Abstract
Super infection in mice at day 7 post-influenza infection exacerbates bacterial pneumonia at least in part via downstream effects of increased IFN-γ signaling. Here we show that up to 3 days post-influenza infection mice have reduced susceptibility to super infection with methicillin-resistant Staphylococcus aureus (MRSA), but that super infection during that time exacerbated influenza disease. This was due to IL-13 signaling that was advantageous for resolving MRSA infection via inhibition of IFN-γ, but was detrimental to clearance of influenza virus. However, if super infection did not occur until the near resolution of influenza infection (day 7), IL-13 signaling was inhibited, at least in part by up regulation of IL-13 decoy receptor (IL-13Rα2), which in turn caused increases in IFN-γ signaling and exacerbation of bacterial infection. Understanding these cytokine sequelae is critical to development of immunotherapies for influenza-MRSA coinfection since perturbations of these sequelae at the wrong time could increase susceptibility to MRSA and/or influenza.
Keywords: Influenza, methicillin-resistant Staphylococcus aureus (MRSA), secondary-bacterial infections, IL-13, IFN-γ
Introduction
Post-influenza bacterial super infections are the primary cause of deaths during influenza pandemics and result in increased morbidity and mortality due to exacerbated bacterial pneumonias [1–5]. Though the precise cause(s) for this increased susceptibility at around day 7 of influenza infection has not yet been established, it has been associated with: disrupted respiratory epithelium [6]; neuraminidase-mediated exposure of pneumococcal receptors [7]; exhaustion of neutrophils and macrophages, and down regulation of Toll-like receptors [2]. More recent evidence indicates that susceptibility to streptococcal super infection at day 7 of influenza is associated with IFN-γ-mediated reduction in MARCO-mediated phagocytosis by alveolar macrophages (AM) [3]. However, the cytokine sequelae early in influenza infection, that eventually determines the later IFN-γ-mediated susceptibility is not understood.
We have shown elsewhere, that IL-13 plays a critical role in resistance to MRSA pneumonia via amplification of bacterial clearance by lung neutrophils and CD11c+ cells [8]. As IL-13, and IFN-γ are known to affect functions of each other [9–12], we hypothesized that IL-13 can regulate IFN-γ during the course of influenza infection, which may affect the susceptibility of mice to bacterial super infection.
Here we show that secondary MRSA pneumonia initiated 2–3 days post-influenza infection was better contained than in MRSA-only infected mice. This reduced susceptibility to MRSA super infection, was mediated by IL-13 that directly suppressed subsequent production of IFN-γ. IL-13 signaling capacity gradually diminished after day 3 of influenza infection, as clinical symptoms emerged. However, if IL-13 signaling was sustained (by either MRSA super infection or mrIL-13 treatment of WT mice) it exacerbated influenza pneumonia. Finally, the presence of IFN-γ and concomitant lack of IL-13 in mice super infected with MRSA 7 days post-influenza was associated with increased expression of IL-13 decoy receptor, IL-13Rα2, and treatment with anti-IL-13Rα2 partially reduced susceptibility. Thus, the switch from reduced susceptibility to increased susceptibility to secondary MRSA pneumonia during the progression of influenza infection occurred as the capacity for IL-13 signaling in response to MRSA challenge waned and was replaced with increased IFN-γ and IL-13Rα2 levels. Therefore, the balance between IL-13 and IFN-γ during the progression of influenza infection dictates the outcome of both primary influenza infection and secondary MRSA pneumonia.
Results
Mice with pre-symptomatic influenza infection are less susceptible to secondary MRSA pneumonia
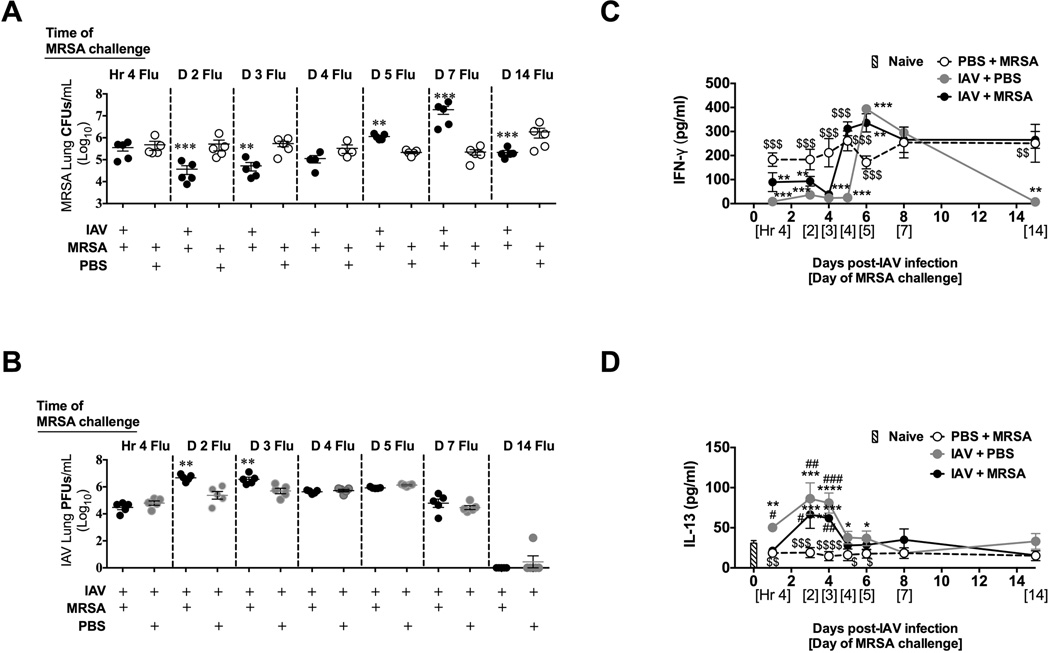
To determine the kinetics of susceptibility to super infection we challenged C57BL/6 mice with MRSA at 0 (4 h), 2, 3, 4, 5, 7, or 14 days post-influenza infection. Mice challenged on day 2 or 3 showed a significant decrease in bacterial burden when compared to challenged, mock-infected mice (Figure 1A). However, mice challenged 5 or 7 days post-influenza infection were more susceptible to MRSA and mice challenged 14 days post-influenza infection or later again showed enhanced bacterial clearance (Figure 1A and S1A). Nearly identical kinetics of susceptibility to post-influenza MRSA super infection was observed in C57BL/6 (Figure 1A) and BALB/c mice (Figure S1A). Susceptibility to super infection with Streptococcus pneumoniae was also reduced in mice at day 3 of influenza infection (Figure S1B).
Figure 1. During pre-clinical influenza infection mice are less sensitive to MRSA super infection and have increased IL-13 and reduced IFN-γ.
C57BL/6 mice were intranasally infected with influenza virus (IAV) on day 0 and then were challenged with 1×108 CFU of MRSA 4 hours, 2 days, 3 days, 4 days, 5 days, 7 days, or 14 days later. MRSA lung bacterial burden (A) and IAV lung viral burden (B) were determined 24 h post-MRSA challenge. Each data point represents a mean ± SEM of 5 mice from one representative experiment. (C, D) The concentration of cytokines was evaluated by ELISA in murine cell-free BALF collected at the terminal time points (24 h post-MRSA challenge for each individual time point). Data points represent mean ± SEM of BALF samples collected from 5 mice per group (each sample was plated in triplicates) from one representative experiment. Statistical significance: In panel A and B **, p < .01; ***; p < .001 vs. PBS + MRSA infected mice (unpaired t-test). In panel C and D “*” corresponds to mice infected with PBS + MRSA, “#” corresponds to naïve (not infected) mice, whereas “$” corresponds to mice infected with IAV + PBS (one-way ANOVA with a Bonferroni’s post test). Experiments of similar design were independently performed at least twice with similar results.
In contrast to improved bacterial clearance in mice super infected with MRSA 2 or 3 days post-influenza infection, these mice showed reduced capacity to clear viral infection when compared to influenza-infected mice not challenged with MRSA (Figure 1B). This improved bacterial clearance and concomitant reduced viral clearance in mice infected with MRSA at either days 2 or 3 of influenza infection was associated with about 4% body weight loss from time 0 to 24 h post-MRSA super infection (Figure S2A), and no mortality (Figure S2B). However, mice challenged between days 4 and 7 after influenza infection lost between 6 and 8.2% of their body weight and exhibited up to 27% mortality at 24 h after MRSA challenge (Figure S2).
Reduced susceptibility to MRSA early in IAV infection is associated with increased IL-13 production
Increased susceptibility to super infection with S. pneumonia 7 days post-influenza infection has been correlated to the increased levels of IFN-γ and associated down regulation of the scavenger receptor MARCO on CD11c+ cells [3]. Indeed we found that IFN-γ was elevated in BALF of C57BL/6 mice at day 7 of influenza infection (both with and without MRSA challenge; Figure 1C) in comparison to either 2 or 3 days when IFN-γ was nearly undetectable. Similar results were found in BALB/c mice (Figure S3A). Mice infected with influenza for up to 3 days also showed significantly higher expression of MARCO when compared to mice infected for 7 days (Figure S4). We have shown elsewhere that increased IL-13 production is required for virus-like particle (VLP)-induced resistance to MRSA pneumonia [8]. Thus, we tested whether reduced susceptibility to secondary MRSA challenge observed early in influenza infection is associated with the presence of this cytokine. We found elevated IL-13 in the BALF of naïve mice consistent with our previous results [8], and interestingly, we also found elevated IL-13 in BALF of mice infected with influenza 2 or 3 days before challenge with MRSA (Figure 1D). However, when mice progressed to clinical influenza infection, we observed a steady decrease in levels of IL-13, and by day 7, when influenza-infected mice showed increased susceptibility to MRSA, almost no IL-13 was detected in their BALF samples (Figure 1D). Similar results were found in BALB/c mice (Figure S3B). The low levels of IL-13 upon MRSA challenge of mock-infected mice was associated with increased bacterial burden (Figure 1A) and decreased survival when compared to influenza-infected mice challenged with MRSA on day 3 (Figure S2B). Thus, the presence of IL-13 was associated with reduced susceptibility to MRSA challenge in the first few days of influenza infection.
IL-13−/− mice are susceptible to MRSA pneumonia early in IAV infection and have increased IFN-γ
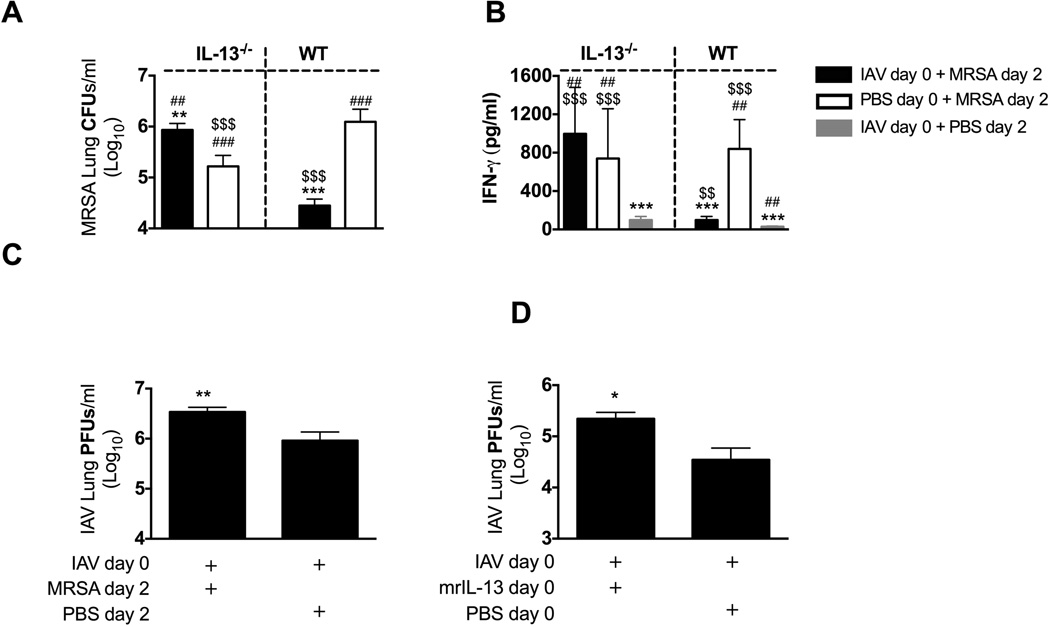
To test whether IL-13 is required for reduced susceptibility to secondary MRSA pneumonia during preclinical influenza infection we super infected IL-13−/− mice with MRSA 2 days after influenza infection. Influenza-infected IL-13−/− mice were more susceptible to MRSA challenge than either mock-infected IL-13−/− mice, or influenza-infected WT mice (Figure 2A). This increased susceptibility of IL-13−/− mice to secondary MRSA pneumonia was associated with increased levels of IFN-γ in their BALF, when compared to super infected WT mice (Figure 2B). Mock-challenged influenza-infected IL-13−/− and WT mice had minimal amounts of IFN-γ in their BALF 2 days post-influenza infection, whereas high levels of IFN-γ were present in BALF of MRSA-challenged IL-13−/− mice (either influenza- or mock- infected) that corresponded to both fewer bacteria cleared from their lungs and reduced survival past 8 days post-infection (Figure 2A and data not shown). Similar to the experiment described in Figure 1, we found that bacterial super infection early (day 2) in influenza infection exacerbated viral titers of WT mice (Figure 2C). Interestingly, we also found that treatment of WT mice with exogenous IL-13 either at the time of influenza infection, or on day 2–4 of influenza infection exacerbated viral burdens when compared to mice infected with influenza and not treated with mrIL-13 (Figure 2D, and data not shown). This suggested that the increased viral burden in mice super infected with MRSA 2–3 days after influenza infection was, at least partially dependent on enhanced IL-13 signaling.
Figure 2. IL-13−/− mice are susceptible to secondary MRSA pneumonia early after influenza infection.
IL-13−/− and C57BL/6 WT mice were infected with influenza (IAV) on day 0 and MRSA on day 2. (A) Bacterial burden was evaluated 24 h after MRSA challenge. (B) Level of IFN-γ was evaluated in cell-free BALF collected at the time of sacrifice. (C) Viral load was measured in mice lungs 24 h after MRSA challenge. (D) WT mice were infected with influenza and treated intratracheally with 0.5 µg mrIL-13 or PBS at the time of challenge with influenza, as well as 3 and 6 h later. Viral burden was evaluated 24 h after influenza infection. Each data point represents mean ± SEM of 5 mice per group from one representative experiment. Experiments of similar design were independently performed at least twice with similar results. Statistical significance: In panels A and B “*” corresponds to respective WT or IL-13−/− mice infected with PBS + MRSA, “#” corresponds to WT mice infected with IAV+ MRSA, whereas “$” corresponds to respective WT of IL-13−/− mice infected with PBS + IAV (one-way ANOVA with a Bonferroni’s post test). In panels C and D “*” corresponds to mice infected with IAV + PBS (unpaired t-test).
IL-13-mediated suppression of IFN-γ is responsible for reduced susceptibility to super infection
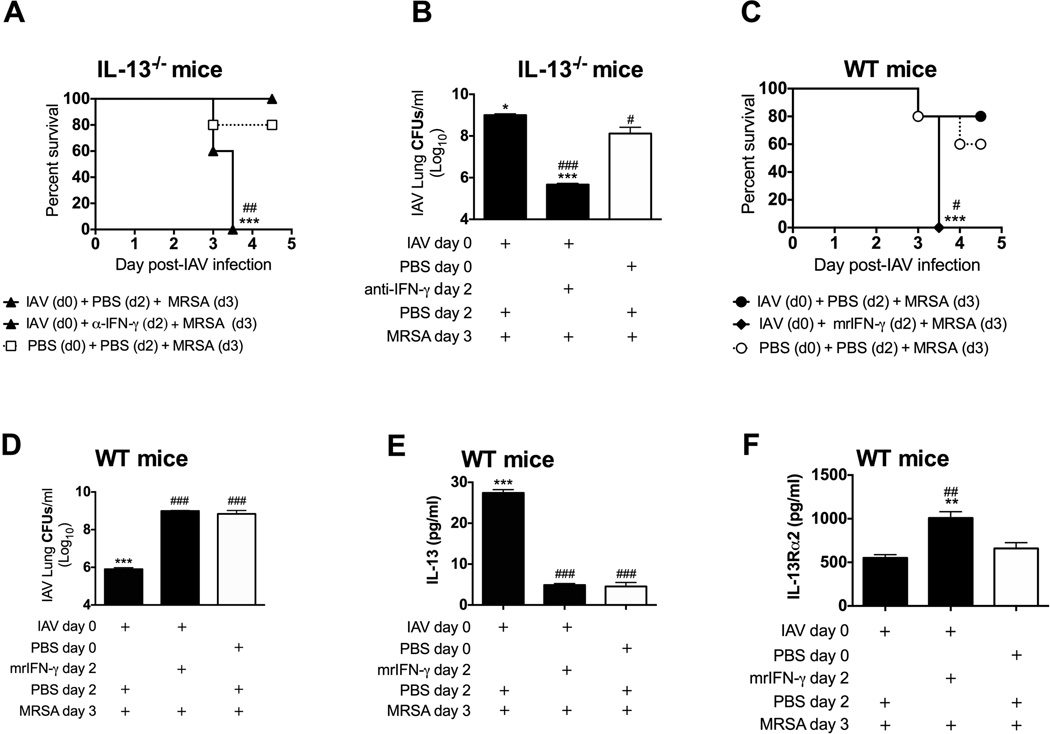
Thus far we have correlated reduced susceptibility of influenza-infected mice to MRSA challenge at either day 2 or 3 with the presence of IL-13 and lack of IFN-γ (Figure 1 and 2). To determine whether IFN-γ,in the absence of IL-13, is responsible for the increased susceptibility of mice to MRSA challenge, we neutralized IFN-γ prior to super infection of IL-13−/− mice. Indeed, anti-IFN-γ treatment increased survival, reduced lung bacterial burden, and thus reduced susceptibility to super infection of IL-13−/− mice (Figure 3A,B).
Figure 3. IL-13-dependent inhibition of IFN-γ is responsible for reduced susceptibility to secondary MRSA infection.
IL-13−/− and WT C57BL/6 mice were infected with influenza and challenged with MRSA 3 days later. One day prior to and at the time of MRSA challenge IL-13−/− mice were intraperitronealy (i.p.) injected with 250 µg anti-IFN-γ antibody or PBS (A, B) and WT mice were intratracheally (i.t.) treated with 1.5 µg of mrIFN-γ or PBS (C–F). (A,C) Survival of mice was evaluated daily. (B,D) In a separate experiment, lung bacterial burdens were evaluated 24 h after MRSA challenge. Levels of IL-13 in BALF samples (E) and IL-13Rα2 in serum samples (F) were evaluated 4 h after MRSA challenge of WT mice treated with mrIFN-γ. Each data point represents mean ± SEM of 5 mice per group from one representative experiment. Experiments of similar design were independently performed at least twice with similar results. Statistical significances: “*” corresponds to PBS + MRSA infected mice (white symbols in each figure panel), whereas “#” corresponds to mice infected with influenza on day 0 and MRSA on day 3 (one-way ANOVA with a Bonferroni’s post test, or Logrank test for differences in survival).
Finally, we determined whether introduction of IFN-γ during pre-clinical influenza infection overcame the state of reduced susceptibility of mice to super infection, as WT mice treated with exogenous mrIFN-γ prior to MRSA infection on day 3 of influenza infection all succumbed to the infection within 24 h (Figure 3C) with increase numbers of MRSA in their lungs (Figure 3D). Interestingly, the exacerbated mortality of mrIFN-γ-treated super infected mice was not entirely due to increased lung bacterial burden, as mice infected with PBS + MRSA had similar number of bacterial in their lungs and despite this showed significantly higher survival (60% survival of PBS + MRSA- infected mice vs. 100% mortality of mrIFN-γ-treated super infected mice). This suggests additional mechanisms other than differences in bacteria numbers contributed to the high mortality rate of mrIFN-γ-treated mice. Super infected WT mice treated with mrIFN-γ early in influenza infection showed significant reduction in IL-13 to the levels observed for MRSA-infected mice, and concomitant increase in serum levels of IL-13Rα2 in response to super infection (Figure 3E,F).
MrIL-13 rescues mice from increased susceptibility to MRSA super infection at day 7 of IAV infection
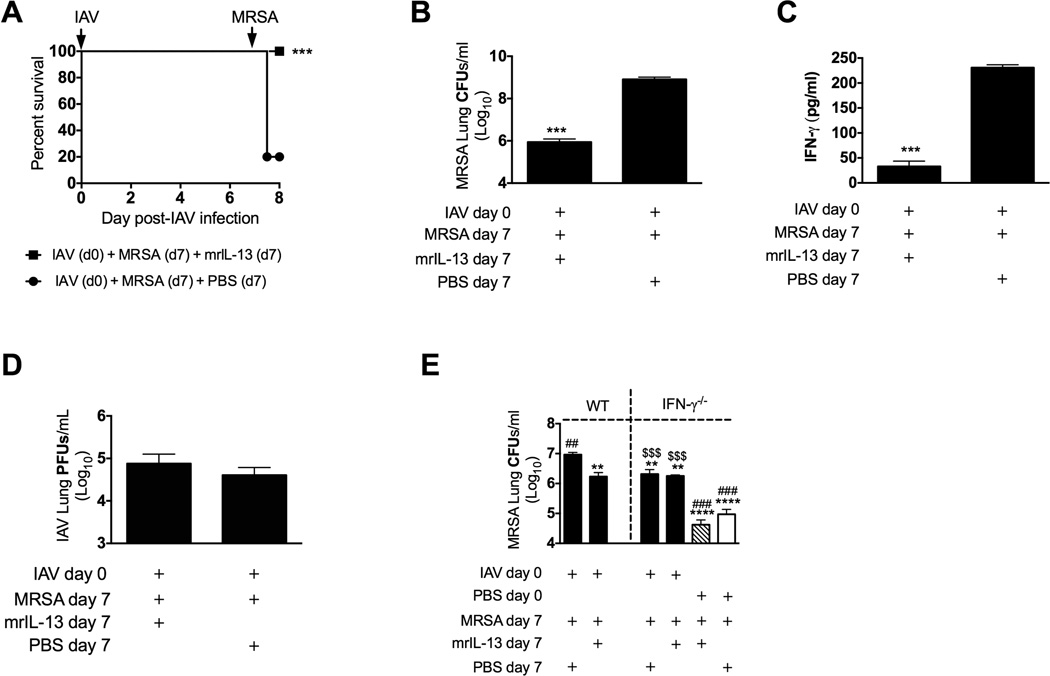
We have reported elsewhere that IL-13 induced in the lung by immunization of mice with VLPs unrelated to influenza was beneficial for the resolution of MRSA pneumonia [8]. To this end we showed that the presence of IL-13 in the lung during preclinical influenza infection was responsible for reduction of susceptibility to MRSA challenge via down regulation of IFN-γ. Because the lung environment at day 7 of influenza infection is characterized by low levels of IL-13 and high levels of IFN-γ (Figure 1C,D), we asked whether treatment of mice with mrIL-13 at the time of challenge with MRSA 7 days after influenza infection could reduce the susceptibility of these mice to MRSA challenge. All mice treated with mrIL-13 at the time of MRSA challenge on day 7 of influenza infection survived the 24 h period post-challenge, which was in contrast to only 20% survival of influenza-infected mice challenged but not treated with mrIL-13 (Figure 4A). Consistent with improved survival, these mice showed significant reduction in bacterial burden and BALF IFN-γ levels, with no change in viral load when compared to mice super infected but not treated with mrIL-13 (Figure 4B–D). To further discern whether IL-13’s role in reduction of susceptibility to super infection is via down regulation of IFN-γ, we treated IFN-γ−/− and WT mice with mrIL-13 at the time of MRSA challenge on day 7 post-influenza infection. Treatment with mrIL-13 had no effect on bacterial burden of IFN-γ−/− mice but significantly reduced bacterial burden in WT mice, confirming that IL-13’s predominant role during super infection is indeed IFN-γ-dependent and functions by down regulation of IFN-γ (Figure 4E). In addition, mrIL-13 treatment reduced bacterial burdens in WT mice infected with MRSA only (data not shown), but did not significantly affect bacterial burdens in IFN-γ−/− mice (Figure 4E).
Figure 4. Treatment with mrIL-13 ameliorates the susceptibility to super infection of mice 7 days after influenza infection.
C57BL/6 mice were infected with influenza and super infected with MRSA 7 days later. One group of mice super infected on day 7 received an intratracheal treatment of 0.5 µg mrIL-13 at the time of MRSA challenge, and 3 and 6 h later. (A) Survival of mice was assessed daily. In a separate experiment lung bacterial (B) and viral (D) burdens as well as BALF levels of IFN-γ (C) were evaluated 24 h after MRSA challenge. (E) IFN-γ−/− and WT mice were infected with influenza virus 3 or 7 days prior to MRSA challenge. A group of IFN-γ−/− mice and WT mice challenged with MRSA 7 days post-IAV infection were treated intratracheally with mrIL-13 at the time of MRSA challenge, 3 and 6 h later. Bacterial burden in mice lungs was evaluated 24 h after MRSA challenge. Each data point represents a mean ± SEM of 5 mice per group from one representative experiment. Experiments of similar design were independently performed at least twice with similar results. Statistical significance: In panels A-D “*” corresponds to control mice super infected with MRSA 7 days after IAV infection that were not treated with mrIL-13 (unpaired t-test or Logrank test for differences in survival). In panel E ”*” corresponds to WT mice infected with influenza on day 0 and MRSA on day 7, “#” corresponds to IFN-γ−/− mice infected with influenza on day 0 and MRSA on day 7, “$” corresponds to IFN-γ−/− mice infected with MRSA but not with influenza (solid white bar) (one-way ANOVA with a Bonferroni’s post test).
Up regulated IL-13Rα2 and suppressed IL-13-inducing cytokines in mice susceptible to super infection
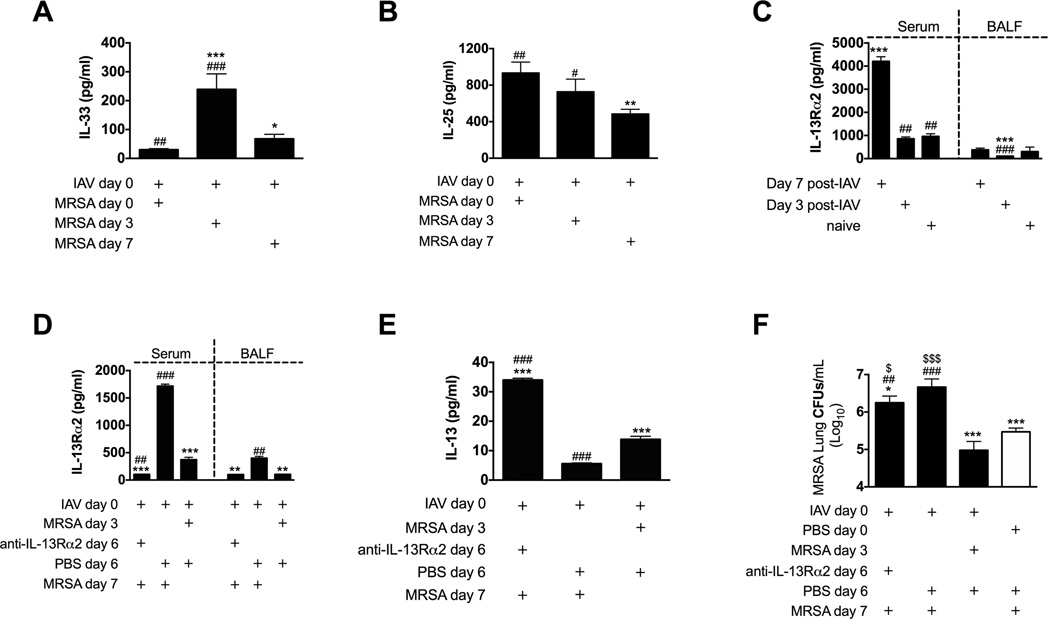
When compared to mice super infected with MRSA on either day 0 or day 7 we found significant increases in the IL-13-inducing cytokine IL-33 in mice super infected 3 days post-influenza infection (Figure 5A). Another IL-13-activating cytokine, IL-25, was also significantly higher in mice super infected at 3 days as compared to 7 days of influenza infection (Figure 5B). Thus, the IL-13-inducing cytokines are higher at the time when IL-13 is higher and susceptibility to MRSA in super infected mice is reduced. Interestingly, only mice with clinical influenza infection (day 7) showed increased expression of IL-13 decoy receptor (IL-13Rα2) (Figure 5C and S5). These data then suggest that decreases in IL-25 and IL-33 signaling, and increases in the IL-13Rα2, between days 3 and 7 of influenza infection could contribute to the loss of IL-13 at 7 days and the resulting increased susceptibility to MRSA challenge at that time of super infection.
Figure 5. Upregulated IL-13Rα2 in mice 7 days post-influenza infection contributes to increased susceptibility to super infection.
(A,B) C57BL/6 mice were infected with influenza on day 0 and super infected with MRSA on day 0, 3 or 7 in relation to influenza infection. Mice were sacrificed 4 h after MRSA challenge and cell-free BALF was evaluated for presence of cytokines IL-33 (A) and IL-25 (IL-17E) (B). (C) C57BL/6 mice were sacrificed 3 or 7 days after infection with influenza. Presence of IL-13Rα2 was determined in their cell-free BALF and serum samples by Quantikine ELISA.(D–F) C57BL/6 mice were infected with influenza and super infected with MRSA 3 or 7 days later. One group of mice super infected on day 7 received an intraperitoneal injection of 250 µg anti-IL-13Rα2 antibody 24 h prior to MRSA challenge. (D)Presence of IL-13Rα2 was evaluated in serum and BALF samples and (E) levels of IL-13 were evaluated in BALF samples 24 h after MRSA challenge. (F) MRSA lung burden was measured 24 h after MRSA challenge. Each data point represents a mean ± SEM of 5 mice per group from one representative experiment. Experiments of similar design were independently performed at least twice with similar results.Statistical significance: In panels A and B“*”corresponds to mice infected with influenza on day 0 and MRSA on day 0, whereas “#” corresponds to mice infected with influenza on day 0 and MRSA on day 7. On panel C “*” corresponds to not infected (naïve) mice, whereas “#” corresponds to mice infected with influenza for 7 days. In panels D–F“*”corresponds to mice infected with influenza on day 0 and MRSA on day 7, “#” corresponds to mice infected with influenza on day 0 and MRSA on day 3, whereas “$” in panel E corresponds to mice not infected with influenza and infected with MRSA (one-way ANOVA with a Bonferroni’s post test).
Blockade of IL-13Rα2 reduces lung MRSA burden in mice susceptible to super infection
When compared to naïve (uninfected) mice, mice infected with influenza for the first 3 days had significant reduction of serum levels of IL-13Rα2 (Figure S5 – grey bar vs. grey trace). However, as mice began progressing into the clinical phase of infection (> day 4 post-influenza infection; Figure S2A), levels of IL-13Rα2 began to increase. MRSA infection of PBS-inoculated mice (white trace) did not alter levels of IL-13Rα2 observed in naïve mice. MRSA super infection 4 h after influenza infection inhibited the down regulation of IL-13Rα2. However MRSA super infection at either day 3 or 4 of influenza infection significantly reduced serum IL-13Rα2 levels when compared to influenza-infected mock-challenged mice. MRSA super infection during the clinical phase of influenza (at either day 5 or 7 post-influenza infection) did not change the influenza-driven increases in IL-13Rα2. Thus, next we determined the possible involvement of this decoy receptor in increased susceptibility of mice to super infection at day 7 of influenza. As such we treated mice with IL-13Rα2 antibody on day 6 of influenza infection and we challenged these mice with MRSA on day 7. Anti-IL-13Rα2 treatment was efficient in blocking IL-13R±2 in both blood and BALF (Figure 5D), and treated mice showed significantly higher levels of IL-13 even compared to mice resistant to super infection (at 3 days post-influenza) (Figure 5E). Anti-IL-13Rα2 treatment improved the anti-bacterial response as determined by a significant reduction (p < .05) in MRSA lung burden when compared to mice super infected on day 7 of influenza infection that were not treated with anti-IL-13Rα2 antibody. However, the bacterial clearance in anti-IL-13Rα2 treated mice was still reduced when compared to either mice super infected on day 3 post-influenza infection (p < .05) or mice infected only with MRSA (p < .05) (Figure 5F).
These results indicate that increases in IL-13Rα2 levels between days 3 and 7 of influenza infection contribute to decreased IL-13 observed during this time, and thus contribute, at least in part, to the increased susceptibility to MRSA challenge at 7 days of influenza infection.
Discussion
The majority of secondary bacterial pneumonias occur within 2 weeks after influenza infection [13–15] and result in significant morbidity and mortality. Recent studies have indicated that dysfunctional innate immune mechanisms including increased production of IFN-γ and subsequent down regulation of scavenger receptor MARCO on the surface of alveolar macrophages [3] is a major contributor to increased susceptibility to secondary infections after influenza [16]. However, little attention has been paid to host responses early in influenza infection that may determine these later responses and increased susceptibility.
Here we report that persistence of IL-13 until 3 days after influenza infection directly inhibits production of IFN-γ reducing susceptibility to bacterial super infection. Whereas up regulation of IL-13Rα2 by day 7 of influenza infection results in sequestration of IL-13 and facilitates increases of IFN-γ that is known to directly inhibit phagocytosis of bacteria [3] and increases susceptibility to bacterial super infection.
Liu et al recently showed that mice infected with WSN influenza are more sensitive to super infection with the linezolid/vancomycin-sensitive MRSA 3 days post-influenza infection [17]. In contrast to this report, in our hands, infection of mice with USA300 MRSA strain 2–3 days following infection with PR8 influenza significantly ameliorated the susceptibility of mice to MRSA challenge. The discrepancy between our results and work by Liu et al may have resulted from different strain and doses of both influenza and MRSA. The goal of our study was to determine how cytokine regulation early in influenza infection (day 2–3) affects susceptibility to super infection later in influenza infection (day 7). Thus, we used higher then reported by some groups [1, 15, 17, 18] infectious doses of the virus and bacteria to measure the susceptibility to both influenza and MRSA soon after MRSA challenge rather then infecting mice with lower doses to allow the bacterial growth over time, which could span different periods of MRSA susceptibility at different times after influenza infection. Though, others have reported decreased long-term survival of mice exposed to S. aureus 3 days post-influenza [1, 18], the low infectious doses and long incubation period in vivo used in these studies preclude being able to detect daily changes in susceptibility and thus, direct comparison to our results.
The importance of IL-13 in regulation of susceptibility to secondary MRSA pneumonia was indicated by the fact that IL-13−/ − mice were susceptible to super infection regardless of the stage of influenza infection. That their BALF contained significantly elevated levels of IFN-γ in response to MRSA super infection, and treatment with anti-IFN-γ antibodies improved survival of these mice and significantly reduced bacterial burden within 24 h after MRSA challenge, suggested that IL-13 functioned by down regulating IFN-γ.In agreement with our results published elsewhere [8], others noted delayed clearance of S. aureus from the lungs of IL-13−/− mice that could be rescued by mrIL-13–treatment at the time of infection [19]. Though the mechanism of this delayed clearance of S. aureus in mice lacking IL-13 remains unknown, the role of IL-13 in shaping immune responses may help to explain its anti-bacterial action.
IL-13 is involved with asthma and allergic responses [20, 21] where it induces goblet cell metaplasia and mucus hypersecretion [22, 23]. The effect of IL-13 on susceptibility to secondary MRSA pneumonia is rapid (<12 h; data not shown), thus it is doubtful that the IL-13-mediated production of mucus is responsible for its anti-bacterial effect in our system. Moreover, the fact that treatment of IFN-γ−/− mice with mrIL-13 had no effect on bacterial burden of these mice 24 h post-MRSA infection, further suggests that the primary mode of IL-13 action in our system is by down regulating IFN-γ. Others have shown that IFN-γ decreases MARCO expression on macrophages reducing bacterial phagocytosis [3]. Consistent with this finding we found that on day 3 of influenza infection expression of MARCO on CD11c+ cells was increased when compared to mice infected with influenza for 7 days. However, that expression of MARCO at day 3, when mice are less susceptible to MRSA, was not higher than that of non-infected mice suggests that at this time MARCO-independent mechanisms of bacterial clearance are also involved.
Increased susceptibility of IL-13−/− mice to super infection early during influenza infection was associated with increased production of IFN-γ. Neutralization of IFN-γ rendered IL-13−/− mice resistant to super infection, whereas treatment of WT mice with mrIFN-γ prior to MRSA challenge at day 3 post-influenza infection significantly worsened the outcome of MRSA pneumonia. Thus, during the first 2–3 days after influenza infection, mice had reduced susceptibility to MRSA super infection, which was mediated by IL-13 that directly inhibited production of IFN-γ. The capacity of IL-13 to inhibit IFN-γ production has been known for almost two decades [9]. To our knowledge we show here for the first time that in super infection, IL-13 mediated levels of IFN-γ, which affected susceptibility to secondary MRSA pneumonia, as treatment of WT mice at day 7 with mrIL-13 reduced IFN-γ levels and reduced susceptibility to MRSA challenge.
The biological effect of IL-13 is typically exerted by its interaction with type I IL-13R [22, 24]. Though IL-13Rα2 has high affinity for IL-13 [22, 24], due to its truncated cytoplasmic tail it is believed to act as a decoy receptor that limits the activity of IL-13 [24, 25]. IL-13Rα2 can be up regulated by both IFN-γ and type I IFNs during an antiviral response [26]. Indeed, we detected up regulation of IL-13Rα2 in serum and BALF samples from mice super infected with MRSA 7 days post-influenza (that had increased IFN-γ), but not in mice super infected on day 3 (that had increased IL-13). Neutralization of IL-13Rα2 increased IL-13 levels and partially ameliorated MRSA pneumonia in mice super infected 7 days post-influenza, indicating that up regulation of IL-13Rα2 contributes to increased susceptibility to super infection at day 7.
Why production of IL-13, which is clearly beneficial for the resolution of MRSA pneumonia, is down regulated during the course of influenza infection is not clear. However, our observation that IL-13 signaling decreases clearance of influenza virus may explain the need to down regulate IL-13 during influenza infection.
In summary, our results indicate that during the first 3 days of influenza infection, the lung is in a state characterized by IL-13-dependent reduced susceptibility to MRSA. Then, as the virus persists during days 3–5, a gradual shift to a state of increased susceptibility to MRSA occurs. This state of increased susceptibility could be overcome with either mrIL-13 or anti-IL-13Rα2-treatement, both which increased IL-13 levels. That this shift in IL-13-dependent mechanisms is required for resistance to influenza was indicated by the observed decrease of influenza clearance that occurred after either MRSA infection or treatment with mrIL-13, both which resulted in sustained IL-13 signaling during influenza infection. Thus, at the time the host is mounting a response to clear influenza it is necessary to shift to a state of increased susceptibility to bacteria because the mechanisms of resistance to MRSA and influenza are not compatible.
Materials and Methods
Mice
Wild type (WT) BALB/c, C57BL/6 mice, IL-13−/− and IFN-γ−/− mice were bred at MSU, Bozeman, MT. 7–8 week old mice were used in all experiments.
Ethics Statement
Mice were maintained at MSU ARC and animal use protocols were approved by the IACUC MSU, (#2011–07), in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. MSU Federal-Wide Assurance number for animal welfare is A3627-01 and MSU is accredited by AAALAC (# 713). Every effort was made to minimize discomfort, pain and distress in the animals.
Infections
For intranasal inoculations (50 µl vol.), mice were lightly anesthetized as described elsewhere [27] and instilled with 1500 PFU of mouse adapted influenza virus strain A/PR8/8/34 (PR8, H1N1) prepared according to the previously described protocol [28]. LAC strain of MRSA (pulsed-field type USA300) was prepared as we previously described [8] and was a kind gift from Dr. Jovanka Voyich at MSU. Doses of 5 × 107 CFU (for BALB/c mice) and 1 × 108 CFU (for C57BL/6 mice background) were used for infection.
Collection and preparation of BALF and serum samples
Mice were sacrificed by i.p. administration of sodium pentobarbital (lethal dose 90 mg/kg) followed by exsanguination and blood collection. Following centrifugation at 10000xg for 5 min in RT, serum samples were collected and stored at −20°C until analyzed. Bronchoalveolar lavage fluid (BALF) was obtained by lavaging the lungs with 2 mls of 3 mM EDTA in PBS. Following centrifugation, the cell-free BALF supernatant was recovered and stored at −80°C for future use to determine levels of cytokines.
Cytokine analyses
Levels of IFN-γ (limit of detection: 15 pg/ml), IL-13 (limit of detection: 4 pg/ml), IL-25 (limit of detection: 16 pg/ml), IL-33 (limit of detection: 25 pg/ml) in cell-free BALF were determined by sandwich ELISA kit in BALF samples (Ready-SET-Go; eBioscience; San Diego, CA), and levels of IL-13Rα2 were determined by Quantikine ELISA Immunoassay (R&D Systems; Minneapolis, MN; limit of detection: 2 pg/ml). BALF and/or serum samples were tested in triplicate.
Survival, body weights and determination of bacterial and viral burdens
Mice were weighed and monitored for signs of morbidity and mortality daily. Our previously described procedures for determining colony forming units (CFU) [8] and plaque forming units (PFU) [27] were followed.
Treatment of mice with mrIL-13, anti-IL-13Rα2, mrIFN-γ and anti-IFN-γ antibodies
For mrIL-13 treatment: mice were treated with 0.5 µg of mouse recombinant IL-13 (BioLegend) by i.t. inoculation 0, 3 and 6 h post-MRSA challenge (for the total of 1.5 µg/mouse). For mrIFN-γ or anti-IFN-γ treatment: mice were treated with 250 µg (0.5 mg/ml) of anti-IFN-γ mAb (BioXcell; West Lebanon, NH) i.p. or with 1.5 µg of mrIFN-γ(R&D Systems) i.t. one day prior and at the time of MRSA challenge. For neutralization of IL-13Rα2: mice were i.p. injected with 250 µg/dose of anti-IL-13Rα2 pAb (R&D Systems) one day prior to MRSA challenge.
Statistical analyses
Statistical significance was determined by one-way ANOVA with a Bonferroni’s post-test of multiple comparisons, or in some cases an unpaired t-test was used. Significance was indicated by *, p < .05; **, p < .01; ***, p < .001; or ****, p < .0001. In some graphs (*) symbols are replaced by other symbols for clarity in comparison between multiple groups, but the number of symbols always corresponds to the appropriate p-value as described above for the asterisk, and explained in individual figure legends. For the differences in survival Kaplan-Meier curves were plotted and analyzed using GraphPad Prism software (Version 4.0; La Jolla, CA) using Gehan-Breslow-Wilcoxon (Logrank) test. Statistical differences of p < .05 were considered significant. All experiments were repeated at least once.
Supplementary Material
Acknowledgments
This work was supported by the following grants: National Institute of Allergy and Infectious Diseases at National Institutes of Health [grant numbers 1R56AI089458, R01AI04905], IDeA Networks for Biomedical Research Excellence at National Institutes of Health [grant number P20GM103474], Centers for Biomedical Research Excellence at National Institutes of Health [grant number P20GM103500], Montana State University Agricultural Experiment Station; and M.J. Murdock Charitable Trust.
Authors would like to thank past and current members of the Harmsen Laboratory at MSU including Katie Rowse, Soo Han and Erin Dobrinen for their help in performing experiments.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- IAV
type A Influenza
- IL-13Rα2
IL-13 receptor alpha 2 – a decoy receptor for IL-13
- MRSA
methicillin-resistant Staphylococcus aureus
Footnotes
Author’s contribution: designed experiments: ARA; LR, AGH; performed experiments: ARA, LR, AH, KL, RM, AE; analyzed results of experiments: ARA, LR, AE, KL, RM, AH, AGH; wrote manuscript: ARA, AGH.
Conflict of Interests
The authors declare no commercial or financial conflict of interests.
References
- 1.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didierlaurent A, Goulding J, Hussell T. The impact of successive infections on the lung microenvironment. Immunology. 2007;122:457–465. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 4.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irizarry-Acosta M, Puius YA. Post-influenza Pneumonia: Everything Old Is New Again. MEDICINE & HEALTH/RHODE ISLAND. 2010;93:204–211. [PubMed] [Google Scholar]
- 6.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23:S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 7.McCullers JA, KC B. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. Journal of Infectious Diseases. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 8.Rynda-Apple A, Dobrinen E, McAlpine M, Read A, Harmsen A, Richert LE, Calverley M, Pallister K, Voyich J, Wiley JA, Johnson B, Young M, Douglas T, Harmsen AG. Virus-Like Particle-Induced Protection Against MRSA Pneumonia Is Dependent on IL-13 and Enhancement of Phagocyte Function. Am J Pathol. 2012;181:196–210. doi: 10.1016/j.ajpath.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muchamuel T, Menon S, Pisacane P, Howard MC, Cockayne DA. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: correlation with down-modulation of TNF-alpha, IFN-gamma, and IL-12 production. J Immunol. 1997;158:2898–2903. [PubMed] [Google Scholar]
- 10.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizukoshi E, Kaneko S, Yanagi M, Ohno H, Matsushita E, Kobayashi K. Up regulation of type I interferon receptor by IFN-gamma. J Interferon Cytokine Res. 1999;19:1019–1023. doi: 10.1089/107999099313235. [DOI] [PubMed] [Google Scholar]
- 12.Meissner N, Swain S, McInnerney K, Han S, Harmsen AG. Type-I IFN signaling suppresses an excessive IFN-gamma response and thus prevents lung damage and chronic inflammation during Pneumocystis (PC) clearance in CD4 T cell-competent mice. Am J Pathol. 2010;176:2806–2818. doi: 10.2353/ajpath.2010.091158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brundage J. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918-–19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–1199. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Moltedo B, Moran TM. Type I IFN induction during influenza infection increases the susceptibility to secondary S. pneumoniae Infection by negative regulation of γδ T cells. Journal of Virology. 2012;86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger DW, Sun K. Immune Dysfunction and Bacterial Coinfections following Influenza. J Immunol. 2013;191:2047–2052. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, He Y, Xiao K, White JR, Fusco DN, GA P. Effect of linezolid on clinical severity and pulmonary cytokines in a murine model of influenza A and Staphylococcus aureus coinfection. PlosONE. 2013;8:e57483. doi: 10.1371/journal.pone.0057483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011;203:880–888. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewski MA, Falkowski NR, Surana R, Sonstein J, Hartman A, Moore BB, Huffnagle GB, Toews GB. Effect of laparotomy on clearance and cytokine induction in Staphylococcus aureus infected lungs. Am J Respir Crit Care Med. 2007;176:921–929. doi: 10.1164/rccm.200606-763OC. [DOI] [PubMed] [Google Scholar]
- 20.Elias JA, Lee CG. IL-13 in asthma. The successful integration of lessons from mice and humans. Am J Respir Crit Care Med. 2011;183:957–958. doi: 10.1164/rccm.201101-0080ED. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunig G, Corry DB, Reibman J, Wills-Karp M. Interleukin 13 and the evolution of asthma therapy. Am J Clin Exp Immunol. 2012;1:20–27. [PMC free article] [PubMed] [Google Scholar]
- 23.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, Steinbrecher KA, Rothenberg ME, Shroyer NF, Matthaei KI, Finkelman FD, Hogan SP. Interleukin-13 (IL-13)/IL-13 receptor alpha1 (IL-13Ralpha1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl- secretion. J Biol Chem. 2011;286:13357–13369. doi: 10.1074/jbc.M110.214965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O'Hara RM, Jr, Beier DR, Turner KJ, Wood CR, Collins M. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 26.Campbell-Harding G, Sawkins H, Bedke N, Holgate ST, Davies DE, Andrews AL. The innate antiviral response upregulates IL-13 receptor alpha2 in bronchial fibroblasts. J Allergy Clin Immunol. 2013;131:849–855. doi: 10.1016/j.jaci.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M. Inducible Bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One. 2009;4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley JA, Tighe MP, Harmsen AG. Upper Respiratory Tract Resistance to Influenza Infection Is Not Prevented by the Absence of Either Nasal-Associated Lymphoid Tissue or Cervical Lymph Nodes. Jounal of Immunology. 2005;175:3186–3196. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.