Abstract
Background:
Sickle cell disease (SCD) in children with a history of repeated hospitalization is distressing for children as well as their parents leading to anxiety and has negative effects on the psychological state of children and their families. Objective: The aim of the study was to determine the overall effect of SCD on the behavior of young children age 1½ to 5 years old who had repeated history of hospitalization, compared to a control group of healthy children attended a vaccination clinic.
Patients and Methods:
Thirty-five children of age 1½ to 5 years who have SCD and repeated history of hospitalization were recruited from pediatric clinic as the study group and matched with same number of healthy children who attended vaccination clinic, as a control group. Both groups were administered the child behavior checklist (CBCL) 1½ to 5 years and diagnostic and statistical (DSM)-oriented scale. Behavior data were collected through a semi-structured questionnaire.
Results:
Children who have SCD had statistically significant behavioral changes on CBCL compared to the control group: Anxiety/depression (65.2 vs. 55.1; P < 0.001), somatic complaint (66.7 vs. 54.4; P < 0.001) withdrawn (63.4 vs. 53.2; P < 0.001), aggressive behavior (60.4 vs. 56; P=0.04), and internalizing symptoms (64.7 vs. 51.5; P < 0.001), respectively. The DSM scale showed that children with SCD scored significantly higher in pervasive developmental disorder compared to the control group (60.9 vs. 53.9; P < 0.001) respectively.
Conclusion:
Children with SCD who had history of repeated hospitalization are at an increased risk of developing behavioral problems. Psychological counseling, social support, and proper pain management could minimize these behavioral consequences.
Keywords: Behavioral, CBCL 1½-5, hospitalization, pediatric, SCD
INTRODUCTION
Sickle cell disease (SCD) is the most common inherited disease in Saudi Arabia, particularly in the eastern and southern provinces and is responsible for a massive health burden. Its main clinical feature is severe pain that is unpredictable and recurrent, in addition to the other acute and chronic features of SCD which may have a huge impact on the quality of life of both the patient and their families and careers.[1,2]
While SCD usually results in anemia, the primary symptomatic manifestation of SCD is pain. Children with homozygous disease face a chronic disease, with onset in childhood resulting in serious complications.[3] Those SCD children are at higher risk of developing many psychological problems including anxiety and depression.[4] Also, chronicity of the disease is distressing for children as well as parents or guardians which has negative effects on the psychological state of children.[5] In addition, frequent problems of pain, withdrawal from normal social surroundings, and frequent or long stays in hospital during the pain episodes may bring also negative consequences to the normal development of a child at stages of emotional, socio-behavioral, cognitive, and academic progress and contribute significantly to impaired psychosocial functioning, altered intra- and interpersonal relationships, and reduced quality of life.[6,7] Indeed, those SCD children are at increased risk of developing internalizing problems as a result of their disease, and often manifest neuro-cognitive impairments and learning problems, problematic interpersonal relationships, low self-esteem, and maladaptive coping patterns.[8,9] Psychological problems that complicate chronic physical illness in children with SCD are common and pediatric liaison services in the developing countries attending to the psychological health needs of those children are limited.[10]
The assessment of pain in young pre-school children is a challenging problem because those children usually lack the verbal and cognitive skills to describe their feeling of pain. Different pain scores have been developed to assess pain at an early childhood which rely on behavioral observations.[11,12,13]
The Toddler-Preschooler Postoperative Pain Scale (TPPPS) was used in our study.[13] It relies on behavioral observations in addition to self-report by children. The TPPPS includes seven parameters; each one is divided into three pain behavior groups: Vocal pain expression (verbal complaint/cry, groan/moan/grunt, scream), facial pain expression (open mouth/lips pulled back at corners, squint/close eyes, brow bulging/furrowed forehead), and bodily pain expression (restless motor behavior/rub or touch painful area). The 7 items were scored as a 1 if the pain behavior was present during a 5 minute observation period or as a 0 if not present. Scores therefore range from 0 to 7. The maximum score obtained was 7, which indicated a high pain intensity. The TPPPS reliability and validity have been demonstrated in children 1 to 5 years of age.[14]
The aim of this study was to determine the overall effect of SCD on the behavior adjustment of children age 1½ to 5 years old with frequent hospital admissions compared to a control group of healthy children attended a vaccination clinic.
PATIENTS AND STUDY METHODS
The study was carried out at Al-Ahsa Hospital-Al-Ahsa city, Saudi Arabia over 8-month period. Thirty-five children with SCD (Group I) were recruited out of those attending pediatric clinic coming for follow up and management of SCD with history of three or more times hospitalization. Inclusion criteria for children with SCD included: Age ranged from 1½ to 5 years, Saudi nationality, free from other acute, or chronic illnesses. The pediatric consultant supervised the data collection. The study group was matched with the control group of children (Group II) who attended a vaccination clinic and who have the same inclusion criteria except SCD.
The research proposal and data collection tools were approved by hospital research and ethics committee. Orientation for parents about study objectives was carried out before obtaining informed consent form with emphasis on the right of the subject of non-participation. Participants’ information confidentiality was maintained through all the study.
Data collection methods
Socio-demographic data were collected including age in months, gender, residence, and weight through an interview with parents particularly mothers in the two study groups. The interviews were carried out on the same day of attending the clinics in the afternoon in a separate room away from the busy clinics by trained nurse under the supervision of the investigators.
Parents accompanying the children were invited to complete a child behavior checklist (CBCL) and Diagnostic and statistical (DSM)-oriented scale which are a group of self-rated questionnaire that survey a wide range of behavioral and cognitive difficulties encountered in children from 1½ to 5 years.[15] The time of completion of CBCL and DSM forms for the SCD group was during the follow-up visit in pediatric clinic after the third admission. However, for the healthy group it was during the vaccination clinic visit. Disease-related SCD data were obtained through the available medical records under the supervision of the attending pediatric consultant including the frequency of hospital admissions.
Pain assessment and management
The most common cause of frequent admission of study children with SCD was pain which was due to vaso-occlusive crisis. The Toddler-Preschooler Postoperative Pain Scale (TPPPS) was used to assess pain in those young children during the admission and for follow up after pain medications. The children were managed with Ibuprofen 10 mg/kg per oral every 8 hours and/or morphine sulfate 0.05-0.1 mg/kg/dose IV every 2 hours until TPPPS became 2 or less. In addition, fluid maintenance according to the formula (4-2-1) was maintained and the children were kept normothermic by covering them with warming blankets and warming of fluids.
Psychiatric measures
A psychiatrist made clinical psychiatric assessments of the children during the last visit to the hospital. The psychiatric diagnosis was given according to a semi-structured interview based on the CBCL 1½-5, and DSM-IV-TR criteria for child and adolescent mental health.[16]
The CBCL 1½-5: It includes 99 items that describe specific kinds of behavioral, emotional, and social problems that characterize preschool children. There are also open-ended items for describing additional problems. The parents were asked to answer the items with a three-point scale: 0 for not true of the child; 1 for somewhat or sometimes true; and 2 for very true or often true. The CBCL behavioral/emotional portion is divided into two broadband dimensions: Internalizing and externalizing and those are divided in subscales. Items are scored on syndrome scales designated as emotionally reactive, anxious/depressed, somatic complaints, withdrawn, attention problems, aggressive behavior, and sleep problems. Open-ended items request information about illnesses and disabilities, what concerns the respondent most about the child, and the best things about the child. The measures’ internal consistency was found to be high and test-retest reliability was observed to be acceptable. High scores mean great behavioral disturbances.
Diagnostic and statistical (DSM)-oriented scale: It includes assessment for five problems: Affective problems, anxiety problems, pervasive developmental problems, attention deficit/hyperactivity problems, and oppositional defiant problems. As in the CBCL questionnaire, parents were asked to answer the items with a three-point scale: 0 for not true of the child; 1 for somewhat or sometimes true, and 2 for very true or often true. Also, high scores mean great suffering. The Arab version of the CBCL and DSM tests were given to Arab participants and the reliability and validity for Arab children was proven and standardized.
Statistical analysis
All data variables were encoded and computerized. Data entry and statistical analysis were performed using the Statistical Package for Social Science (SPSS) version 17.0 (SPSS Inc., Chicago, Illinois). Data was presented in the form of mean and standard deviation, number and percent. The chi-square test was used for categorical data, while the Student t-test was used for continuous data for comparison of two groups. The level of significance was set at P < 0.05.
RESULTS
Table 1 shows baseline characteristics of the two studied groups. There was no statistically significant difference between the two groups in age, gender, or residence. However, children with SCD have significantly lower mean weight than the control group (13.2 vs. 14.6; P = 0.02). About 95% of children with SCD were re-admitted because of vaso-occlusive crisis with an average TPPPS mean of 5.25 ± 1.2 and discharged with an average score of 1.2 ± 0.8. Other causes of SCD re-admission were recurrent fever and splenic sequestration.
Table 1.
Demographic characteristics of the study population
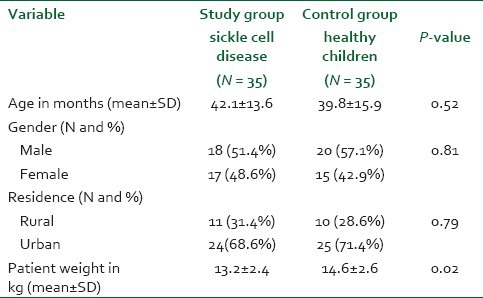
Table 2 shows the mean scores of children with SCD and control healthy group based on the results of CBCL. The mean score of emotional reactivity (62.9 vs. 53.4; P < 0.001) was significantly higher among the SCD group than the control healthy group. However, the mean score of attention problems shows no statistically significant difference between the two study groups. The SCD children have a statistically significant difference in behavioral changes on CBCL compared to the control group (healthy children); anxiety/depression (65.2 vs. 55.1, P < 0.001); somatic complaint (66.7 vs. 54.4, P < 0.001); withdrawn (63.4 vs. 53.2, P < 0.001); aggressive behavior (60.4 vs. 56; P = 0.04), internalizing (64.7 vs. 51.5; P < 0.001), and externalizing symptoms (56.5 vs. 50.7, P = 0.01), respectively. The mean score of sleep problems was higher in SCD children compared to the control group (60.2 vs. 56.3; P = 0.02), respectively [Table 2].
Table 2.
Dimensional CBCL 1½-5 empirically based scale for the study population (Mean ± SD)
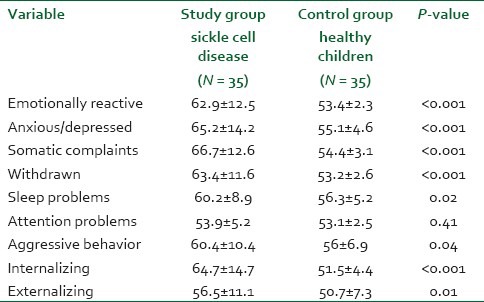
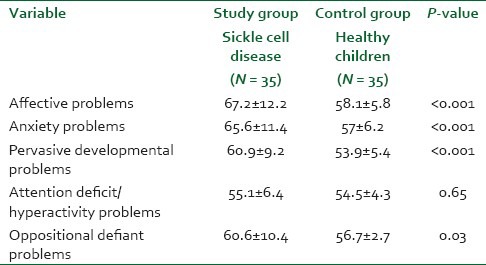
Table 3 shows the mean scores of children with SCD and control group based on the results of DSM-oriented scale. There was statistically significant difference between the study group and control group in means score of affective (67.2 vs. 58.1; P < 0.001), anxiety problems (65.6 vs. 57; P < 0.001), pervasive developmental problems (60.9 vs. 53.9, P < 0.001), and oppositional defiant problems (60.6 vs. 56.7; P = 0.03), respectively. However, the mean score of attention deficit hyperactivity problems shows no statistically significant difference between study and control groups.
Table 3.
Categorical-Diagnostic and statistical (DSM) oriented scale of the study population (Mean ± SD)
DISCUSSION
The present study highlighted the behavioral consequences of SCD on pre-school children who reported repeated hospitalization. It demonstrated that there was no significant difference between the SCD group and control group regarding demographic characteristics notably age, gender, residence but the mean patient weight was significantly lower in the SCD group than in the control group.
However; Iloeje reported that young boys with SCD had significantly higher mean scores of psychiatric morbidity than girls, and older children having higher scores than younger ones.[17] On the other hand, Asnani et al. recognized that utilization of comprehensive SCD services has been shown to be lower for individuals with the disease living in rural areas than for those living in urban areas.[18] However, the residence in our study was non-significant. In line with the present work, Hurtig and Park mentioned that children with SCD are often underweight compared to normal children.[19] In a study of a sample of children with SCD in Al-Ahsa city-Saudi Arabia, Amr et al. observed that male gender was an independent protective factor against psychiatric disorder in adolescents population.[20]
The present work revealed high emotional reactivity among children with SCD (62.9 ± 12.5) in comparison to the control group (53.4 ± 2.3) which is consistent with the results of Bakare et al. who found that emotional and behavioral disorders among Nigerian children with SCD were more prevalent than among group of healthy children.[10] Hijmans et al. explained that emotional and behavioral problems in children with SCD may be related to disease factors or to demographic factors.[8]
The rate of depression and anxiety among children with SCD in the present work was found to be higher than among the control group (65.2 ± 14.2 vs. 55.1 ± 4.6) which goes along with the study of Anie et al. who conceptualize that children with SCD reported high rate of depression and anxiety.[21] Also Amr et al.[20] stated that older children with SCD displayed high rate of depression and anxiety compared to their healthy peers. Levenson explained that depression and anxiety in children with SCD may result from living with a chronic stigmatizing disease, associated with chronic pain, unpredictable painful crises, and cognitive dysfunction primarily during childhood, while Hurtig and Park found that children with SCD encounter problems with self-esteem, dissatisfaction with body image, and social isolation.[3,19]
The present work showed that somatic complaints were manifested in higher rate among children with SCD (66.7 ± 12.6) than the control group (54.4 ± 3.1) similar to the results of Collin et al. who recognized that children with SCD are experiencing myriad medical problems.[9] In line with Hurtig and Park, our results revealed that children with SCD are usually withdrawn which may be interpreted by frequent hospitalization and chronic pain of children with SCD.[19] Our results implied that no significant difference between children with SCD and control group regarding attention problems, but contrary findings denote that children with SCD have significant deficits in neuro-cognitive functions compared to case control.[22] However, the most possible explanation was cultural and methodological differences. Hariman et al. mentioned that brain disease from SCD complication may begin early in life and children with SCD may experience a wide variety of neurologic syndromes, including cognitive difficulties, intellectual deficits (borderline to moderate mental retardation), and reduced language function.[23] Noll et al. explained that chronic hemolytic anemia in children with SCD can result in hypoxia because of the shortage of red blood cells to supply oxygen to the brain.[22]
Our findings revealed that children with SCD showed more aggressive behavior (60.4 ± 10.4) than the control group (56 ± 6.9). However, the reverse was observed by Noll et al. who mentioned that relative to comparison peers children with SCD described to be more prosocial and less aggressive.[24] This may be related to the age difference between the two study population as Noll et al. studied the children between the ages of 8 and 15 year old.[24]
The present study demonstrated that the mean score of internalizing symptoms was higher in children with SCD than the control group which is parallel to the results of Trazepecz et al. who hypothesized that children with SCD would have more internalizing (emotional) and social problems and fewer externalizing (behavioral) problems than comparison peers.[25] On the other hand, Simon et al. observed that internalizing symptoms among children with SCD and healthy siblings did not differ and were within non-clinical range.[26] Our study found that children with SCD scored higher in pervasive developmental problems (60.9 ± 9.2) than the control group (53.9 ± 5.4). On the other hand, Schatz hypothesized that SCD might decrease the risk or ameliorate Autism Spectrum Disorders severity and therefore the Autism Spectrum Disorders prevalence among children with SCD would be lower than expected. This was explained that SCD may provide postnatal physiologic stress that has a cellular protective effect during brain development.[27] In line with Iloeje, behavioral deviance was more common among children with SCD than the control group.[17]
We believe that this is the first research on the behavioral and psychological aspects of children with SCD at an early age of 1½ to 5 years in the eastern area of Saudi Arabia in which SCD is common.
The major limitation in this study was the small sample size not sufficient enough to give firm conclusions. In addition, data were collected retrospectively during interview with children's parents that may have possibility of recalling bias.
CONCLUSION
Children with SCD and who had history of repeated hospitalization are at an increased risk of developing behavioral problems. Psychological counseling, social support, and proper pain management could minimize these appalling consequences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.el-Hazmi MA. Heterogeneity and variation of clinical and haematological expression of haemoglobin S in Saudi Arabs. Acta Haematol. 1992;88:67–71. doi: 10.1159/000204654. [DOI] [PubMed] [Google Scholar]
- 2.Howard J, Thomas VJ, Rawle HM. Pain management and quality of life in sickle cell disease. Expert Rev Pharmacoecon Outcomes Res. 2009;9:347–52. doi: 10.1586/erp.09.32. [DOI] [PubMed] [Google Scholar]
- 3.Levenson JL. Psychiatric issues in adults with sickle cell disease. Prim Psychiatry. 2008;15:45–9. [Google Scholar]
- 4.Dumaplin CA. Avoiding admission for afebrile paediatric sickle cell pain: Pain management methods. J Pediatr Health Care. 2006;20:115–22. doi: 10.1016/j.pedhc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–45. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 6.Caldas JC, Pais-Ribeiro JL, Carneiro SR. General anesthesia, surgery and hospitalization in children and their effects upon cognitive, academic, emotional and sociobehavioral development - A review. Paediatr Anaesth. 2004;14:910–5. doi: 10.1111/j.1460-9592.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 7.Edwards CL, Scales MT, Loughlin C, Bennett GG, Harris-Peterson S, De Castro LM, et al. A brief review of the pathophysiology, associated pain, and psychosocial issues in sickle cell disease. Int J Behav Med. 2005;12:171–9. doi: 10.1207/s15327558ijbm1203_6. [DOI] [PubMed] [Google Scholar]
- 8.Hijmans CT, Grootenhuis MA, Oosterlaan J, Last BF, Heijboer H, Peters M, et al. Behavioral and emotional problems in children with sickle cell disease and healthy siblings: Multiple informants, multiple measures. Pediatr Blood Cancer. 2009;53:1277–83. doi: 10.1002/pbc.22257. [DOI] [PubMed] [Google Scholar]
- 9.Collins M, Kaslow N, Doepke K, Eckman J, Johnson M. Psychosocial interventions for children and adolescents with Sickle Cell Disease (SCD) J Black Psychol. 1998;24:432–54. [Google Scholar]
- 10.Bakare MO, Omigbodun OO, Kuteyi OB, Meremikwu MM, Agomoh AO. Psychological complications of childhood chronic physical illness in Nigerian children and their mothers: The implication for developing paediatric liaison services. Child Adolesc Psychiatry Ment Health. 2008;2:34. doi: 10.1186/1753-2000-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath PJ, Johnson G, Goodman JT, Schillinger J, Dunn J, Chapman J. CHEOPS: A Behavioral scale for rating postoperative pain in children. Adv Pain Res Ther. 1985;9:395–402. [Google Scholar]
- 12.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A Behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 13.Tarbell SE, Cohen IT, Marsh JL. The Toddler-Preschooler Postoperative Pain Scale: An observational scale for measuring postoperative pain in children aged 1-5.Preliminary report. Pain. 1992;50:273–80. doi: 10.1016/0304-3959(92)90031-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartrick CT, Kovan JP. Pain assessment following general anesthesia using the Toddler Preschooler Postoperative Pain Scale: A comparative study. J Clin Anesth. 2002;14:411–5. doi: 10.1016/s0952-8180(02)00389-6. [DOI] [PubMed] [Google Scholar]
- 15.Achenbach TM, Rescorla LA. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. Manual for the Achenbach System of Empirically Based Assessment (ASEBA): Preschool Forms and Profiles. [Google Scholar]
- 16.American Psychiatric Association. Text Revision. 4th ed. Washington, DC: American Psychiatric Press; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 17.Iloeje SO. Psychiatric morbidity among children with sickle-cell disease. Dev Med Child Neurol. 1991;33:1087–94. doi: 10.1111/j.1469-8749.1991.tb14831.x. [DOI] [PubMed] [Google Scholar]
- 18.Asnani MR, Reid ME, Ali SB, Lipps G, Williams-Green P. Quality of life in patients with sickle cell disease in Jamaica: Rural-urban differences. Rural Remote Health. 2008;8:890. [PubMed] [Google Scholar]
- 19.Hurtig AL, Park KB. Adjustment and coping in adolescents with sickle cell disease. Ann N Y Acad Sci. 1989;565:172–82. doi: 10.1111/j.1749-6632.1989.tb24164.x. [DOI] [PubMed] [Google Scholar]
- 20.Amr MA, Amin TT, Hablas HR. Psychiatric Disorders in a Sample of Saudi Arabian Adolescents with Sickle Cell Disease. Child Youth Care Forum. 2010;39:151–66. [Google Scholar]
- 21.Anie KA. Psychological complications in sickle cell disease. Br J Haematol. 2005;129:723–9. doi: 10.1111/j.1365-2141.2005.05500.x. [DOI] [PubMed] [Google Scholar]
- 22.Noll RB, Stith L, Gartstein MA, Ris MD, Grueneich R, Vannatta K, et al. Neuropsychological functioning of youths with sickle cell disease: Comparison with non-chronically ill peers. J Pediatr Psychol. 2001;26:69–78. doi: 10.1093/jpepsy/26.2.69. [DOI] [PubMed] [Google Scholar]
- 23.Hariman LM, Griffith ER, Hurtig AL, Keehn MT. Functional outcomes of children with sickle-cell disease affected by stroke. Arch Phys Med Rehabil. 1991;72:498–502. [PubMed] [Google Scholar]
- 24.Noll RB, Reiter-Purtill J, Vannatta K, Gerhardt CA, Short A. Peer relationships and emotional well-being of children with sickle cell disease: A controlled replication. Child Neuropsychol. 2007;13:173–87. doi: 10.1080/09297040500473706. [DOI] [PubMed] [Google Scholar]
- 25.Trzepacz AM, Vannatta K, Gerhardt CA, Ramey C, Noll RB. Emotional, social, and behavioral functioning of children with sickle cell disease and comparison peers. J Pediatr Hematol Oncol. 2004;26:642–8. doi: 10.1097/01.mph.0000139456.12036.8d. [DOI] [PubMed] [Google Scholar]
- 26.Simon K, Barakat LP, Patterson CA, Dampier C. Symptoms of depression and anxiety in adolescents with sickle cell disease: The role of intrapersonal characteristics and stress processing variables. Child Psychiatry Hum Dev. 2009;40:317–30. doi: 10.1007/s10578-009-0129-x. [DOI] [PubMed] [Google Scholar]
- 27.Schatz J. Brief report: Academic attainment in children with sickle cell disease. J Pediatr Psychol. 2004;29:627–33. doi: 10.1093/jpepsy/jsh065. [DOI] [PubMed] [Google Scholar]


