4.Shmarakov IO, Jiang H, Yang KJ, Goldberg IJ, Blaner WS. Hepatic retinoid stores are required for normal liver regeneration. J Lipid Res. 2013;54:893–908. doi: 10.1194/jlr.M029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Liu HX, He Y, Fang Y, Fang J, Wan YJ. Transcriptome profiling and genome-wide DNA binding define the differential role of fenretinide and all-trans RA in regulating the death and survival of human hepatocellular carcinoma Huh7 cells. Biochemical pharmacology. 2013;85:1007–17. doi: 10.1016/j.bcp.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falasca L, Favale A, Gualandi G, Maietta G, Conti Devirgiliis L. Retinoic acid treatment induces apoptosis or expression of a more differentiated phenotype on different fractions of cultured fetal rat hepatocytes. Hepatology. 1998;28:727–37. doi: 10.1002/hep.510280319. [DOI] [PubMed] [Google Scholar]
- 7.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Advanced drug delivery reviews. 2010;62:1285–98. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature reviews Cancer. 2001;1:181–93. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda H, Fujiwara K. Retinoic acid inhibits DNA and albumin synthesis stimulated by growth factor in adult rat hepatocytes in primary culture. Biochemical and biophysical research communications. 1993;191:675–80. doi: 10.1006/bbrc.1993.1270. [DOI] [PubMed] [Google Scholar]
- 10.Ohtake Y, Maruko A, Abe S, Fukumoto M, Ohkubo Y. Effect of retinoic acid-induced transglutaminase on cell growth in regenerating liver. Biomed Res-Tokyo. 2006;27:75–80. doi: 10.2220/biomedres.27.75. [DOI] [PubMed] [Google Scholar]
- 11.Kohno H, Hoshino Y, Katoh S, Ohkubo Y. Effect of Retinoic Acid on Liver Transglutaminase Activity and Carbon Tetrachloride-Induced Liver-Damage in Mice. Experientia. 1992;48:386–8. doi: 10.1007/BF01923436. [DOI] [PubMed] [Google Scholar]
- 12.Ohtake Y, Maruko A, Ohishi N, Kawaguchi M, Satoh T, Ohkubo Y. Effect of retinoic acid on transglutaminase and ornithine decarboxylase activities during liver regeneration. Cell Biochem Funct. 2008;26:359–65. doi: 10.1002/cbf.1451. [DOI] [PubMed] [Google Scholar]
- 13.Ohtake Y, Maruko A, Abe S, Nagashima T, Fukumoto M, Ohkubo Y. Involvement of retinoic acid-induced transglutaminase activity in zonal differences of hepatocyte proliferation after partial hepatectomy. J Gastroen Hepatol. 2006;21:1726–30. doi: 10.1111/j.1440-1746.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- 14.Ozeki A, Tsukamoto I. Retinoic acid repressed the expression of c-fos and c-jun and induced apoptosis in regenerating rat liver after partial hepatectomy. Biochimica et biophysica acta. 1999;1450:308–19. doi: 10.1016/s0167-4889(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 15.Satoh M, Yamazaki M. In-Vitro DNA-Synthesis of Mouse Hepatocytes Stimulated by Tumor-Necrosis-Factor Is Inhibited by Glucocorticoids and Prostaglandin-D(2) but Enhanced by Retinoic Acid. J Cell Physiol. 1993;157:104–9. doi: 10.1002/jcp.1041570114. [DOI] [PubMed] [Google Scholar]
- 16.Ledda-Columbano GM, Pibiri M, Molotzu F, Cossu C, Sanna L, Simbula G, et al. Induction of hepatocyte proliferation by retinoic acid. Carcinogenesis. 2004;25:2061–6. doi: 10.1093/carcin/bgh221. [DOI] [PubMed] [Google Scholar]
- 17.Ohmura T, Columbano GL, Columbano A, Katyal SL, Locker J, Shinozuka H. 9-cis retinoic acid is a direct hepatocyte mitogen in rats. Life Sci. 1996;58:Pl211–Pl6. doi: 10.1016/0024-3205(96)00039-2. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M, Watanabe M, Ishibashi N, Yanagida S, Ogihara M. Acyclic retinoid NIK-333 accelerates liver regeneration and lowers serum transaminase activities in 70% partially hepatectomized rats, in vivo. European journal of pharmacology. 2010;643:267–73. doi: 10.1016/j.ejphar.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Perea G, Salar A, Altes A, Brunet S, Sierra J. Acute hepatomegaly with severe liver toxicity due to all-trans-retinoic acid. Haematologica. 2000;85:551–2. [PubMed] [Google Scholar]
- 20.Rao J, Qian X, Wang P, Pu L, Zhai Y, Wang X, et al. All-trans retinoic acid preconditioning protects against liver ischemia/reperfusion injury by inhibiting the nuclear factor kappa B signaling pathway. The Journal of surgical research. 2013;180:e99–e106. doi: 10.1016/j.jss.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Dai GL, He L, Bu PL, Wan YJY. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology. 2008;47:1277–87. doi: 10.1002/hep.22129. [DOI] [PubMed] [Google Scholar]
- 22.Yang XX, Guo ML, Wan YJY. Deregulation of Growth Factor, Circadian Clock, and Cell Cycle Signaling in Regenerating Hepatocyte RXRα-Deficient Mouse Livers. Am J Pathol. 2010;176:733–43. doi: 10.2353/ajpath.2010.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu HX, Fang Y, Hu Y, Gonzalez FJ, Fang J, Wan YJ. PPARbeta Regulates Liver Regeneration by Modulating Akt and E2f Signaling. PloS one. 2013;8:e65644. doi: 10.1371/journal.pone.0065644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS) Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmon-Divon M, Dvinge H, Tammoja K, Bertone P. PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC bioinformatics. 2010;11:415. doi: 10.1186/1471-2105-11-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sever CE, Locker J. Expression of retinoic acid alpha and beta receptor genes in liver and hepatocellular carcinoma. Molecular carcinogenesis. 1991;4:138–44. doi: 10.1002/mc.2940040209. [DOI] [PubMed] [Google Scholar]
- 28.Zhan Q, Fang Y, He Y, Liu HX, Fang J, Wan YJ. Function annotation of hepatic retinoid x receptor alpha based on genome-wide DNA binding and transcriptome profiling. PloS one. 2012;7:e50013. doi: 10.1371/journal.pone.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Valencia V, Rangel P, Rodriguez S, Hernandez-Munoz R. Involvement of alcohol and aldehyde dehydrogenase activities on hepatic retinoid metabolism and its possible participation in the progression of rat liver regeneration. Biochemical pharmacology. 2007;73:586–96. doi: 10.1016/j.bcp.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Omori M, Muto Y, Nagao T. Cellular retinoid-binding proteins in reginerating rat liver: demonstration of a novel cellular retinoid-binding protein. J Lipid Res. 1981;22:899–904. [PubMed] [Google Scholar]
- 31.Grant GD, Brooks L, 3rd, Zhang X, Mahoney JM, Martyanov V, Wood TA, et al. Identification of cell cycle-regulated genes periodically expressed in U2OS cells and their regulation by FOXM1 and E2F transcription factors. Molecular biology of the cell. 2013;24:3634–50. doi: 10.1091/mbc.E13-05-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 33.Panariello L, Quadro L, Trematerra S, Colantuoni V. Identification of a novel retinoic acid response element in the promoter region of the retinol-binding protein gene. The Journal of biological chemistry. 1996;271:25524–32. doi: 10.1074/jbc.271.41.25524. [DOI] [PubMed] [Google Scholar]
- 34.Horie S, Ishii H, Matsumoto F, Kusano M, Kizaki K, Matsuda J, et al. Acceleration of thrombomodulin gene transcription by retinoic acid: retinoic acid receptors and Sp1 regulate the promoter activity through interactions with two different sequences in the 5′-flanking region of human gene. The Journal of biological chemistry. 2001;276:2440–50. doi: 10.1074/jbc.M004942200. [DOI] [PubMed] [Google Scholar]
- 35.Chae JI, Jeon YJ, Shim JH. Downregulation of Sp1 is involved in honokiol-induced cell cycle arrest and apoptosis in human malignant pleural mesothelioma cells. Oncology reports. 2013;29:2318–24. doi: 10.3892/or.2013.2353. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Huang K, Li XR, Du M, Kang X, Luo X, et al. Identification of Poly(ADP-Ribose) Polymerase-1 as a Cell Cycle Regulator through Modulating Sp1 Mediated Transcription in Human Hepatoma Cells. PloS one. 2013:8. doi: 10.1371/journal.pone.0082872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan YJ, Yang X, Guo M. HEPATOCYTE RXR alpha - A KEY ELEMENT OF LIVER REGENERATION. J Hepatol. 2009;50:S314–S5. [Google Scholar]
- 38.Yang X, Guo M, Wan YJ. Deregulation of growth factor, circadian clock, and cell cycle signaling in regenerating hepatocyte RXRalpha-deficient mouse livers. Am J Pathol. 2010;176:733–43. doi: 10.2353/ajpath.2010.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang XH, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. P Natl Acad Sci USA. 2002;99:16881–6. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchet E, Annicotte JS, Lagarrigue S, Aguilar V, Clape C, Chavey C, et al. E2F transcription factor-1 regulates oxidative metabolism. Nature cell biology. 2011;13:1146–52. doi: 10.1038/ncb2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevins JR. The Rb/E2F pathway and cancer. Human molecular genetics. 2001;10:699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 42.Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, et al. All-Trans-Retinoic Acid as a Differentiating Agent in the Treatment of Acute Promyelocytic Leukemia. Blood. 1995;85:2643–53. [PubMed] [Google Scholar]
- 43.Bollag W. Effects of Vitamin-a Acid (Nsc-122758) on Transplantable and Chemically Induced Tumors. Cancer Chemoth Rep 1. 1971;55:53. [PubMed] [Google Scholar]
- 44.Li C, Wan YJY. Differentiation and antiproliferation effects of retinoic acid receptor beta in hepatoma cells. Cancer Lett. 1998;124:205–11. doi: 10.1016/s0304-3835(97)00475-8. [DOI] [PubMed] [Google Scholar]
- 45.Faria TN, Mendelsohn C, Chambon P, Gudas LJ. The targeted disruption of both alleles of RAR beta(2) in F9 cells results in the loss of retinoic acid-associated growth arrest. Journal of Biological Chemistry. 1999;274:26783–8. doi: 10.1074/jbc.274.38.26783. [DOI] [PubMed] [Google Scholar]
- 46.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 47.Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. Journal of the National Cancer Institute. 2001;93:605–18. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 48.Khuri FR, Lee JJ, Lippman SM, Kim ES, Benner SE, Winn R, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. Journal of the National Cancer Institute. 2006;98:441–50. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 49.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 50.Rao GN, Ney E, Herbert RA. Effect of retinoid analogues on mammary cancer in transgenic mice with c-neu breast cancer oncogene. Breast cancer research and treatment. 1998;48:265–71. doi: 10.1023/a:1005957620881. [DOI] [PubMed] [Google Scholar]
- 51.Chiesa MD, Passalacqua R, Michiara M, Franciosi V, Di Costanzo F, Bisagni G, et al. Tamoxifen vs Tamoxifen plus 13-cis-retinoic acid vs Tamoxifen plus Interferon alpha-2a as first-line endocrine treatments in advanced breast cancer: updated results of a phase II, prospective, randomised multicentre trial. Acta bio-medica: Atenei Parmensis. 2007;78:204–9. [PubMed] [Google Scholar]
- 52.Singletary SE, Atkinson EN, Hoque A, Sneige N, Sahin AA, Fritsche HA, et al. Phase II clinical trial of N-(4-hydroxyphenyl)retinamide and tamoxifen administration before definitive surgery for breast neoplasia. Clinical Cancer Research. 2002;8:2835–42. [PubMed] [Google Scholar]
- 53.Heinonen OP, Huttunen JK, Albanes D, Haapakoski J, Palmgren J, Pietinen P, et al. Effect of Vitamin-E and Beta-Carotene on the Incidence of Lung-Cancer and Other Cancers in Male Smokers. New Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 54.Albright CD, Salganik RI, Van Dyke T. Dietary depletion of vitamin E and vitamin A inhibits mammary tumor growth and metastasis in transgenic mice. J Nutr. 2004;134:1139–44. doi: 10.1093/jn/134.5.1139. [DOI] [PubMed] [Google Scholar]
- 55.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer I. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 56.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. JNCI Journal of the National Cancer Institute. 1996;88:1550–9. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 57.Liu XX, Nugoli M, Laferrierea J, Saleh SM, Rodrigue-Gervais IG, Saleh M, et al. Stromal retinoic acid receptor beta promotes mammary gland tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:774–9. doi: 10.1073/pnas.1011845108. [DOI] [PMC free article] [PubMed] [Google Scholar]