Abstract
Objectives:
The objective of this study is to evaluate the efficacy and safety of sealing pressure as an inflation technique of the Microcuff pediatric tracheal cuffed tube.
Materials and Methods:
A total of 60 children were enrolled in this study. After induction of anesthesia and intubation with Microcuff pediatric tracheal tube, patients were randomly assigned, to one of the three groups. Control group (n = 20) the cuff was inflated to a cuff pressure of 20 cm H2O; sealing group (n = 20) the cuff was inflated to prevent the air leak at peak airway pressure of 20 cm H2O and the finger group (n = 20) the cuff was inflated to a suitable pressure using the finger estimation. Tracheal leak, incidence and severity of post-extubation cough, stridor, sore throat and hoarseness were recorded.
Results:
The cuff pressure as well as the volume of air to fill the cuff was significantly low in the sealing group when compared with the control group (P < 0.001); however, their values were significantly high in the finger group compared with both the control and the sealing group (P < 0.001). The incidence and severity of sore throat were significantly high in the finger group compared with both the control and the sealing group (P = 0.0009 and P = 0.0026). Three patients in the control group developed air leak around the endotracheal tube cuff. The incidence and severity of other complications were similar in the three groups.
Conclusion:
In pediatric N2O, free general anesthesia using Microcuff pediatric tracheal tub, sealing cuff pressure is safer than finger palpation technique regarding post-extubation morbidities and more reliable than recommended safe pressure in prevention of the air leak.
Keywords: Airway morbidity, children, cuff pressure, Microcuff endotracheal tube
INTRODUCTION
For a long time, the cuffed endotracheal tube (CETT) were not recommended by most pediatric anesthetists for children younger than 5-8 years.[1,2] Primary because the diameter of a CETT had to be 1-2 sizes smaller than the un-cuffed tube, Consequently, flow resistance and the work of breathing would be drastically increased in those spontaneously breathing children.[3] The second reason was the earlier reports of airway morbidity[4,5] caused by poor design and material[6,7,8,9,10,11] of earlier CETT. With the introduction of a new pediatric tracheal tube with an ultrathin (10 μm) polyurethane high volume-low pressure (HVLP) cuff (Microcuff pediatric tracheal tube), as well as, mechanical ventilation became a common practice,[3] cuffed tracheal tubes in neonates and smaller children have become more popular in pediatric anesthesia during the last decade.[12,13,14,15]
Although the design and tracheal sealing characteristics of newer pediatric cuffed tracheal tubes have been markedly improved,[16,17,18,19] the risk of inadvertent cuff hyperinflation remains a matter of concern as a potential cause of airway damage in children. The upper limit of safety for cuff pressure in adults is 25-30 cm H2O, but there is no data in children and it is speculated that a lower cuff pressure (20 cm H2O) would possibly be safe.[18,20] The aim of this study is to evaluate the safety and reliability of the cuff sealing pressure of the Micro-cuff pediatric tracheal tube in the prevention of the post-intubation airway morbidity compared with the other inflation techniques.
MATERIALS AND METHODS
After approval from local Research and Ethics Committee and written informed consents, 60 children with age between 6 and 12 years old and American Society of Anesthesiologists physical status I or II were enrolled in this prospective controlled, randomized, blinded study. All patients were scheduled for elective dental surgery under N2O free general anesthesia with expected duration of 120 min or more. Any patients who had a recent attack of upper respiratory tract infection, history of bronchial asthma, or in whom intubation was difficult (two or more attempts) were excluded from the study.
All the patients were pre-medicated with 0.2 mg/kg oral diazepam 90 min before anesthesia induction. After fixation of routine monitors (pulse oximetry, blood pressure, electrocardiogram) and pre-oxygenation for 2-3 min, a standard anesthetic technique was conducted by consultant anesthesiologist who was blind to the study design. Anesthesia was induced with fentanyl 2 μg/kg and propofol 2.5 mg/kg. Tracheal intubation was facilitated with rocuronium bromide 0.6 mg/kg, using oral Microcuff pediatric endotracheal tube (ETT) (Microcuff GmbH, Weinheim, Germany) with HVLP cuff [Figures 1 and 2] and with an inner diameter calculated according to the Khine's et al. formula (ID = age/4 + 3).[12]
Figure 1.
Microcuff endotracheal tube with deflated cuff
Figure 2.
Microcuff endotracheal tube with inflated cuff
Patients were randomly assigned, by opening a sealed envelope, to one of three groups. In the control group (n = 20), the ETT cuff was aspirated as much as possible and then inflated with air to achieve a cuff pressure of 20 cm H2O. In the sealing group (n = 20), the ETT cuff was aspirated as much as possible and then inflated with air to prevent air leaks during the inspiratory phase of mechanical ventilation of the patient when peak airway pressure was 20 cm H2O. In the finger group (n = 20) the ETT cuff was aspirated as much as possible and then inflated to a suitable pressure using finger estimation. The volume used to fill the cuff and the cuff pressure were recorded in each group using the hand-held cuff pressure gauge (Mallinckrodt Medical Athlone, Ireland) by an Anesthesiologist who was blind to the studied group [Figure 3]. The same anesthesiologist assessed tracheal leak by both audible technique and by observing the difference between inspiratory and expiratory tidal volume. Mechanical ventilation was controlled and adapted to maintain end-tidal carbon dioxide at 30-35 mmHg. Anesthesia was maintained with sevoflurane (1-2% end-tidal) and 30% oxygen in the air. Additional boluses of Fentanyl (1-2 μg/kg/body weight) were administered to maintain surgical analgesia. At the end of the surgery, sevoflurane was discontinued, the lungs were ventilated with 100% O2 and the pharynx was gently suctioned. The residual muscle paralysis was reversed by neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg. The tracheas of the patients were extubated when they fulfilled the following criteria; (1) efficient spontaneous respiration (2) ability to follow the verbal commands and ability to do purposeful movement (attempting self-extubation) (3) full reversal of neuromuscular block (ulnar nerve T4/T1 ratio = 1). The duration of the surgery and intubation as well as post-extubation stridor, were recorded. Post-extubation coughing was graded and recorded based on the modified four points scale as follows; Grade 0 = No cough; Grade 1 = (Mild) single bout of cough; Grade 2 = (Moderate) more than one episodes of unsustained (≤5 s) coughing and Grade 3 = (Severe) sustained (>5 s) bouts of coughing. Paracetamol suppository (15 mg/kg) was inserted for post-operative analgesia. The same blinded anesthesiologist recorded coughing as above grading and recorded sore throat and hoarseness using visual analog scale score (VAS: 0-10 cm) before discharge from post-anesthesia care unit (PACU) and 24 h after tracheal extubation.
Figure 3.
Mallinckrodit cuff pressure gauge connected to Microcuff endotracheal tube inflated to a pressure 20 cm H2O
Statistics
To calculate the sample size, we estimated that using sealing pressure inflation technique would decrease the incidence of post-operative sore throat by 50-60% than the finger estimation technique as evaluated by the pilot study. Based on this estimation and at a significance level of 0.05 with a power of 80%, 20 subjects were needed in each group. Demographic data, duration of surgery and intubation, dose of fentanyl, the volume of air injected in the cuff and intracuff pressure were analyzed by one-way ANOVA-test with Bonferroni correction. Unpaired Student's t-test was used to compare the previous data between the control group and the other two groups. Male to female ratio and the differences in the incidence of the laryngotracheal symptoms were analyzed by Fisher's exact or Chi-square tests. Kruskal-Wallis and Mann-Whitney U-tests were used for non-parametric data. Probability (P < 0.05) was considered to be statistically significant. Analysis was performed using Statistica software version 7.0 for windows (Statsoft Inc., Tulsa, USA).
RESULTS
A total of 60 patients participated in our study and none was excluded. There was no significant difference between the three groups regarding demographic data, duration of surgery and intubation or in the total intraoperative fentanyl doses [Table 1]. The cuff pressure as well as the volume of air to fill the cuff were significantly low in the sealing group compared with the control group (P < 0.001), however their values were significantly high in the finger group compared to both the control and the sealing group (P < 0.001) [Table 1]. Three patients (15%) in the control group developed air leak around the ETT cuff compared to zero patients in the other two groups (P = 0.0439). The incidence of sore throat, was similar for the control and sealing groups in the PACU and at 24 h, whereas for the finger group the incidence of sore throat was significantly higher than the other two groups in the PACU and at 24 h (P = 0.0009 and P = 0.0229). The incidences of hoarseness, cough and post-extubation stridor were comparable in the three groups at all recording times [Table 2].
Table 1.
Patient's demographic and operative data values are mean (SD) or number (proportion)
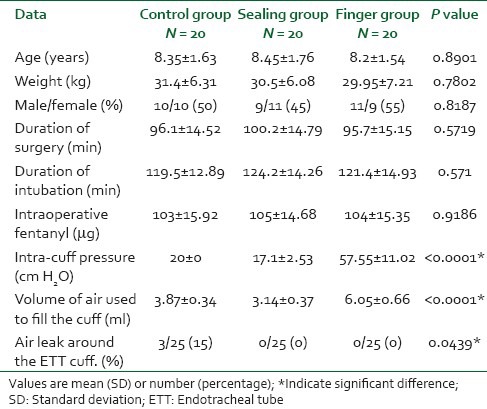
Table 2.
Incidence of tracheal tube-induced emergence phenomena
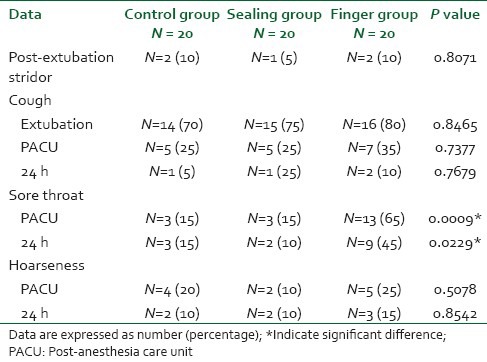
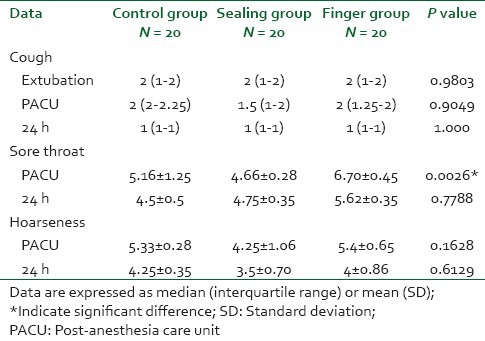
The severity (visual analog scale) of sore throat was significantly high in the finger group compared to both the control and the sealing group at PACU (P = 0.0026) even though; the three groups were similar at 24 h. The severity of hoarseness and cough were comparable in the three groups at all recording times [Table 3].
Table 3.
Severity of tracheal tube-induced emergence phenomena
DISCUSSION
Our prospective randomized controlled double-blind study showed significant higher incidence of sore throat in the finger group than both sealing and control groups. Whereas, the incidences of hoarseness, cough and post-extubation stridor were similar in the three groups.
In the present study and similar to our previous study in adults,[21] though significant low mean cuff pressures in the sealing group (17.1 ± 2.53 cm H2O) compared to the control group (20 cm H2O), the frequency or intensity of post-operative laryngotracheal complaints was not reduced. This was expected as the cuff pressure in the control group was in the safe recommended range (20-25 cm H2O), which unlikely to impair tracheal capillary mucosal perfusion in children.[18,20] On the other hand, the mean cuff pressure in the finger group (57.55 ± 11.02 cm H2O) greatly exceeded the critical value, which could explain the higher significant incidence and severity of sore throat in this group compared to the sealing and control groups.
In our present study, the incidence of sore throat in the finger group was 65%, which is comparable to the result of Holzki[6] study who reported 82% incidence of airway trauma in children with CETT. This could be explained by the in vitro study of Bernet et al.[22] who found that the external diameter of pediatric CETTs can expand to more than twice the age-corresponding tracheal internal diameter when overinflated, leading to considerable increases in cuff pressure above the perfusion pressures of the tracheal mucous membrane. This indicates that palpation of the external balloon (contiguous with the cuff) after intubation as a rough estimation of the intracuff pressure is unreliable unsafe technique for ETT cuff inflation.
In the present study, nevertheless the incidence of sore throat, was significantly higher in the finger group compared to both sealing and control group, the incidences of post-extubation stridor, cough and hoarseness were similar in the three groups.
This finding have been supported by our and other studies on the adult population.[21,23,24] This could be explained by the direct relation between the tracheal cuff pressure and the development of tracheal mucosal ulcerations, which correlate with the incidence of post-operative sore throat. While the other post-operative symptoms principally correlated with tracheal intubation and airway management, explaining their similar incidence in all groups. On the other hand, although the severity of sore throat was comparable in the three groups in similar adult studies,[21,23,24] one of the interesting difference in this study is the significant high severity of sore throat in the finger group than both control and sealing groups. This could be explained by the delicate and tiny structure of the children tracheal mucosa, which might be deeply injured by high cuff pressure than adult mucosa resulting in more severe sore throat.
Our present study showed that Microcuff pediatric tracheal tube with an ultrathin HVLP cuff membrane allows an effective tracheal sealing at very low mean cuff pressures. This confirm the preliminary study of Dullenkopf et al.[19] who reported a significant low sealing pressure (11 cm H2O)[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] of the new Microcuff pediatric tracheal tube compared with three conventional pediatric cuffed tracheal tubes. This was explained by the ability of the ultrathin polyurethane cuff membrane of the Microcuff tracheal tube to avoids the formation of longitudinal folds and provides “all-around” surface contact with the tracheal mucosa. This allows tracheal sealing by filling the tracheal lumen and not by pressure against the tracheal wall, producing low sealing pressure.
Although, the mean cuff pressure in the control group (20 cm H2O) was significantly higher than the mean cuff pressure of the sealing group (17.1 ± 2.53 cm H2O), three patients in the control group developed air leak around the cuff. This could be explained by the individual variation in the age related size and length of the pediatric trachea as well as the site of cuff placement. This finding pointed out the reliability of the sealing cuff pressure as a cuff inflation technique than cuff inflation to a recommended low pressure in prevention of the air leak.
The limitation of this study is that, we did not compare the pediatric Microcuff tracheal tube with the conventional pediatric cuffed tracheal tubes regarding the post-extubation morbidity. However, the present study and Dullenkopf et al. study[19] reported a lower sealing cuff pressure with the use of Microcuff tracheal tube, which would predict a lower stress exerted on the airway mucosa and hereafter, a lower incidence of post-extubation morbidity than with the use of conventional tracheal tube.
CONCLUSION
In anesthesia in which N2O is not used, inflation of the cuff of the pediatric Micro-cuff tracheal tube to a sealing pressure is safer than finger palpation technique regarding post-extubation laryngotracheal morbidity. Moreover, it is more convenient and reliable than inflation of the cuff to a safe lower pressure (20 cm H2O) regarding prevention of the air leak.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fisher DM. Anesthesia equipment for pediatrics. In: Gregory GA, editor. Pediatric Anesthesia. New York: Churchill Livingstone; 1989. pp. 464–5. [Google Scholar]
- 2.Uejima T. Cuffed endotracheal tubes in pediatric patients. Anesth Analg. 1989;68:423. doi: 10.1213/00000539-198903000-00062. [DOI] [PubMed] [Google Scholar]
- 3.Gronert BJ, Motoyama EK. Induction of anesthesia and endotracheal intubation. In: Motoyama EK, Davis PJ, editors. Smith's Anesthesia for Infants and Children. 6th ed. St. Louis: Mosby; 1996. pp. 281–312. [Google Scholar]
- 4.Hawkins DB. Glottic and subglottic stenosis from endotracheal intubation. Laryngoscope. 1977;87:339–46. doi: 10.1288/00005537-197703000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Honig EG, Francis PB. Persistent tracheal dilatation: Onset after brief mechanical ventilation with a “soft-cuff” endotracheal tube. South Med J. 1979;72:487–90. [PubMed] [Google Scholar]
- 6.Holzki J. Laryngeal damage from tracheal intubation. Paediatr Anaesth. 1997;7:435–7. doi: 10.1046/j.1460-9592.1997.d01-127.x. [DOI] [PubMed] [Google Scholar]
- 7.Dillier CM, Trachsel D, Baulig W, Gysin C, Gerber AC, Weiss M. Laryngeal damage due to an unexpectedly large and inappropriately designed cuffed pediatric tracheal tube in a 13-month-old child. Can J Anaesth. 2004;51:72–5. doi: 10.1007/BF03018551. [DOI] [PubMed] [Google Scholar]
- 8.Ho AM, Aun CS, Karmakar MK. The margin of safety associated with the use of cuffed paediatric tracheal tubes. Anesthesia. 2002;57:173–5. doi: 10.1046/j.0003-2409.2001.02364.x. [DOI] [PubMed] [Google Scholar]
- 9.Goel S, Lim SL. The intubation depth marker: The confusion of the black line. Paediatr Anaesth. 2003;13:579–83. doi: 10.1046/j.1460-9592.2003.01103.x. [DOI] [PubMed] [Google Scholar]
- 10.Weiss M, Dullenkopf A, Gysin C, Dillier CM, Gerber AC. Shortcomings of cuffed paediatric tracheal tubes. Br J Anaesth. 2004;92:78–88. doi: 10.1093/bja/aeh023. [DOI] [PubMed] [Google Scholar]
- 11.Deakers TW, Reynolds G, Stretton M, Newth CJ. Cuffed endotracheal tubes in pediatric intensive care. J Pediatr. 1994;125:57–62. doi: 10.1016/s0022-3476(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 12.Khine HH, Corddry DH, Kettrick RG, Martin TM, McCloskey JJ, Rose JB, et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86:627–31. doi: 10.1097/00000542-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Murat I. Cuffed tubes in children: A 3-year experience in a single institution. Paediatr Anaesth. 2001;11:748–9. doi: 10.1046/j.1460-9592.2001.0774d.x. [DOI] [PubMed] [Google Scholar]
- 14.Orliaguet GA, Renaud E, Lejay M, Meyer PG, Schmautz E, Telion C, et al. Postal survey of cuffed or uncuffed tracheal tubes used for paediatric tracheal intubation. Paediatr Anaesth. 2001;11:277–81. doi: 10.1046/j.1460-9592.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Newth CJ, Rachman B, Patel N, Hammer J. The use of cuffed versus uncuffed endotracheal tubes in pediatric intensive care. J Pediatr. 2004;144:333–7. doi: 10.1016/j.jpeds.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Weiss M, Dullenkopf A, Gerber AC. Microcuff pediatric tracheal tube. A new tracheal tube with a high volume-low pressure cuff for children. Anaesthesist. 2004;53:73–9. doi: 10.1007/s00101-003-0604-x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss M, Gerber AC, Dullenkopf A. Appropriate placement of intubation depth marks in a new cuffed paediatric tracheal tube. Br J Anaesth. 2005;94:80–7. doi: 10.1093/bja/aeh294. [DOI] [PubMed] [Google Scholar]
- 18.Dullenkopf A, Schmitz A, Gerber AC, Weiss M. Tracheal sealing characteristics of pediatric cuffed tracheal tubes. Paediatr Anaesth. 2004;14:825–30. doi: 10.1111/j.1460-9592.2004.01316.x. [DOI] [PubMed] [Google Scholar]
- 19.Dullenkopf A, Gerber AC, Weiss M. Fit and seal characteristics of a new paediatric tracheal tube with high volume-low pressure polyurethane cuff. Acta Anaesthesiol Scand. 2005;49:232–7. doi: 10.1111/j.1399-6576.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 20.Dullenkopf A, Bernet-Buettiker V, Maino P, Weiss M. Performance of a novel pressure release valve for cuff pressure control in pediatric tracheal tubes. Paediatr Anaesth. 2006;16:19–24. doi: 10.1111/j.1460-9592.2005.01686.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Metwalli RR, Al-Ghamdi AA, Mowafi HA, Sadek S, Abdulshafi M, Mousa WF. Is sealing cuff pressure, easy, reliable and safe technique for endotracheal tube cuff inflation? A comparative study. Saudi J Anaesth. 2011;5:185–9. doi: 10.4103/1658-354X.82795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernet V, Dullenkopf A, Maino P, Weiss M. Outer diameter and shape of paediatric tracheal tube cuffs at higher inflation pressures. Anesthesia. 2005;60:1123–8. doi: 10.1111/j.1365-2044.2005.04359.x. [DOI] [PubMed] [Google Scholar]
- 23.Combes X, Schauvliege F, Peyrouset O, Motamed C, Kirov K, Dhonneur G, et al. Intracuff pressure and tracheal morbidity: Influence of filling with saline during nitrous oxide anesthesia. Anesthesiology. 2001;95:1120–4. doi: 10.1097/00000542-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Braz JR, Navarro LH, Takata IH, Nascimento P., Júnior Endotracheal tube cuff pressure: Need for precise measurement. Sao Paulo Med J. 1999;117:243–7. doi: 10.1590/s1516-31801999000600004. [DOI] [PubMed] [Google Scholar]


