Abstract
Aims
Lysyl oxidase (LOX) expression is elevated in colorectal cancer (CRC) tissue and associated with disease progression. A blood test may form a more acceptable diagnostic test for CRC although LOX has not previously been measured in the serum. We therefore sought to determine the clinical usefulness of a serum LOX test for CRC in a symptomatic population.
Methods
Adult patients referred to a hospital colorectal clinic with bowel symptoms completed a questionnaire and provided a blood sample for serum LOX measurement. Associations between presenting symptoms, serum LOX concentrations and outcomes of investigations were tested by univariate and multivariate analyses to determine if serum LOX was clinically useful in the prediction of CRC. LOX expression in CRC and adjacent colon biopsies was evaluated by ELISA and immunohistochemistry.
Results
ThirtyJone cases of colorectal cancer and 16 high-risk polyps were identified from a total of 962 participants. There was no association between serum LOX concentration and the presence of CRC, high-risk polyps or cancers at any site. LOX expression was significantly increased in CRC tissue compared to adjacent colon.
Conclusion
Despite overexpression of LOX in CRC tissue, elevated serum levels could not be demonstrated. Serum LOX measurement is therefore not a clinically useful test for CRC.
Keywords: colorectal cancer, diagnosis, serum, lysyl oxidase
Introduction
There are over 40,000 new cases of colorectal cancer (CRC) diagnosed each year in the UK and the disease accounts for 10% of all cancer deaths [1]. Early diagnosis is associated with improved survival rates and the NHS Bowel Cancer Screening Programme aims to identify early stage disease. Screening, in the form of faecal occult blood tests on a 2-yearly basis from the age of 60 years, has been shown to reduce the incidence of colorectal cancer [2]. Other screening tests are in the process of being introduced following clinical trials of their effectiveness [3]. Approximately half of patients decline screening. Research has found that barriers to screening uptake include fear of the outcome and lack of time, and crucially, many patients may decline screening due to the nature of the test, which requires patients to come into close contact with faecal matter [4]. There is therefore an urgent need for alternative procedures, such as a blood test, to detect tumour markers that could be used to diagnose bowel cancer at an early stage.
The majority of bowel cancer diagnoses are made following presentation with bowel symptoms to primary care [5]. Only a minority of patients referred to secondary care with bowel symptoms are subsequently diagnosed with bowel cancer [6]. A blood test that could reliably exclude bowel cancer would therefore reduce the morbidity and mortality associated with performing colonoscopy in a healthy population, and might be more acceptable to patients than a stool test.
Lysyl oxidase (LOX) is an extra-cellular matrix-modifiying enzyme that has been linked to CRC cell proliferation, metastasis and angiogenesis [7,8]. High expression of LOX correlated with poor prognosis in a variety of different solid tumours [9] and LOX-inhibition reduces metastasis in mice. LOX is a copper-dependent amine oxidase that oxidises lysine residues in collagen and elastin resulting in cross-linking of these fibres, and this modulation of the extra-cellular matrix is thought to play an important role in the process of metastasis [10]. LOX has been demonstrated in the circulation by way of serum enzyme activity measurement [11]. However, no published studies to date have directly measured serum LOX protein concentration.
Given the overexpression of LOX in CRC tissue, an evaluation of serum LOX levels as a potential diagnostic marker is warranted. This study measured serum LOX levels in a large cohort of patients who had been referred to secondary care with bowel symptoms to determine whether serum LOX levels, either alone, or in combination with bowel symptoms and patient factors, could predict the incidence of CRC. Tissue LOX levels in biopsies from CRC and matched adjacent colon were also evaluated by ELISA and immunohistochemistry.
Patients and methods
Patients and samples
We had access to stored serum samples from a historical cohort of adults who had been referred to the colorectal clinic at the University Hospital Birmingham NHS Foundation Trust. All patients had been recruited into a previous study evaluating serum MMP-9 levels in colorectal cancer, the details of which have been reported in full elsewhere [12,13]. Patients had given consent for serum samples to be stored and used in the future for the purpose of evaluation of diagnostic tests and new potential biomarkers. Patients were aged 18 or over and had completed a questionnaire detailing bowel symptoms, recent injuries and chronic illnesses experienced in the 3 months prior to questionnaire completion, and personal or family history of bowel cancer. Following the completion of this questionnaire, patients were seen in the clinic and a blood sample taken. All details of clinical examination and investigations together with their outcomes were recorded.
Justification of sample size
The sample size calculation for the MMP9 study was based on the precision with which the sensitivity of MMP9 levels for predicting the presence of colorectal cancer could be estimated. Pilot work had shown MMP9 to have a sensitivity of 99%, thus 60 cases of colorectal cancer were required within the study population to estimate this sensitivity with 95% confidence. A sample size of 1000 patients was therefore chosen, assuming a 6% prevalence of colorectal cancer in the population. In our study, serum LOX sensitivity was unknown, but a conservative assumption that serum LOX sensitivity may be as low as 0.5 was made. Therefore, 32 cases of colorectal cancer would be sufficient to estimate a serum LOX sensitivity of 75% at 90% confidence.
Serum LOX measurement
Blood samples were taken into a red-topped Vacuette tube (Greiner Bio-One Ltd., Gloucester, UK) with no additive and kept on ice until the end of the colorectal clinic. The samples were transported on ice to the laboratory where they were centrifuged and the serum fraction separated and stored at −80°C until further use. Serum samples were diluted with an equal volume of phosphate-buffered saline and duplicate diluted samples were assayed for serum LOX concentration using an ELISA kit (USCN Life Science Inc., China). ELISA assays were conducted at the same time by 3 individuals who were blinded to all patient and sample details. Linearity of the assay has been demonstrated over a concentration of range of 0.156–10 ng/ml with a sensitivity of 0.053 ng/ml.
Tissue LOX measurement
Tissue samples were obtained from patients undergoing resection for colorectal cancer having given informed consent at the University Hospital Birmingham NHS Foundation Trust, Birmingham, UK. The study was approved by the Local Research & Ethics Committee (South Birmingham 2003/242, renewed 2012). Fresh pieces of tumour tissue and strips of colonic tissue, a minimal distance of 10 cm from the tumour, were taken with the help of an experienced pathologist and snap frozen in liquid nitrogen. Protein was extracted from snap-frozen tissue by incubation in ice-cold lysis buffer (CellLytic MT, 20 μL/mg tissue, Sigma-Aldrich, UK) containing a proteinase inhibitor cocktail (Sigma-Aldrich UK) and 5 U/mL DNase I (Roche Diagnostics, UK). The tissue was mechanically dissociated in the buffer solution using the GentleMACS tissue dissociator (Miltenyi Biotech, UK). Samples were pelleted and the supernatant collected. Protein concentrations were determined against a bovine serum albumin standard using a bicinchoninic acid assay and sample protein concentrations adjusted to 2 mg/mL by dilution in cell lysis buffer. Protein samples were diluted 1 in 10 parts phosphate-buffered saline and tissue LOX concentration was then measured using the LOX ELISA kit as above.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues were deparaffinised and rehydrated by passing the sections through fresh solutions of Clearene (Leica Biosystems, UK) and graduated alcohols. Sections for immunohistochemistry were incubated with 0.3% hydrogen peroxide solution in methanol and all sections underwent antigen retrieval by microwaving in pre-heated EDTA buffer for 15 minutes. Sections were blocked with 10% casein solution for 30 minutes and then incubated with either anti-LOX antibody (sc-66947, 1 in 50 dilution, Santa Cruz Biotechnology, USA) or an isotype control antibody at equal concentration (Dako, UK) for 1 hour at room temperature. The sections were then washed twice in TBS containing 0.1% Tween-20 and incubated with an HRP-conjugated development system (Vector ImmPress, Vector Laboratories, USA). Following a final wash with TBS/0.1% Tween-20, immunoreactive cells were visualised using 3,3′-diaminobenzidine (Vector) and counter-stained with Meyer’s haematoxylin (Sigma-Aldrich, UK) before mounting in DPX (Leica Biosystems, UK). Tissue expression was visualised using a Leica DM6000 (chromogenic) microscope and the manufacturer’s software.
Data analysis
Based on patients’ ‘final outcome’ diagnosis, patients were classified into two groups: a ‘CRC group’ consisting of confirmed cases of colorectal cancer and a ‘non-CRC group’ consisting of polyps, cancer at other sites, benign inflammatory disease of the colon/rectum, other benign conditions of the colon/rectum, other benign conditions or no abnormality. Patients were also classified into a ‘neoplasia’ group consisting of cases of confirmed colorectal cancer together with polyps with moderate dysplasia (high-risk polyps), and a ‘non-neoplasia’ group consisting of low-risk polyps, cancer at other sites, benign inflammatory disease of the colon/rectum, other benign conditions of the colon/rectum, other benign conditions or no abnormality.
Continuous variables, including serum LOX levels, were compared across the ‘CRC’ versus ‘no-CRC’ groups using the non-parametric Mann-Whitney U-test. Categorical variables, such as the presence or absence of particular symptoms, were compared using the Chi-squared test.
Binary logistic regression was performed using variables which had been shown to be associated with CRC and/or neoplasia by univariate analysis to determine the best combination of factors to predict CRC. In the first model, serum LOX level as a categorical variable was forcibly retained even though there was no association with CRC by univariate analysis. In the second model, only factors shown to be associated with CRC by univariate analysis were entered into the model.
Differences in tissue LOX levels were compared using the Mann-Whitney U-test. All P-values <0.05 were considered statistically significant.
Results
Of 1002 participants from the original MMP-9 study [13], 962 had given consent for future use of stored serum samples and formed the final number of participants for this study. The median age of participants was 57 years (IQR: 44-68). 48.5% were male and the majority (89.7%) were from a white ethnic group. The median body-mass index (BMI) was 26 (IQR: 23-29). Approximately one-third (34%) of participants had been referred urgently to the colorectal clinic.
Reported symptoms, medical conditions and the outcomes of investigations are presented in table 1. There were 31 cases of CRC and 16 cases of high risk polyps.
Table 1. Patient clinical characteristics.
| Characteristic | Yes (%) | No (%) | Median symptom duration (IQR) |
|---|---|---|---|
|
| |||
| Patient reported symptoms, duration and median duration in weeks | |||
|
| |||
| Abdominal pain | 414 (43.0) | 548 (57.0) | 12 (4-14+) |
| Weight loss | 138 (14.3) | 824 (85.7) | 14 (6-14+) |
| Tiredness | 485 (50.4) | 477 (49.6) | 14 (8-14+) |
| Blood in stools | 329 (34.2) | 633 (65.8) | 8(2-14+) |
| Rectal bleeding | 467 (48.5) | 495 (51.5) | 12 (3-14+) |
| Bowel habit - harder stools | 320 (33.3) | 642 (66.7) | 12 (6-14+) |
| Bowel habit - softer stools | 483 (50.2) | 479 (49.8) | 12 (5-14+) |
| Opening bowels less frequently | 218 (22.7) | 744 (77.3) | 12 (6-14+) |
| Opening bowels more frequently | 431 (44.8) | 531 (55.2) | 12 (6-14+) |
| Anal pain | 522 (54.3) | 440 (45.7) | 14 (8-14+) |
|
| |||
| Medical conditions (self-reported) | |||
|
| |||
| Angina | 113 (11.7) | 849 (88.3) | |
| Hypertension | 284 (29.5) | 678 (70.5) | |
| Diabetes | 71 (7.4) | 891 (92.6) | |
| Asthma | 162 (16.8) | 800 (83.2) | |
| Inflammatory Bowel Disease (IBD) | 47 (4.9) | 915 (95.1) | |
| Arthritis | 308 (32.0) | 654 (68.0) | |
| Muscular problems | 141 (14.7) | 821 (85.3) | |
| Epilepsy | 20 (2.1) | 942 (97.9) | |
| Other condition | 360 (37.4) | 602 (62.6) | |
| Injury | 85 (8.8) | 877 (91.2) | |
| Previous bowel polyps | 87 (9.0) | 875 (91.0) | |
| Previous bowel cancer | 17 (1.8) | 945 (98.2) | |
| Bowel cancer family history | 226 (23.5) | 736 (76.5) | |
|
| |||
| Blood test results | Number (%) | Median | Range (IQR) |
|
| |||
| Serum LOX (ng/mL) | 959 (99.7) | 4.92 | 1.70-39.73 ( 3.70-7.68) |
|
| |||
| Outcome of clinical investigations | Number (%) | ||
|
| |||
| Adenocarcinoma of colon/rectum | 31 (3.2) | ||
| High risk polyp | 16 (1.7) | ||
|
| |||
| Total ‘neoplasia’ group | 47 (4.9) | ||
|
| |||
| Cancer at site other than colon/rectum | 22 (2.3) | ||
| Low-risk polyp | 49 (5.1) | ||
| Intraepithelial neoplasia at other site | 2 (0.2) | ||
| Inflammatory benign condition of colon/rectum | 45 (4.7) | ||
| Inflammatory benign condition at other site | 11 (1.1) | ||
| Other benign condition of colon/rectum | 257 (26.7) | ||
| Other benign condition at other site | 311 (32.3) | ||
| No abnormality | 218(22.7) | ||
Abbreviations: IQR = interquartile range; LOX = lysyl oxidase
Serum LOX concentrations
The median serum LOX concentration was 5.11 ng/ml (IQR: 3.61-9.28) in the ‘CRC’ group and 4.91 ng/ml (IQR: 3.71-7.67) in the ‘non-CRC’ group, although this difference did not reach statistical significance (P=0.689). Similarly, the median serum LOX concentration was 5.11 ng/ml (IQR: 3.72-8.32) in the ‘neoplasia’ group and 4.90 ng/ml (IQR: 3.70-7.67) in the ‘non-neoplasia’ group, P=0.712. Serum LOX concentrations were compared against demographic, clinical and outcome data (see table 2). Higher serum LOX concentrations were significantly associated with a white ethnic group, diabetes and the poorly-defined group of patients with documented other medical conditions.
Table 2. Factors associated with serum LOX concentration.
| Median serum LOX concentration, ng/mL (IQR) |
|||
|---|---|---|---|
| Characteristic | Yes | No | P-value |
|
| |||
| Patient reported symptoms and demographics | |||
|
| |||
| Age, >57years | 4.81 (3.78-7.72) | 4.97 (3.57-7.67) | 0.588 |
| BMI, >26 | 5.00 (3.79-7.90) | 4.79 (3.64-7.69) | 0.288 |
| Gender, male | 4.99 (3.79-7.67) | 4.76 (3.61-7.69) | 0.317 |
| Ethnic group, white | 4.95 (3.75-7.71) | 4.25 (3.24-6.60) | 0.022 |
| Pre-menopausal (women only) | 4.61 (3.55-7.30) | 4.88 (3.66-7.78) | 0.305 |
| Abdominal pains | 4.96 (3.81-7.26) | 4.85 (3.62-8.02) | 0.779 |
| Weight loss | 4.93 (3.81-7.71) | 4.92 (3.67-7.67) | 0.475 |
| Tiredness | 5.04 (3.81-7.87) | 4.75 (3.56-7.51) | 0.078 |
| Blood in stools | 4.95 (3.70-7.15) | 4.86 (3.70-7.92) | 0.805 |
| Bleeding from rectum | 4.93 (3.62-7.17) | 4.86 (3.78-7.92) | 0.340 |
| Bowel habit - harder stools | 4.92 (3.65-8.07) | 4.92 (3.74-7.49) | 0.773 |
| Bowel habit - looser stools | 4.91 (3.81-7.44) | 4.92 (3.60-8.05) | 0.793 |
| Opening bowels less frequently | 4.93 (3.59-7.51) | 4.90 (3.74-7.71) | 0.723 |
| Opening bowels more frequently | 4.95 (3.85-7.50) | 4.86 (3.59-7.86) | 0.450 |
| Anal pain | 4.91 (3.63-7.14) | 4.92 (3.79-8.09) | 0.285 |
|
| |||
| Medical conditions (self-reported) | |||
|
| |||
| Angina | 5.00 (3.92-8.45) | 4.90 (3.64-7.65) | 0.192 |
| Hypertension | 5.04 (3.86-7.47) | 4.81 (3.62-7.71) | 0.114 |
| Diabetes | 5.84 (3.81-8.59) | 4.86 (3.68-7.49) | 0.046 |
| Asthma | 5.07 (3.78-7.44) | 4.85 (3.66-7.71) | 0.666 |
| IBD | 4.51 (3.29-8.95) | 4.92 (3.72-7.60) | 0.905 |
| Arthritis | 4.99 (3.77-8.07) | 4.86 (3.68-7.51) | 0.386 |
| Muscular problems | 5.06 (3.54-6.78) | 4.86 (3.72-7.71) | 0.722 |
| Epilepsy | 4.42 (4.08-6.39) | 4.93 (3.70-7.69) | 0.960 |
| Other condition | 5.12 (3.81-8.14) | 4.77 (3.62-7.39) | 0.037 |
| Injury | 5.00 (3.71-7.7) | 4.90 (3.70-7.67) | 0.812 |
| Previous bowel polyps | 5.00 (3.75-7.35) | 4.91 (3.69-7.70) | 0.673 |
| Previous bowel cancer | 3.93 (3.53-7.18) | 4.93 (3.72-7.68) | 0.425 |
| Bowel cancer family history | 4.70 (3.48-7.45) | 4.95 (3.77-7.70) | 0.170 |
|
| |||
| Outcome of clinical investigations | |||
|
| |||
| Confirmed colorectal cancer | 5.11 (3.61-9.28) | 4.91 (3.71-7.67) | 0.689 |
| Neoplasia | 5.11 (3.72-8.32) | 4.90 (3.7-7.67) | 0.712 |
| Cancer (colorectal or other site) | 5.00 (3.63-8.44) | 4.91 (3.7-7.66) | 0.845 |
Abbreviations: IQR = interquartile range; BMI = body mass index; IBD = inflammatory bowel disease; LOX = lysyl oxidase
Serum LOX concentrations and factors predictive of CRC
Factors associated with CRC were increasing age, the absence of anal pain and a history of previous bowel polyps or bowel cancer (see table 3). This univariate analysis was repeated for ‘neoplasia’ and ‘non-neoplasia’ groups (see supplementary tables). In addition to the above factors, patient gender, the presence of blood in the stools and a history of diabetes were significantly associated with neoplasia. Factors associated with CRC and/or neoplasia were entered into a binary logistic regression model together with serum LOX concentration. Serum LOX concentrations, expressed either as quartiles, or absolute values (absolute values not shown), did not show any association with CRC (see table 4) or neoplasia (see supplementary tables) in this model.
Table 3. Univariate analysis of factors predictive of colorectal cancer.
| CRC, | Non-CRC, | |||||
|---|---|---|---|---|---|---|
| Continuous variables | Number | median (IQR) | median (IQR) | P-value | ||
|
| ||||||
| Age | 962 | 68 (58-76) | 57 (44-68) | <0.001 | ||
| BMI | 745 | 26 (24-27) | 26 (23-30) | 0.194 | ||
|
| ||||||
| Categorical variables | Category | Number | n (Row %) | n (Row %) | OR | P-value |
|
| ||||||
| Gender | Male | 467 | 20 (4.3) | 447 (95.7) | 1.95 (0.94-4.30) | 0.074 |
| Female | 495 | 11 (2.2) | 484 (97.8) | |||
|
| ||||||
| Ethnic group | White | 863 | 28 (3.2) | 835 (96.8) | 0.96 (0.33-4.24) | 0.951 |
| Non-white | 93 | 3 (3.2) | 90 (96.8) | |||
|
| ||||||
| Referral type | Urgent | 328 | 13 (4) | 315 (96) | 2.09 (0.90-5.00) | 0.086 |
| Routine | 519 | 10 (1.9) | 509 (98.1) | |||
|
| ||||||
| Pre-menopausal (women only) | Yes | 154 | 154 (100) | 0 (0) | OR could not be calculated | |
| No | 340 | 329 (96.7) | 11 (3.3) | |||
|
| ||||||
| Abdominal pains | Yes | 414 | 11 (2.7) | 403 (97.3) | 0.73 (0.33-1.51) | 0.398 |
| No | 548 | 20 (3.6) | 528 (96.4) | |||
|
| ||||||
| Weight loss | Yes | 138 | 7 (5.1) | 131 (94.9) | 1.81 (0.70-4.10) | 0.206 |
| No | 824 | 24 (2.9) | 800 (97.1) | |||
|
| ||||||
| Tiredness | Yes | 477 | 20 (4.2) | 457 (95.8) | 0.53 (0.24-1.11) | 0.095 |
| No | 485 | 11 (2.3) | 474 (97.7) | |||
|
| ||||||
| Blood in stools | Yes | 329 | 14 (4.3) | 315 (95.7) | 1.61 (0.77-3.33) | 0.202 |
| No | 633 | 17 (2.7) | 616 (97.3) | |||
|
| ||||||
| Bleeding from rectum | Yes | 467 | 12 (2.6) | 455 (97.4) | 0.66 (0.31-1.38) | 0.273 |
| No | 495 | 19 (3.8) | 476 (96.2) | |||
|
| ||||||
| Bowel habit – harder stools | Yes | 320 | 8 (2.5) | 312 (97.5) | 0.70 (0.29-1.53) | 0.382 |
| No | 642 | 23 (3.6) | 619 (96.4) | |||
|
| ||||||
| Bowel habit - looser stools | Yes | 483 | 13 (2.7) | 470 (97.3) | 0.71 (0.34-1.47) | 0.357 |
| No | 479 | 18 (3.8) | 461 (96.2) | |||
|
| ||||||
| Opening bowels less frequently | Yes | 218 | 5 (2.3) | 213 (97.7) | 0.66 (0.22-1.62) | 0.393 |
| No | 744 | 26 (3.5) | 718 (96.5) | |||
|
| ||||||
| Opening bowels more frequently | Yes | 431 | 13 (3) | 418 (97) | 0.89 (0.42-1.84) | 0.753 |
| No | 531 | 18 (3.4) | 513 (96.6) | |||
|
| ||||||
| Anal pain | Yes | 522 | 9 (1.7) | 513 (98.3) | 0.34 (0.14-0.72) | 0.005 |
| No | 440 | 22 (5) | 418 (95) | |||
|
| ||||||
| Angina | Yes | 113 | 2 (1.8) | 111 (98.2) | 0.55 (0.08-1.85) | 0.376 |
| No | 849 | 29 (3.4) | 820 (96.6) | |||
|
| ||||||
| Hypertenson | Yes | 284 | 10 (3.5) | 274 (96.5) | 1.15 (0.51-2.43) | 0.723 |
| No | 678 | 21 (3.1) | 657 (96.9) | |||
|
| ||||||
| Diabetes | Yes | 71 | 5 (7) | 66 (93) | 2.58 (0.83-6.45) | 0.093 |
| No | 891 | 26 (2.9) | 865 (97.1) | |||
|
| ||||||
| Asthma | Yes | 162 | 2 (1.2) | 160 (98.8) | 0.36 (0.05-1.20) | 0.106 |
| No | 800 | 29 (3.6) | 771 (96.4) | |||
|
| ||||||
| IBD | Yes | 47 | 1 (2.1) | 46 (97.9) | 0.73 (0.03-3.50) | 0.752 |
| No | 915 | 30 (3.3) | 885 (96.7) | |||
|
| ||||||
| Arthritis | Yes | 308 | 6 (1.9) | 302 (98.1) | 0.51 (0.19-1.19) | 0.123 |
| No | 654 | 25 (3.8) | 629 (96.2) | |||
|
| ||||||
| Muscular problems | Yes | 141 | 2 (1.4) | 139 (98.6) | 0.42 (0.06-1.42) | 0.187 |
| No | 821 | 29 (3.5) | 792 (96.5) | |||
|
| ||||||
| Epilepsy | Yes | 20 | 0 (0) | 20 (100) | OR could not be calculated | |
| No | 942 | 31 (3.3) | 911 (96.7) | |||
|
| ||||||
| Other condition | Yes | 360 | 11 (3.1) | 349 (96.9) | 0.92 (0.42-1.93) | 0.835 |
| No | 602 | 20 (3.3) | 582 (96.7) | |||
|
| ||||||
| Injury | Yes | 85 | 2 (2.4) | 83 (97.6) | 0.75 (0.11-2.57) | 0.696 |
| No | 877 | 29 (3.3) | 848 (96.7) | |||
|
| ||||||
| Previous bowel polyps | Yes | 87 | 8 (9.2) | 79 (90.8) | 3.79 (1.53-8.48) | 0.006 |
| No | 875 | 23 (2.6) | 852 (97.4) | |||
|
| ||||||
| Previous bowel cancer | Yes | 17 | 5 (29.4) | 12 (70.6) | 14.82 (4.34-43.91) | <0.001 |
| No | 945 | 26 (2.8) | 919 (97.2) | |||
|
| ||||||
| Bowel cancer family history | Yes | 226 | 3 (1.3) | 223 (98.7) | 0.36 (0.08-1.02) | 0.056 |
| No | 736 | 28 (3.8) | 708 (96.2) | |||
|
| ||||||
| Serum LOX, quartiles | 1 (lower) | 241 | 9 (3.7) | 232 (96.3) | Reference | |
| 2 | 240 | 5 (2.1) | 235 (97.9) | 0.54 (0.17-1.61) | 0.288 | |
| 3 | 238 | 7 (2.9) | 231 (97.1) | 0.78 (0.27-2.13) | 0.630 | |
| 4 (higher) | 240 | 10 (4.2) | 230 (95.8) | 1.12 (0.44-2.87) | 0.808 | |
Abbreviations: IQR = interquartile range; OR = odds ratio; BMI = body mass index; IBD = inflammatory 1 oxidase
Table 4.
Binary logistic regression models for probability of colorectal cancer
| Variable | ß | P-value | Odds ratio ( 95% CI) |
|---|---|---|---|
|
| |||
| Intercept | −6.23 | <0.001 | |
|
| |||
| Age | 0.038 | 0.015 | 1.04 (1.01-1.07) |
|
| |||
| Gender | 0.684 | 0.134 | 1.98 (0.83-5.08) |
|
| |||
| Mode of referral | 0.271 | 0.551 | 1.31 (0.54-3.27) |
|
| |||
| Blood in stools | 0.977 | 0.026 | 2.65 (1.12-6.35) |
|
| |||
| Anal pain | −1.055 | 0.034 | 0.34 (0.12-0.88) |
|
| |||
| Diabetes | 0.273 | 0.681 | 1.31 (0.29-4.27) |
|
| |||
| Previous bowel polyps | −0.016 | 0.984 | 0.98 (0.15-3.79) |
|
| |||
| Previous bowel cancer | 0.844 | 0.468 | 2.33 ( 0.11-16.9) |
|
| |||
| LOX quartile | |||
|
| |||
| 2 | −0.458 | 0.465 | 0.63 (0.18-2.20) |
| 3 | −0.511 | 0.423 | 0.60 ( 0.16-2.10) |
| 4 | −0.091 | 0.876 | 0.91 (0.29-2.98) |
| LOX not included in model | |||
|---|---|---|---|
|
| |||
| Variable | ß | P-value | Odds ratio ( 95% CI) |
|
| |||
| Intercept | −6.372 | <0.001 | |
|
| |||
| Age | 0.037 | 0.017 | 1.04 (1.01-1.07) |
|
| |||
| Gender | 0.645 | 0.154 | 1.91 (0.80-4.86) |
|
| |||
| Mode of referral | 0.244 | 0.591 | 1.28 (0.52-3.17) |
|
| |||
| Blood in stools | 0.956 | 0.028 | 2.60 (1.10-6.21) |
|
| |||
| Anal pain | −1.052 | 0.035 | 0.50 (2.23-1.05) |
|
| |||
| Diabetes | 0.321 | 0.625 | 1.38 (0.31-4.40) |
|
| |||
| Previous bowel polyps | −0.076 | 0.923 | 0.93 (0.14-3.51) |
|
| |||
| Previous bowel cancer | 0.923 | 0.422 | 2.52 (0.13-18.01) |
Abbreviations: CI = confidence interval; LOX = lysyl oxidase
Tissue LOX concentration
Protein lysates at a concentration of 2mg of total protein per ml from 5 samples of matched CRC tissue and adjacent colon were analysed for LOX concentration using the same ELISA as described for the serum samples. The median colonic tissue LOX concentration was 50.62 ng/mL (IQR: 44.31-74.68) while the median CRC concentration was 281.65 ng/mL (IQR: 219.26-308.73), a difference that was statistically significant (P<0.01).
LOX immunostaining
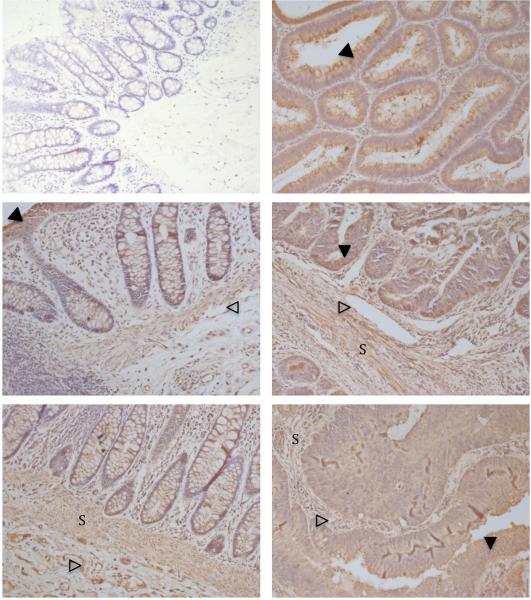
Sections from 8 different CRC samples and one bowel polyp, all with matched colon tissue were stained for LOX by immunohistochemistry. Representative images are shown in Figure 1. LOX staining is evident not just in the cancer epithelium, but also in the epithelium from polyp tissue and colon. LOX staining is also seen in the endothelium and in cells sited in the stroma of all tissues.
Figure 1. LOX immunohistochemistry.
Isotype-matched control (top-left), polyp (top-right), two matched colon and CRC samples (middle, bottom). ×200 magnification. Epithelial staining (filled arrows), endothelial staining open arrows) and stromal staining (‘S’).
Discussion
LOX cross-links collagen and elastin to increase the stiffness of the extra-cellular matrix. Increased stiffness, driven by LOX, has been measured in CRC tumour models and shown to play a role in metastasis [14] leading the authors to suggest a therapeutic potential for LOX inhibition in CRC. Tumour markers based on tumour cell phenotype are dependent on marker expression by a clone of tumour cells with the necessary underlying genetic alterations, acquired as part of the natural history of cancer progression [15]. The existence of a tumour marker, derived from a common process such as extra-cellular matrix remodelling, is attractive as it may be common to all cancer cell genotypes.
This study investigated whether serum LOX measurement, either alone or in combination with symptoms and other patient factors, could predict the presence of colorectal cancer or high-risk polyps. Serum samples were taken from symptomatic patients referred from primary care to a hospital colorectal clinic. No association was found between serum LOX concentration and the presence of colorectal cancer, high-risk polyps or cancers at any site. Serum LOX concentrations, both as a continuous variable and as an ordinal variable derived from LOX concentration quartiles, were analyzed separately in a binary logistic regression model to determine the potential use of serum LOX measurement in combination with other predictive factors. Serum LOX concentrations did not demonstrate any significant association with the presence of cancer or neoplasia in this multivariate model (table 4). Patient age and the symptoms of blood in the stools and absence of anal pain were independently associated with the presence of colorectal neoplasia, as published previously [13].
The serum samples used in this study had been stored for 6-7 years at −80°C. Although tumour marker studies frequently employ the use of stored samples [16], it has been demonstrated that protein concentrations can vary between fresh and stored serum samples with time [17,18]. The stability of LOX in stored frozen serum samples has not been investigated and therefore deterioration in LOX could have a bearing on our results. Despite this, all samples were stored for a similar period of time.
Recent studies have shown that LOX plays an important role in CRC metastasis: in a mouse CRC model, LOX-overexpressing cell lines displayed enhanced proliferation and invasion while the opposite effects were seen using cell lines in which LOX had been silenced [8]. The same study reported enhanced LOX expression by immunohistochemistry in CRC tissue compared to adjacent colon, with a positive association between LOX expression and disease stage. Our data demonstrated increased LOX expression in CRC tissue compared to adjacent colon by ELISA and is therefore in keeping with the findings from this study. Immunostaining revealed greater LOX expression in CRC compared to adjacent colon, however LOX expression appeared to be fairly ubiquitously expressed by epithelium, endothelium and stromal cells throughout the colon (Figure 1). Such high background expression may help to explain why serum LOX concentrations were not significantly raised in patients with colorectal neoplasia despite increased expression in the CRC tissue. Another possible explanation is that active LOX, cleaved from the pro-enzyme in the extra-cellular environment, is able to re-enter cells, preventing a rise in serum levels [19].
This study is the first to report the direct measurement of serum LOX protein concentrations. We have shown an association between serum LOX concentration and diabetes. Although this has not been reported previously, others have shown an association between serum LOX enzyme activity and diabetes [20]. It has long been known that there are widespread changes in collagen structure due to increased cross-linking of collagen fibres in patients with diabetes. This is partly explained by non-enzymatic glycosylation of lysine and hydroxylysine residues, but increased levels of lysyl oxidaseJdependent crossJlinking have also been demonstrated in the skin of diabetic individuals [21]. The mechanism for increased LOX activity in diabetic skin was not investigated, although a more recent study has shown that endothelial cells are able to upregulate LOX when cultured in high glucose conditions [22]. Elevated serum LOX enzyme activity has also been demonstrated in liver diseases such as active and chronic hepatitis, alcoholic liver disease and primary biliary cirrhosis [11,23]. Unfortunately, the absence of information concerning the presence or absence of liver disease in our cohort precluded the testing for any association between liver disease and serum LOX concentrations. LOX has been shown to have a complex relationship with atherosclerosis. High LOX levels are associated with early endothelial dysfunction, while lower levels are associated with neointimal thickening and stenosis [24]. The cholesterolJlowering class of drugs, statins, have been shown to increase LOX expression by human endothelium [25]. It was not possible to determine any association between statin use and serum LOX concentration in our study as patient drug history was not recorded. LOX belongs to a family of molecules, of which there are 5 members (LOX, LOXL1, LOXL2, LOXL3, LOXL4). They have a highlyJconserved C-terminal end and are all presumed to have amine oxidase activity [9]. Aside from LOX, LOXL2 is the best characterised and has been shown to be upregulated in a variety of solid tumours, including CRC [26]. It is therefore possible that serum LOXL2 concentration may be significantly elevated in patients with CRC and account for the increase in lysyl oxidase-dependent catalytic activity described previously.
In conclusion, in this large study involving symptomatic patients referred to a colorectal clinic we have not detected any significant association between serum LOX concentration and colorectal cancer. This is despite previous reports that LOX is upregulated in CRC tissue and our corroborative findings of increased tissue LOX in CRC compared to adjacent colon by ELISA. Serum LOX was increased with diabetes and the concentration may be elevated in a number of other conditions, suggesting that serum LOX levels would exhibit poor specificity as a tumour marker.
Supplementary Material
Acknowledgements
The lead author received an educational grant to support this study from the Shire Innovation Fund for SpRs, which is a pharmaceutical company. The authors would like to acknowledge Roger Holder and Linda Nichols who provided the framework for the statistical analysis, Sue Wilson who was the chief investigator of the original MMP-9 study [13] and Jonathon James who handled, processed and stored the blood/serum samples.
Role of funding source
The study sponsors had no role in the design of the study, in the collection, analysis and interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest: none
References
- 1.Office for National Statistics (ONS) Mortality Statistics: Deaths registered in 2010, England and Wales. 2011 http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2010/stb-deaths-by-cause-2010.html.
- 2.McClements PL, Madurasinghe V, Thomson CS, et al. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol. 2012;36:e232–242. doi: 10.1016/j.canep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Hawkes N. New screening tests for bowel and cervical cancer are to be piloted in NHS. BMJ. 2012;345:e8455. doi: 10.1136/bmj.e8455. [DOI] [PubMed] [Google Scholar]
- 4.Chapple A, Ziebland S, Hewitson P, et al. What affects the uptake of screening for bowel cancer using a faecal occult blood test (FOBt): a qualitative study. Soc Sci Med 1982. 2008;66:2425–35. doi: 10.1016/j.socscimed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Goodyear SJ, Stallard N, Gaunt A, et al. Local impact of the English arm of the UK Bowel Cancer Screening Pilot study. Br J Surg. 2008;95:1172–9. doi: 10.1002/bjs.6230. [DOI] [PubMed] [Google Scholar]
- 6.Thorne K, Hutchings HA, Elwyn G. The effects of the Two-Week Rule on NHS colorectal cancer diagnostic services: a systematic literature review. BMC Health Serv Res. 2006;6:43. doi: 10.1186/1472-6963-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker A-M, Bird D, Welti JC, et al. Lysyl Oxidase Plays a Critical Role in Endothelial Cell Stimulation to Drive Tumor Angiogenesis. Cancer Res. 2013;73:583–94. doi: 10.1158/0008-5472.CAN-12-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker A-M, Cox TR, Bird D, et al. The Role of Lysyl Oxidase in SRC-Dependent Proliferation and Metastasis of Colorectal Cancer. J Natl Cancer Inst. 2011;103:407–24. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka T, Eustace A, West C. Lysyl oxidase: from basic science to future cancer treatment. Cell Struct Funct. 2012;37:75–80. doi: 10.1247/csf.11015. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Q, Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron Off J Int Cancer Microenviron Soc. 2012;5:261–73. doi: 10.1007/s12307-012-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murawaki Y, Kusakabe Y, Hirayama C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatol Baltim Md. 1991;14:1167–73. [PubMed] [Google Scholar]
- 12.Ryan AV, Wilson S, Wakelam MJ, et al. A prospective study to assess the value of MMP-9 in improving the appropriateness of urgent referrals for colorectal cancer. BMC Cancer. 2006;6:251. doi: 10.1186/1471-2407-6-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damery S, Nichols L, Holder R, et al. Assessing the value of matrix metalloproteinase 9 (MMP9) in improving the appropriateness of referrals for colorectal cancer. Br J Cancer. 2013;108:1149–56. doi: 10.1038/bjc.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker A-M, Bird D, Lang G, et al. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. 2013;32:1863–8. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Cano SJ. Tumor Heterogeneity: Mechanisms and Bases for a Reliable Application of Molecular Marker Design. Int J Mol Sci. 2012;13:1951–2011. doi: 10.3390/ijms13021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Männistö T, Surcel H-M, Bloigu A, et al. ShortJ and Long-Term Storage on Serum Thyrotropin, Thyroid Hormones, The Effect of Freezing, Thawing, and and Thyroid Autoantibodies: Implications for Analyzing Samples Stored in Serum Banks. Clin Chem. 2007;53:1986–7. doi: 10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- 18.Kugler KG, Hackl WO, Mueller LA, et al. The impact of sample storage time on estimates of association in biomarker discovery studies. J Clin Bioinforma. 2011;1:9. doi: 10.1186/2043-9113-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Pischon N, Palamakumbura AH, et al. Intracellular distribution of the lysyl oxidase propeptide in osteoblastic cells. Am J Physiol H Cell Physiol. 2007;292:C2095–C2102. doi: 10.1152/ajpcell.00613.2006. [DOI] [PubMed] [Google Scholar]
- 20.Yuen CT, Easton D, Misch KJ, et al. Increased activity of serum amine oxidases in granuloma annulare, necrobiosis lipoidica and diabetes. Br J Dermatol. 1987;116:643–9. doi: 10.1111/j.1365-2133.1987.tb05897.x. [DOI] [PubMed] [Google Scholar]
- 21.Buckingham B, Reiser KM. Relationship between the content of lysyl oxidase-dependent cross-links in skin collagen, nonenzymatic glycosylation, and long-term complications in type I diabetes mellitus. J Clin Invest. 1990;86:1046–54. doi: 10.1172/JCI114807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronopoulos A, Tang A, Beglova E, et al. Oxidase Expression and Activity in Retinal Endothelial Cells: Mechanism for High Glucose Increases Lysyl Compromised Extracellular Matrix Barrier Function. Diabetes. 2010;59:3159–66. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto M, Murawaki Y, Hirayama C. Serum lysyl oxidase activity in patients with various liver diseases. Gastroenterol Jpn. 1987;22:730–6. doi: 10.1007/BF02776746. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez C, Martínez-González J, Raposo B, et al. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez C, Alcudia JF, Martínez-González J, et al. Statins normalize vascular lysyl oxidase down Jregulation induced by proatherogenic risk factors. Cardiovasc Res. 2009;83:595–603. doi: 10.1093/cvr/cvp136. [DOI] [PubMed] [Google Scholar]
- 26.Fong SFT, Dietzsch E, Fong KSK, et al. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46:644–55. doi: 10.1002/gcc.20444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.