Abstract
Background
It is unknown if the reduction in HIV-1 reservoirs observed following allogeneic hematopoietic stem cell transplantation (HSCT) with susceptible donor cells is sufficient to achieve sustained HIV-1 remission.
Objective
To characterize HIV-1 reservoirs in blood and tissues, and to perform analytical antiretroviral treatment interruptions to determine the potential for allogeneic HSCT to lead to sustained antiretroviral-free HIV-1 remission.
Design
Characterization of HIV-1 reservoirs and immunity before and after antiretroviral interruption.
Setting
Tertiary care center.
Patients
Two HIV-infected men with undetectable HIV-1 following allogeneic HSCT for hematologic malignancies.
Measurements
Quantification of HIV-1 in various tissues after HSCT and the duration of antiretroviral-free HIV-1 remission after treatment interruption.
Results
No HIV-1 was detected from peripheral blood or rectal mucosa prior to analytical treatment interruption. Plasma HIV-1 RNA and cell-associated HIV-1 DNA remained undetectable until 12 to 32 weeks after antiretroviral cessation. Both patients experienced rebound viremia with the development of acute retroviral syndrome within one to two weeks of the most recent negative viral load measurement. One patient developed new efavirenz resistance after re-initiation of antiretroviral therapy. Re-initiation of active therapy led to viral decay and resolution of symptoms in both patients.
Limitations
The study was limited to 2 patients.
Conclusions
Allogeneic HSCT may lead to loss of detectable HIV-1 from blood and gut tissue and variable periods of antiretroviral-free HIV-1 remission, but viral rebound can occur despite a minimum 3-log10 reduction in reservoir size. Long-lived tissue reservoirs may have contributed to viral persistence. Defining the nature and half-life of such reservoirs is essential in order to achieve durable antiretroviral-free HIV-1 remission.
Introduction
A major challenge in eradicating HIV-1 infection is the persistence of latently infected cells, which are established by integration of the viral genome into host cell chromosomes (1, 2). Combination antiretroviral therapy (ART) reduces plasma HIV-1 RNA levels to below the limit of detection of clinical assays. However, low-level plasma viremia and cell-associated HIV-1 DNA are detected in a majority of patients on ART, even after intensification of the antiretroviral regimen (3-5). Furthermore, virus typically rebounds within 1 to 8 weeks after treatment interruption in patients on long-term suppressive ART (6-11). As a result, ART-free HIV-1 remission (i.e. “functional” cure) remains elusive.
Sustained HIV-1 remission for over 7 years has been demonstrated in a chronically infected patient (the “Berlin patient”) who underwent myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia using cells from a donor homozygous for a 32-base pair deletion in the gene encoding CCR5 (ccr5Δ32), a coreceptor for HIV-1 (12-14). The extent of reduction in the pool of latently infected cells in the blood and other tissues required to achieve sustained HIV-1 remission is unknown.
We previously reported reduction in peripheral blood HIV-1 reservoirs following reduced-intensity conditioning allogeneic HSCT in two chronically infected male patients. Both patients were heterozygous for ccr5Δ32, but received HIV-1-susceptible, wild-type donor cells (15). Neither HIV-1 DNA nor HIV-1 RNA were detectable in circulating CD4+ cells or plasma, respectively, after donor cells replaced host cells under the cover of suppressive ART. Anti-HIV antibody levels and avidity declined after HSCT, a phenomenon also observed in the Berlin patient (14, 15). However, extensive sampling of tissues and large numbers of peripheral blood mononuclear cells (PBMCs) for the presence of HIV-1 is necessary to understand the full impact of allogeneic HSCT on HIV-1 persistence and ultimately, analytical antiretroviral treatment interruption (ATI) is necessary to establish that viral remission has been achieved. We therefore conducted in-depth analysis of HIV-1 persistence in various tissues from our two patients and performed closely monitored ATIs.
Methods
Tissue Collection, Apheresis, and Sample Processing
The Dana-Farber/Harvard Cancer Center Office for Human Research Studies approved this study. Both patients previously provided consent for blood and excess tissue sampling and were aware of these results prior to participation in this study. A second informed consent process was initiated after full human research committee review that offered patients the option to undergo optional collection of PMBCs by apheresis, cerebral spinal fluid (CSF) collection, gut-associated lymphoid tissue sampling by anoscopy, and carefully monitored ATIs following reservoir characterization and with permission of clinical care providers. PBMCs were collected by leukapheresis involving 1.5 to 2.5 total blood circulating volumes and were purified by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation. In addition, up to 120 ml of whole blood were obtained from patients at 3-month intervals for plasma collection and isolation of PBMCs. Cells were used fresh or cryopreserved for later testing. Rectal biopsy samples were either flash frozen or cells disassociated and cryopreserved after Percoll (Sigma-Aldrich) centrifugation as previously described (16, 17).
Assays to Quantify and Characterize HIV-1 Reservoirs
DNA was extracted from PBMCs and gut tissue using the QIAamp DNA Blood Mini Kit or the All Prep DNA/RNA Mini Kit (Qiagen, Valencia, CA). HIV-1 DNA was quantified using a sensitive real-time PCR assay as previously described. (18, 19) Parallel PCRs were performed in up to 42 wells. A single copy assay capable of detecting plasma HIV-1 RNA at a lower limit of detection of 0.4 copies/mL was performed.(20) Viral outgrowth assays to determine the presence of replication-competent HIV-1 pre-ATI were performed in 30 to 32 replicate assays using aliquots of 5 million purified CD4 + T cells for a total of ≥150 million CD4+ cells (21-23). The COBAS TaqMan HIV-1 Test, v2.0 (Roche Diagnostics) was used to quantify HIV-1 RNA in CSF. Homology between pre-HSCT proviral DNA and primers and probes used in the quantitative PCR assays was verified for each patient, and positive controls were incorporated in each assay as previously described (15). Cells from a patient who underwent autologous HSCT with detectable HIV-1 DNA were used as positive controls in the viral outgrowth experiments.
Single-genome analysis of near full-length HIV-1 envelope sequences (approximately 2.5 kb) was performed on cell-associated DNA prior to loss of detectable HIV-1 DNA and from plasma RNA following viral rebound as previously described (24). Maximum likelihood phylogenetic trees of single-genome sequences were constructed using PhyML (Geneious v.7, Biomatters Ltd.)
HIV-1 Specific Immune Responses and Host Microchimerism
Longitudinal quantification of HIV-specific antibody avidity by limiting-antigen (LAg) enzyme immunoassay and antibody levels by the less sensitive (LS; 1:400 diluted plasma) VITROS Anti-HIV-1+2 assay were performed as previously described (25). PBMCs were used to perform interferon -γ enzyme-linked immunospot assays incorporating overlapping peptides representing the Gag and Nef consensus protein sequence of Clade B HIV, and selected HIV-1 peptides (spanning gp160, Vif, Nef, p24, p17, reverse transcriptase, protease) described to be presented by the patients' HLA class I molecules to determine HIV-1-specific cellular immune responses (26). Highly sensitive allele-specific PCR tests targeting HLA and insertion-deletion polymorphisms unique to the patient or donor were used to determine levels of host microchimerism in blood (i.e., the proportion of residual host PBMCs post HSCT) (27, 28). The microchimerism assay is highly specific and sensitive to a single copy of target DNA allowing detection of host cells present as a very low proportion of the PBMC population depending on the number of cells surveyed (27).
Analytical Treatment Interruption
Carefully monitored ATIs were performed incorporating weekly virus load (VL) testing by the COBAS TaqMan HIV-1 Test, v2.0 (limit of quantification 20 RNA copies/ml, limit of detection as low as 5 to 10 copies/ml) during the first 10 weeks after ART discontinuation and every one to two weeks thereafter. Qualitative testing of HIV-1 DNA from whole blood (Quest Diagnostics, Chantilly, VA) was performed approximately every two weeks. As per our institutional research guidelines, we were only able to provide patients with live-time results from approved clinical assays during this study. We planned to restart a patient's prior ART at the first sign of plasma viral rebound (any HIV-1 RNA >1000 copies/mL or any confirmed value >200 copies/ml). Large volume blood collections for detailed immunologic and virologic reservoir characterization as described above were planned every 12 weeks prior to and after cessation of ART. HIV-1 genotyping of plasma virus was performed either by Quest Diagnostics or by our laboratory as previously described (29).
Role of the Funding Source
This study was supported by grants from the American Foundation for AIDS Research, the National Institute of Allergy and Infectious Diseases, the Delaney AIDS Research Enterprise, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology and Harvard, and by the Bill & Melinda Gates Foundation. The funding sources had no role in the design, analysis, decision to publish or preparation of the manuscript.
Results
Virologic and Immunologic Characteristics Prior to ART Discontinuation
Patient A is a perinatally infected male who underwent reduced-intensity conditioning allogeneic HSCT for recurrent Hodgkin lymphoma. Patient B is a male with sexually acquired HIV who underwent reduced-intensity conditioning allogeneic HSCT for myelodysplastic syndrome following treatment for non-Hodgkin lymphoma and subsequent Hodgkin lymphoma. Both patients received sirolimus, tacrolimus and short course methotrexate to prevent acute graft-versus-host disease following HSCT. Patient A developed chronic involvement of the skin, eye and liver and was treated with prednisone 9 months after HSCT with initial response. Pt B developed skin, liver and oropharynx graft-versus-host disease on post-HSCT day 220 requiring intermittent oral prednisone.
Given the lack of detectable HIV-1 from peripheral blood as previously reported (15), patients A and B were re-approached and provided written informed consent to undergo in-depth sampling. Leukapheresis was performed 4.3 years after HSCT for patient A and 2.6 years after HSCT for patient B. Table 1 provides a summary of virologic tests performed prior to ATI. No HIV-1 DNA was detected from PBMCs by sensitive quantitative PCR assay and no replication competent HIV-1 was recovered from co-culture assays involving ≥150 million purified CD4+ T cells from either patient. Patient B consented to undergo rectal biopsies, in which no HIV-1 DNA could be detected. Microchimerism testing revealed that only 0.0004%-0.001% of peripheral blood cells were of host origin 1416 and 736 days after transplantation for patients A and B, respectively.
Table 1. Studies to Assess HIV-1 Reservoirs after Allogeneic Stem Cell Transplantation and Before Antiretroviral Treatment Interruption.
| Sample Type | Quantity | Total Cells Tested | No. of Positive Replicates/ No. of Wells |
|---|---|---|---|
| Patient A (4.3 years post-HSCT) | |||
| Total HIV-1 PBMC DNA | <0.12 copies/106 cells | 26×106 PBMCs | 0/42 |
| Infectious Virus by Co-Culture | <0.007 IUPMa | 150×106 CD4+ T Cells | 0/30 |
| Patient B (2.6 years post-HSCT) | |||
| Total HIV-1 PBMC DNA | <0.13 copies/106 cells | 24×106 PBMCs | 0/42 |
| Infectious Virus by Co-Culture | <0.006 IUPMa | 160×106 CD4+ T Cells | 0/32 |
| Rectal Tissue | <2.4 copies/106 cells | 1.23×106 nucleated cellsb | 0/22 |
Abbreviations: HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; PBMC, peripheral blood mononuclear cell; IUPM, infectious units per million cells
No replication-competent HIV-1 was recovered
25% CD4 + T cells determined by flow cytometry after tissue disaggregation and cell separation by density centrifugation
No significant HIV-1-specific cellular immune responses were detected in PBMC of either patient before or after HSCT and prior to ATI by enzyme-linked immunospot assay (<30 spots per 106 PBMCs). However, PBMCs from patient B, who had a history of cytomegalovirus infection, demonstrated activation upon stimulation with pooled cytomegalovirus/Epstein–Barr virus/influenza peptides 652 days post-HSCT.
Treatment Interruption and Viral Rebound
Given the lack of detectable HIV-1 despite extensive sampling, ATI with close monitoring for virologic rebound was performed. The study team and all clinical providers, including oncologists and infectious disease specialists, were aware of results from additional reservoir testing and made joint decisions to offer treatment interruption to each patient. After thorough discussion of the uncertain significance of virologic assays and potential risks of an ATI, including viral rebound, acute retroviral syndrome, and/or graft-versus-host disease exacerbation, both patients provided consent to interrupt ART. Figure 1 shows the clinical, virologic and immunologic course after ART discontinuation; Table 2 shows results from frequent blood sampling for quantification of cell and plasma virus. During ATI, patient A and B had no detectable plasma RNA or cell-associated HIV-1 DNA until 12 and 32 weeks after ART cessation, respectively. Patient A continued treatment with tacrolimus for persistent graft-versus-host disease during ATI; patient B required no treatment during this period.
Figure 1. Detection of HIV-1 and Clinical Course Following Analytical Treatment Interruption.
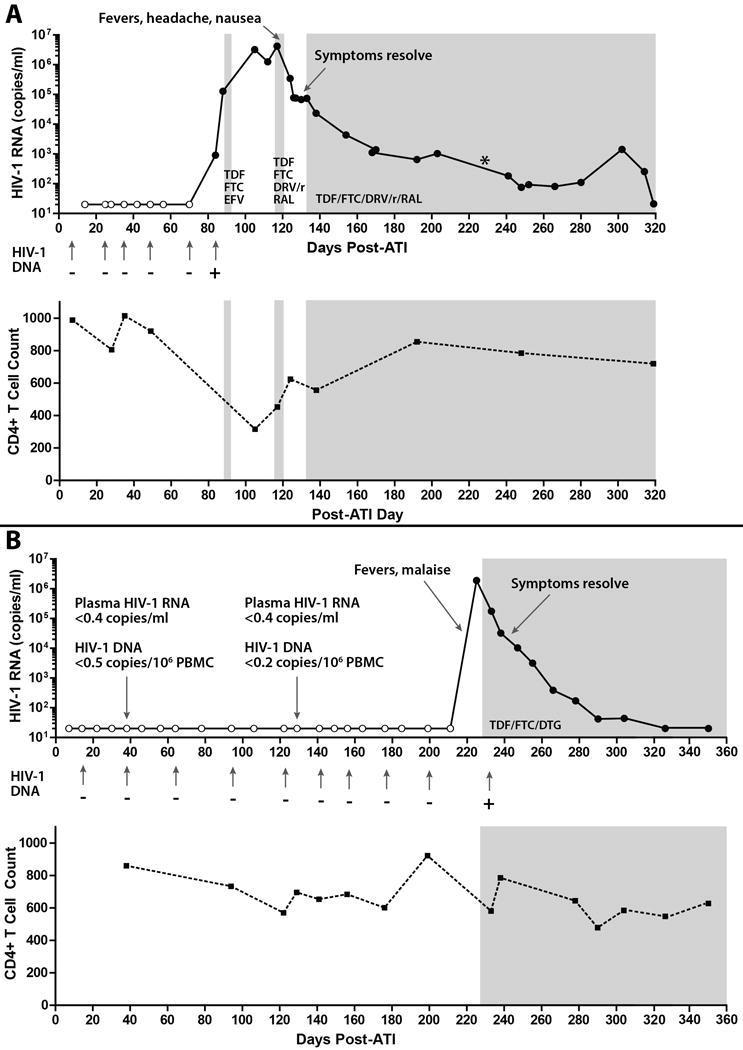
Panel A shows results from clinical monitoring of quantitative HIV-1 plasma RNA and qualitative whole blood HIV-1 DNA testing for patient A. Open circles or minus signs (-) denote samples in which no HIV-1 was detected. Shaded areas represent the use of combination antiretroviral therapy (ART). HIV-1 was first detected 12 weeks after ART interruption (+). Clinical symptoms of acute retroviral syndrome followed a rapid rise in plasma viral load, and resolved at the time of active ART re-initiation. A plasma RNA level of 20, 202 copies/ml was recorded 226 days after treatment interruption (*), but this may have been due to sample switching in the clinical laboratory. CD4+ T cell counts transiently declined during the time of peak viremia.
Panel B shows results for patient B. HIV-1 was first detected 32 weeks after ART interruption. In addition, no HIV-1 DNA or plasma RNA was detected by sensitive research assays 38 and 129 days after ART discontinuation. Clinical symptoms of acute retroviral syndrome occurred approximately 7 days after the last negative viral load test. Symptoms resolved with prompt initiation of ART and subsequent viral suppression. TDF denotes tenofovir; FTC emtricitabine, EFV efavirenz, RAL raltegravir, DRV/r ritonavir boosted darunavir, DTG dolutegravir, ATI analytical treatment interruption.
Table 2. HIV-1 Plasma RNA and Cell-Associated DNA During Analytical Treatment Interruption.
| Post-ATI Sample Day | Plasma RNA (copies/ml) | Whole Blood or PBMC DNA |
|---|---|---|
| Patient A | ||
| 7, 25, 35, 49, 70 | DNA not detected on all days (blood)a | |
| 14, 25, 28, 35, 42, 49, 56, 70 | <20b | |
| 84 | 904 | DNA detected (blood) |
| 88c | 127843 | 13 copies/106 PBMCs |
| 105 | 3225526 | |
| 112 | 1255960 | |
| 117 | 4173922 | 676 copies/106 PBMCs |
| 124 | 345634 | |
| 126 | 77504 | |
| 130 | 66977 | |
| 133 | 73369 | |
| 138 | 23222 | |
| 154 | 4344 | |
| 170 | 1388 | |
| 168 | 1114 | |
| 192 | 646 | 105 copies/106 PBMCs |
| 203 | 1028 | |
| 226 | (20202) d | |
| 241 | 183 | |
| 248 | 75 | |
| 252 | 93 | |
| 266 | 81 | |
| 280 | 110 | |
| 302 | 1420 | |
| 314 | 255 | |
| 319 | 21 | |
| Patient B | ||
| 14, 30, 64, 94, 122, 141, 156, 176, 199 | DNA not detected on all days (blood)a | |
| 38 | <0.4e | <0.5 copies/106 PBMCsb |
| 129 | <0.4e | <0.2 copies/106 PBMCsb |
| 7, 14, 22, 30, 38, 46, 56, 64, 78, 94, 106, 122, 129, 141, 149, 156, 164, 176, 185, 199, 211 | <20b | |
| 225 | 1900000 | |
| 233 | 174577 | |
| 238f | 32185 | 1100 copies/106 PBMCs |
| 247 | 10269 | |
| 255 | 3160 | |
| 266 | 385 | |
| 278 | 171 | 318 copies/106 PBMCs |
| 290 | 42 | |
| 304 | 44 | |
| 326 | <20 (detected) | |
| 350 | <20 (detected) | |
Abbreviations: ATI, analytical treatment interruption; PBMC, peripheral blood mononuclear cell
Clinical laboratory validated detection threshold was 250 HIV-1 DNA copies/ml of whole blood, but the assay may detect fewer DNA copies
No HIV-1 detected
RNA detected in cerebral spinal fluid, but <20 copies/ml
Positive value may have been due to sample mix-up at clinical laboratory, unable to verify sample was from day 226 and not an earlier time point
No RNA detected by single-copy assay
Cerebral spinal fluid viral load = 269 copies/ml
Viremia was first detected in plasma from Patient A on post-ATI day 84 (904 RNA copies/ml) by clinical viral load assay, 14 days after a negative clinical VL test. He was initially asymptomatic and was asked to immediately restart his prior ART regimen (tenofovir/emtricitabine/efavirenz) as per protocol after follow up testing 4 days later revealed a VL increase to 127, 843 copies/ml and HIV-1 was detected in CSF, but <20 copies/ml. His VL subsequently peaked at 4.2 million RNA copies/ml on post-ATI day 117, which may have been due, in part, to suboptimal medication adherence. HIV resistance testing performed on post ATI 112 revealed a new efavirenz resistance mutation (K103N), as well as resistance to several protease inhibitors (consistent with his history of previous treatment regimens) with the exception of tipranavir and darunavir. Given the emergence of drug resistance and rapidly increasing plasma viremia of uncertain etiology, the patient was prescribed tenofovir/emtricitabine/raltegravir and ritonavir-boosted darunavir and reduced-dose tacrolimus on post-ATI day 117. However, he developed nausea, vomiting, headache and fevers thought to be secondary to acute retroviral syndrome, and was only able to take several doses. He was evaluated at an outside hospital emergency department on post-ATI day 120 where he underwent CSF sampling and found to have a low-grade lymphocytic pleocytosis (11 white blood cells per high powered field) consistent with HIV-associated meningitis, but HIV-1 RNA was not measured in the CSF at this time. His symptoms may have been exacerbated by ART and tacrolimus co-administration.
The patient was discharged, but continued to have difficulty taking medication given central nervous system symptoms and vomiting though to be due to acute retroviral syndrome and potential medication side-effects, and was admitted to our hospital for workup and observed re-initiation of his ART. The tacrolimus trough level peaked at 104 ng/ml (desired range <10 ng/ml) shortly after admission in the setting of concomitant ritonavir. He also had an elevated serum creatinine of 1.7, but renal function and tacrolimus levels returned to normal within four days after both ART and tacrolimus were with held. His symptoms resolved with decreasing viral RNA prior to ART re-initiation; ART was resumed along with very low-dose tacrolimus (0.5 mg every three weeks) on post-ATI day 133 with continued viral decay.
Patient B had frequent negative HIV-1 clinical laboratory testing during ATI and undetectable PBMC-associated HIV-1 DNA and plasma HIV-1 RNA by quantitative PCR assays (limits of detection, 0.2 and 0.5 DNA copies/106 PBMCs and 0.4 RNA copies/ml, respectively) 5 and 18 weeks after ATI (Table 2). However, he developed fevers, malaise, and fatigue on post-ATI day 219, 8 days after a negative clinical VL test. The patient presented to an outpatient urgent care center with worsening symptoms on post-ATI day 225 and was found to have a plasma HIV-1 RNA level of 1.9 million copies/ml.
He promptly resumed ART on post-ATI day 228 with tenofovir/emtricitabine and dolutegravir, and symptoms resolved with subsequent decay of plasma viremia (Figure 1). No resistance-associated mutations were detected in plasma virus on post-ATI day 233 despite a pre-HSCT history of NNRTI resistance. In addition, he had 269 HIV-1 RNA copies/ml in CSF post-ATI day 238.
Single genome, near full-length HIV-1 envelope sequences from rebound viremia from both patient A (N=44) and B (N=50) were related to peri-HSCT HIV-1 DNA sequences in phylogenetic analyses. Viral sequences from each patient after rebound were monophyletic with a high degree of intra-sample sequence homology (Online Figure 2).
HIV-1 Specific Immunity after Treatment Discontinuation
No activation of PBMCs in response to HLA- matched peptides or pooled overlapping HIV-1 peptides in enzyme-linked immunospot assays were detected in pre-ATI samples from patients A and B, or from samples from patient B obtained prior to viral rebound at post-ATI days 38 and 129 (PBMCs were not collected for post-ATI time-points prior to rebound on patient A). Activation was observed in response to an HLA-A02-restricted Nef peptide in PBMC obtained from patient B 13 days after first detectable viral RNA (post-ATI day 238). No activation to HIV-1 peptides was detected in PBMC collected from patient A 5 days after viral rebound (post-ATI day 89), but low-level activation to Gag and Nef peptides and higher level activation to HLA-A02-restricted epitopes in p24 and p17 were detected in PBMC collected 108 days after viral rebound (post-ATI day 193).
Figure 3 shows HIV-1 antibody levels by LS Vitros assay and avidity over time. Antibody avidity declined in both patients during the period of virologic suppression post-HSCT. Antibody levels and avidity increased following viral rebound in patient A, in whom virologic control was not immediately re-established. Despite declining avidity after rebound in patient B, who promptly resumed ART, antibody levels increased 13 days after the first detectable VL measurement.
Figure 3. Anti-HIV-1 Antibody Avidity by Limiting-Antigen Assay Before and After Analytical Treatment Interruption.

HIV-1 antibody levels and avidity declined in both patients during the period of virologic suppression post-allogeneic stem cell transplantation [including pre-ATI time-points as previously reported (15)]. Antibody levels measured by the less sensitive (LS) VITROS Anti-HIV- 1+2 assay increased in both patients shortly after viral rebound. Antibody avidity increased slightly following viral rebound in patient A, in whom virologic control was not immediately reestablished, but continued to decline in patient B, who promptly resumed ART after the first detectable VL measurement. O Dn denotes normalized optical density measured by limitingantigen avidity (LAg) enzyme immunoassay, S/C signal to cutoff ratio of the VITROS assay, HSCT hematopoietic stem cell transplantation, and ATI analytical treatment interruption.
Discussion
Substantial reductions in HIV-1 reservoirs were observed in two patients who underwent allogeneic HSCT with HIV-1-susceptible donor cells while receiving continuous ART. Despite readily detectable proviral DNA prior to HSCT (15), HIV-1 RNA, proviral HIV-1 DNA and viral outgrowth were undetectable years after HSCT using highly sensitive assays applied to plasma, PBMC and GALT. Nevertheless, HIV-1 rebound was first detected 12 and 32 weeks after ART interruption. Rebound of viremia and the development of symptoms consistent with acute retroviral syndrome occurred within one to two weeks after the last negative plasma HIV-1 RNA result. Based on previously reported proviral DNA levels prior to or immediately following HSCT in both patients (96 to 144 copies/106 PBMCs) and the detection limits of sensitive quantitative assays used in this study, our findings suggest a minimum 3-log10 reduction in the number of circulating cells harboring proviral HIV-1 DNA after HSCT. Although allogeneic HSCT may lead to significant, sustained reductions in the HIV-1 reservoir, infected tissue or cell-bound virus persists. Persistence of these small numbers of residual infected cells appears to be sufficient to rekindle HIV-1 replication.
Our patients differed from the Berlin patient, who achieved sustained HIV-1 remission after two myeloablative HSCTs with resistant donor cells incapable of supporting HIV-1 replication in the event of reactivation of residual latent reservoirs (12-14). However, phylogenetic analyses indicated that only one or a small number of cells or latent proviruses contributed to viral rebound after ATI in our patients. The role of specific conditioning regimens and the use of various anti-inflammatory medications after HSCT and HIV-1 persistence are largely unknown and warrant further study.
HIV-1 typically rebounds 1 to 8 weeks after ART discontinuation (6-11). One person with undetectable HIV-1 DNA in blood or tissue, but very low levels of detectable infectious virus from virus outgrowth assays, experienced viral rebound 7 weeks following ATI (22). Post-treatment control of viral replication has been observed in patients initiating ART during acute HIV-1 infection and several of these individuals experienced delayed rebound after stopping ART (30, 31). However virus has been detected intermittently in early-treated patients with subsequent control of virus after ART discontinuation, and durable control was achieved in relatively few individuals (30, 31). Despite frequent sampling, neither of our patients had detectable HIV-1 in PBMC or plasma for several months following ART discontinuation prior to viral rebound. In addition, ultrasensitive microchimerism testing showed that nearly all PBMCs were of donor origin years after HSCT.
It is possible that a chronic, ongoing graft-versus-host reaction was responsible for the continued surveillance and clearance of residual recipient hematopoietic cells that survived conditioning chemotherapy in our patients and the Berlin patient, some of which happened to harbor latent HIV-1. Graft-versus-tumor and more generalized graft-versus-hematopoietic effects may exist without the development or persistence of clinical graft-versus-host disease, and are mediated by innate immunity and natural killer (NK) cells in addition to T lymphocyte activity (32, 33). Furthermore, the lack of detectable HIV-1 DNA and replication competent virus prior to ATI supports the hypothesis that donor cells in various tissues (e.g. blood and gut) were largely protected from infection by ART.
Reductions in the HIV-1 reservoir have been described in patients undergoing myeloablative allogeneic HSCT in the setting of AZT monotherapy or suppressive ART (34-38). Detailed data on ART interruption following allogeneic HSCT are limited to the report of an individual who experienced a reduction in HIV-1 DNA shortly after myeloablative HSCT and full donor chimerism (34). That patient developed grade III graft-versus-host disease of the skin and gastrointestinal tract, and experienced rapid viral rebound within 16 days of stopping ART 4 months after transplantation (34). In contrast, our patients had been on ART for 2 or more years post-HSCT (15), and achieved months of ART-free viral remission. It is possible that chronic graft-versus-host effects without clinically significant disease led to more profound reductions in viral reservoirs and ultimately delayed return of virus. The longer interval between HSCT and ATI may also have contributed to a longer period of HIV-1 remission in our patients.
Long-lived tissue reservoirs, including host macrophages that are replaced more slowly than T-lymphocytes following HSCT (12), may have contributed to viral rebound. It is possible that residual pre-transplant recipient lymphoid tissue persisted despite a very high degree of donor blood chimerism, or that donor cells inaccessible to peripheral blood and tissue sampling had become infected. For example, only a limited number of CD4+ T cells were able to be surveyed from gut tissue, and more intensive sampling may have led to detection of HIV-1. Low levels of detectable HIV-1 RNA were identified in CSF following viral rebound, but were orders of magnitude lower than peripheral blood viral loads. We were unable to obtain CSF during ATI prior to rebound, and further studies of tissue localization and cellular composition of this reservoir are needed.
Patients undergoing allogeneic HSCT have variable HIV-specific cellular immune responses after transplantation (38). Little to no T cell response to HIV-1 peptides was observed in our patients after HSCT or during ATI until at least 13 days after viral rebound. Thus, it appears that virus-specific adaptive immunity played little role in controlling HIV-1 replication prior to rebound. The extent to which non-specific innate immunity and graft-versus-host effects influenced the duration of ART-free remission prior to viral rebound is not well defined and warrants further investigation. Both patients also experienced a decrease in and subsequent low-level persistence of HIV-1-specific antibodies and avidity following allogeneic HSCT. Antibody levels increased shortly after viral rebound in both patients, but avidity continued to decrease in samples from patient B, who promptly re-initiated ART. Although the Berlin patient experienced an even further decrease in antibody levels and avidity following HSCT, his antibodies persisted after transplantation for more than 5 years (14). The sources of residual antibodies in the HSCT patients are unknown and warrant further study.
As a result of allogeneic HSCT from HIV-unexposed donors, the reconstituted immune systems of our patients were HIV-naïve, as reflected by the absence of detectable virus-specific cellular immune responses. Consequently, when HIV-1 rebound occurred, it mimicked the kinetics observed during acute HIV-1 infection (39). Given the rapid virologic rebound, the development of accompanying symptoms, and the emergence of a new NNRTI mutation despite closely monitored ART re-initiation, any future studies of ATI in the setting of allogeneic HSCT should proceed with utmost caution. Given the limited sensitivity of currently available assays for detecting viral persistence, however, analytical treatment interruption remains the only reliable means of assessing the extent of HIV-1 reservoir depletion following therapeutic interventions.
In summary, our results suggest that allogeneic HSCT with CCR5 wild-type donor cells may lead to loss of detectable HIV-1 from blood and rectal mucosa, but viral rebound may nevertheless occurr after antiretroviral treatment interruption despite a significant reduction in reservoir size. Defining the nature and half-life of residual viral reservoirs is essential in order to achieve durable ART-free HIV-1 remission.
Supplementary Material
Phylogenetic trees of near full-length, single genome HIV-1 envelope sequences for patients A (left) and B (right) are shown. Compared with an outgroup of HIV-1 subtype B sequences from the Los Alamos National Laboratory HIV Sequence Database Compendium, rebound HIV-1 envelope sequences were highly related to per-HSCT cell-associated viral DNA. Rebound HIV-1 envelope sequences were monophyletic with a very high degree of intra-sample sequence homology, suggesting that viral reseeding occurred from only one or a small number of cells or proviruses. Maximum likelihood trees were constructed using a general time-reversible model after stripping gaps and ambiguous alignment regions. Distance scale bars denote nucleotide substations per site.
Acknowledgments
T.J.H had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We acknowledge A. Tsibris and S. Deeks for helpful discussions, M. Lebedeva for performing antibody quantitation, L. Wen for performing microchimerism testing, A. LaCasce for helping with initial study design, and C. Palmer for helping with flow cytometry and immune assays.
Funding: This work was supported by the Foundation for AIDS Research (amfAR) ARCHE grant; the National Institutes of Health/National Institute of Allergy and Infectious Disease 1K23AI098480-01A1; UM1 AI068636 (AIDS Clinical Trials Group Virology Support Laboratory); P30 AI060354 (Harvard CFAR Program in Therapeutics); U19 AI096109 (DARE Collaboratory); and funding from the Ragon Institute of MGH, MIT and Harvard (M.A.). This publication is also based on research funded in part by the Bill & Melinda Gates Foundation (Global Health Grant Number OPP1017716). The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation or the NIH.
Major Funding Sources: Foundation for AIDS Research; National Institute of Allergy and Infectious Diseases
Footnotes
Conflict of Interest: D.R.K. is a consultant to and has received honoraria from Abbott, Boehringer - Ingelheim, Bristol-Myers Squibb, Celera, Gilead, Glaxo Smith Kline, Inno VirVax, Koronis, Merck, Roche, Sangamo and ViiV; he has received grant support from Gilead and Merck, and speaking honoraria from Gilead and ViiV. M.A. has received speaking honoraria from Gilead and Merck.
Authorship: T.J.H designed the study, designed and performed experiments, performed data analysis, interpreted results and wrote the manuscript; J.Z.L. E.H., M.S., A.H., S.K. T-H. L., and B.T.D. designed and performed experiments; Y.R. coordinated clinical research and sample collection; A.L.H. performed data analysis and interpreted assay results; F.F.M., P.A. and R.J.S. helped design the study and provided clinical care/oversight; M.P.B. and M.A. helped design experiments and interpreted results; D.R.K. helped design the study, interpreted results, and aided in manuscript preparation.
References
- 1.Siliciano RF. What do we need to do to cure HIV infection. Top HIV Med. 2010;18(3):104–8. [PubMed] [Google Scholar]
- 2.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 3.Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106(23):9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi RT, Bosch RJ, Aga E, Albrecht M, Demeter LM, Dykes C, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201(2):293–6. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, Hafner R, Schneider C, Trkola A, Joos B, Joller H, et al. HIV RNA in plasma rebounds within days during structured treatment interruptions. Aids. 2003;17(2):195–9. doi: 10.1097/00002030-200301240-00009. [DOI] [PubMed] [Google Scholar]
- 8.Taylor S, Boffito M, Khoo S, Smit E, Back D. Stopping antiretroviral therapy. Aids. 2007;21(13):1673–82. doi: 10.1097/QAD.0b013e3281c61394. [DOI] [PubMed] [Google Scholar]
- 9.Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. Aids. 1999;13(8):F59–62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz L, Martinez-Picado J, Romeu J, Paredes R, Zayat MK, Marfil S, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. Aids. 2000;14(4):397–403. doi: 10.1097/00002030-200003100-00013. [DOI] [PubMed] [Google Scholar]
- 11.Frost SD, Martinez-Picado J, Ruiz L, Clotet B, Brown AJ. Viral dynamics during structured treatment interruptions of chronic human immunodeficiency virus type 1 infection. J Virol. 2002;76(3):968–79. doi: 10.1128/JVI.76.3.968-979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2010 doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 13.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta 32/Delta 32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 14.Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9(5):e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207(11):1694–702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, et al. The Distribution of HIV DNA and RNA in Cell Subsets Differs in Gut and Blood of HIV-Positive Patients on ART: Implications for Viral Persistence. J Infect Dis. 2013;208(8):1212–20. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malnati MS, Scarlatti G, Gatto F, Salvatori F, Cassina G, Rutigliano T, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc. 2008;3(7):1240–8. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- 19.Henrich TJ, Gallien S, Li JZ, Pereyra F, Kuritzkes DR. Low-level detection and quantitation of cellular HIV-1 DNA and 2-LTR circles using droplet digital PCR. J Virol Methods. 2012;186(1-2):68–72. doi: 10.1016/j.jviromet.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 22.Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. Aids. 2010;24(18):2803–8. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 24.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105(21):7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7(3):e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altfeld MA, Trocha A, Eldridge RL, Rosenberg ES, Phillips MN, Addo MM, et al. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J Virol. 2000;74(18):8541–9. doi: 10.1128/jvi.74.18.8541-8549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee TH, Chafets DM, Reed W, Wen L, Yang Y, Chen J, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006;46(11):1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Paglieroni T, Utter GH, Chafets D, Gosselin RC, Reed W, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45(8):1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 29.Li JZ, Gallien S, Do TD, Martin JN, Deeks S, Kuritzkes DR, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56(11):5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann DE, Lichterfeld M, Altfeld M, Addo MM, Johnston MN, Lee PK, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;1(2):e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Besien K. Allogeneic transplantation for AML and MDS: GVL versus GVHD and disease recurrence. Hematology Am Soc Hematol Educ Program. 2013;2013:56–62. doi: 10.1182/asheducation-2013.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Murphy WJ, Parham P, Miller JS. NK cells--from bench to clinic. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S2–7. doi: 10.1016/j.bbmt.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avettand-Fenoel V, Mahlaoui N, Chaix ML, Milliancourt C, Burgard M, Cavazzana-Calvo M, et al. Failure of bone marrow transplantation to eradicate HIV reservoir despite efficient HAART. Aids. 2007;21(6):776–7. doi: 10.1097/QAD.0b013e3280b01836. [DOI] [PubMed] [Google Scholar]
- 35.Contu L, La Nasa G, Arras M, Pizzati A, Vacca A, Carcassi C, et al. Allogeneic bone marrow transplantation combined with multiple anti-HIV-1 treatment in a case of AIDS. Bone Marrow Transplant. 1993;12(6):669–71. [PubMed] [Google Scholar]
- 36.Holland HK, Saral R, Rossi JJ, Donnenberg AD, Burns WH, Beschorner WE, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Ann Intern Med. 1989;111(12):973–81. doi: 10.7326/0003-4819-111-12-973. [DOI] [PubMed] [Google Scholar]
- 37.Huzicka I. Could bone marrow transplantation cure AIDS?: review. Medical Hypotheses. 1999;52(3):247–57. doi: 10.1054/mehy.1997.0638. [DOI] [PubMed] [Google Scholar]
- 38.Woolfrey AE, Malhotra U, Harrington RD, McNevin J, Manley TJ, Riddell SR, et al. Generation of HIV-1-specific CD8 + cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112(8):3484–7. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324(14):961–4. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic trees of near full-length, single genome HIV-1 envelope sequences for patients A (left) and B (right) are shown. Compared with an outgroup of HIV-1 subtype B sequences from the Los Alamos National Laboratory HIV Sequence Database Compendium, rebound HIV-1 envelope sequences were highly related to per-HSCT cell-associated viral DNA. Rebound HIV-1 envelope sequences were monophyletic with a very high degree of intra-sample sequence homology, suggesting that viral reseeding occurred from only one or a small number of cells or proviruses. Maximum likelihood trees were constructed using a general time-reversible model after stripping gaps and ambiguous alignment regions. Distance scale bars denote nucleotide substations per site.


