Abstract
Protein immune detection requires secondary antibodies which must be carefully selected in order to avoid interspecies cross-reactivity, and is therefore restricted by the limited availability of primary/secondary antibody pairs. Here we present a versatile DNA-based protein detection system using a universal adapter to interface between IgG antibodies and DNA-modified reporter molecules. As a demonstration of this capability, we successfully used DNA nano-barcodes, quantum dots, and horseradish peroxidase enzyme to detect multiple proteins using our DNA-based labeling system. Our system not only eliminates secondary antibodies but also serves as a novel method platform for protein detection with modularity, high capacity, and multiplexed capability.
Antibody-based protein detection methods including Western blot, enzyme-linked immunosorbent assay (ELISA), dot blot, and immunohistochemistry are widely used analytical techniques in both research and clinical settings. In these methods, sample proteins are first bound with specific primary antibodies and then detected with secondary antibodies carrying a label such as a fluorescent dye, radioactive marker, or enzyme.1 However, the secondary antibodies must be carefully selected to match the species specificity of the primary antibodies in order to prevent undesirable cross-reactivity.2 Moreover, there are only a limited number of commercially available primary/secondary antibody pairs,2 which severely constrains the capacity of multiplexed protein detection. For example, most primary antibodies are derived from mouse or rabbit, and as a result most secondary antibodies are either antimouse IgG or anti-rabbit IgG. In vivo experimental models typically involve mice or rabbits and therefore obviate the use of mouse or rabbit secondary antibodies, respectively.
Here, we present a DNA-based protein detection system which does not require secondary antibodies. Thus, our system allows multiple primary antibodies of the same isotype or species to be used together in a single experiment. Furthermore, our system can label proteins/antibodies with any other materials that can be attached to DNA. As a demonstration of this capability, we successfully used DNA nano-barcodes, quantum dots (QDs), and horseradish peroxidase (HRP) to detect multiple proteins using our DNA-based protein detection system.
The key feature of our system is the universal adapter (UA), a bifunctional protein–DNA hybrid molecule that includes both an antibody-binding component and a DNA tag, as shown in Figure 1 (middle panel). DNA–protein conjugates, with the functions of both nucleic acid and protein, have been previously explored for many applications, including biosensing and molecular self-assembly.3 Inspired by these examples, we chose EZZ protein, an engineered variant of protein A, which recognizes and binds to most types of IgG primary antibodies4 as the antibody-binding component. The DNA tag is a short oligonucleotide which can be hybridized to DNA-modified signal-carrying molecules, such as DNA nano-barcodes, QDs, enzymes, etc. (Figure 1, right panel). Therefore, the combination of UA, IgG primary antibodies, and reporter molecules generates a modular library of pre-labeled primary IgG antibodies that can be used for all applications of protein detection without using secondary antibodies.
Figure 1.
(Middle) UA, a bifunctional protein–DNA hybrid molecule, which binds to most types of IgG antibodies (left) and any DNA-modified reporter molecules (right) to generate a modular library of pre-labeled primary IgG antibodies for any applications of protein detection in place of secondary antibodies.
In order to create UA, we used a self-catalyzing protein (SNAP)5 to form an EZZ protein–DNA hybrid molecule at 1:1 ratio with a high yield (Supplementary Figure 1). More specifically, a DNA tag was first conjugated to maleimidebenzyl guanine (BG) that served as the substrate for the SNAP enzyme. This BG-modified DNA tag was then linked to EZZ protein through SNAP catalysis, and the hybrid molecule was purified by gel electrophoresis to eliminate free proteins and DNA. After purification, the bifunctional binding of UA was tested against both DNA nano-barcodes and IgG primary antibodies by gel electrophoresis and dot blot, respectively (Supplementary Figure 2). DNA nano-barcodes, previously developed in our laboratory, utilize branched DNA to carry multiple fluorescent dyes with pre-determined color ratios, which were successfully used for multiplexed detection of DNA targets.6
As a demonstration of our DNA-based protein detection system using DNA nano-barcodes (termed IgG nano-barcodes), we used a Y-shaped DNA structure to obtain three different color ratios of the DNA nano-barcodes: R2G0, R1G1, and R0G2. Here, “R” represents the red color fluorophore (Alexa 546), and “G” represents the green color fluorophore (Alexa 488), and the subscripts correspond to the ratio of the two colors in the nano-barcodes. For the DNA binding function, UA was hybridized separately to three distinct DNA nano-barcodes. The resulting hybridization products of UA and DNA nano-barcodes displayed clear electrophoretic shifts compared to DNA nano-barcodes alone (Supplementary Figure 2b). In addition, each shifted band exhibited a unique and expected fluorescence color ratio, suggesting the modular nature of our system in which the color labels can be preassigned. On the other hand, for testing IgG binding function, the UA-DNA nano-barcodes were incubated with three different IgG primary antibodies on a dot blot membrane. The resulting IgG nano-barcodes showed distinct fluorescence signals corresponding to the pre-assigned color ratios (Supplementary Figure 2c), confirming that EZZ protein remained fully functional after conjugation. Thus, UA that can bind to both DNA nano-barcodes and IgG primary antibodies demonstrated that we can use DNA to label IgG antibodies to replace secondary antibodies in a protein detection system.
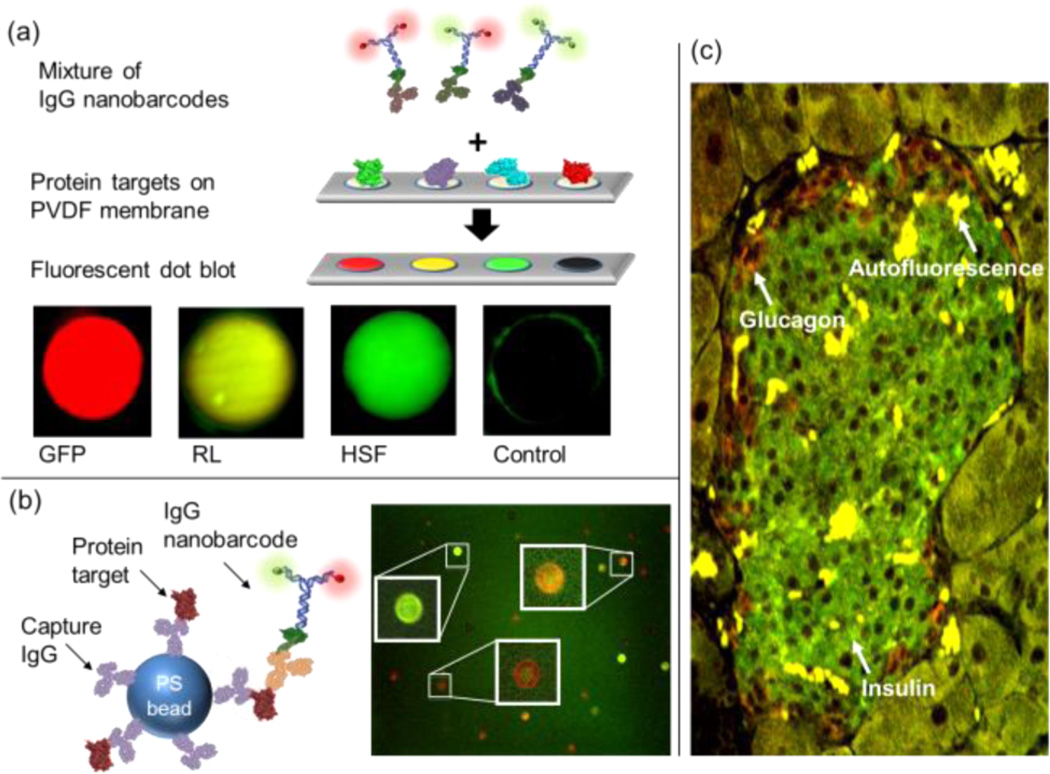
We first tested our IgG nano-barcodes for protein detection using the dot blot technique, one of the most commonly used methods to detect proteins. In this approach, we chose three different protein targets: green fluorescent protein (GFP), renilla luciferase (RL), and heat shock factor (HSF). Each protein was dot blotted onto polyvinylidene difluoride (PVDF) membranes and was individually detected with the corresponding IgG nano-barcode (Supplementary Figure 3a). To further show the use of our system in a multiplexed format, the aforementioned three protein targets were blotted together onto a membrane and incubated in the mixture of all three IgG nano-barcodes. As shown in Figure 2a, all three proteins were detected simultaneously and distinctively by IgG nano-barcodes using dot blot technique without any secondary antibodies. We note that the nano-barcode color ratios remained easily distinguishable, despite the potential for exchange reactions between the antibodies and DNA labels on different IgG nano-barcodes, suggesting that our IgG nano-barcodes were sufficiently stable for multiplexed detection under the conditions used here.
Figure 2.
Using IgG nano-barcodes for protein detection. (a) Multiplexed protein detection with dot blot. Proteins were spotted on a PVDF membrane and then incubated with a detection solution, which contained a mixture of IgG nano-barcodes. The dots with fluorescence signals showed specific binding between protein targets and the corresponding IgG nano-barcodes. From left to right: GFP, RL, HSF, and sortase (negative control) proteins were labeled with R2G0-anti-GFP, R1G1-anti-RL, and R0G2-anti-HSF IgG antibodies, respectively. Dot diameters were 250 µm for all images. Background fluorescence from GFP alone was subtracted from the measurement with IgG nano-barcodes. (b) Multiplexed bead-based protein detection. PS beads were tagged with the first IgG antibody which captured the antigen and then sandwiched with IgG nano-barcodes. GFP, RL, and HSF protein targets were specifically detected on microbeads with R2G0-anti-GFP (red), R1G1-anti-RL IgG antibodies (orange), and R0G2-anti-HSF (green), respectively. (c) IgG nano-barcodes for immunostaining of insulin and glucagon proteins in a diabetic mouse pancreas tissue. Insulin was stained with R0G2-antiinsulin (green) antibody, and glucagon was stained with R2G0-antiglucagon (red) antibody. Intense yellow regions are the autofluorescence of contaminated red blood cells.
In addition to the blot-based protein detection methods, microbeads have been increasingly employed as a convenient format for immunoassays such as ELISA, agglutination, and flow cytometry. For testing our IgG nano-barcode system with microbeads, we utilized the traditional sandwich strategy except that no secondary antibodies were involved. We first coated polystyrene (PS) microbeads with capture primary antibodies which specifically recognized target antigens, and we then used our IgG nano-barcodes as reporters to visualize the fluorescence signals (Figure 2b, left panel). We successfully detected three different targets with only two colors in E. coli lysates, which mimicked the realistic sample matrices (Supplementary Figure 3b). In the absence of targets, there was no fluorescence detected from the beads (Supplementary Figure 3b, bottom right). Moreover, when these targets were combined together, the multiplexed color ratios were not scrambled, confirming the robustness of the link between IgG and DNA nano-barcodes via our universal adapter (Figure 2b, right panel).
In contrast to dot blot and bead-based detection methods, in situ detection methods, including immunostaining for protein detection and fluorescence in situ hybridization (FISH) for DNA detection, can reveal not only the presence but also the spatial localization of targets. Combining the concepts of both immunostaining and FISH and further utilizing our UA, we carried out DNA-based protein detection in situ. We chose diabetic mouse pancreas tissue for in situ detection of insulin, and glucagon proteins. Individual proteins could be detected using IgG nano-barcodes with sensitivity comparable to that of traditional immunostaining methods using secondary antibodies (Supplementary Figure 4). In addition, IgG nano-barcodes allowed us to implement multiplexed protein detection in situ in which insulin and glucagon proteins were stained simultaneously in one step and visualized at their expected locations (Figure 2c). Conventional multiplexed immunostaining is often restricted due to the limited availability of secondary antibodies. Therefore, DNA nano-barcodes system in combination with UA provides a simple and flexible approach for labeling in multiplexed immunostaining applications.
To further explore the capability of our DNA-based protein detection system beyond fluorescence dyes, we replaced fluorophores with other reporter molecules. Using QDs and enzyme labels, we demonstrated the versatility of our system via dot blot and Western blot (WB). QDs are excellent labels for diagnostic applications due to their high brightness, stability, and compatibility with multiplexing.7 In order to use QDs for their higher quantum efficiency, we modified QDs with DNA which was subsequently hybridized to our universal adapter (UA-QDs). IgG primary antibodies were incubated with proteins on a PVDF membrane, and then UA-QDs were simply added to the membrane to recognize the IgG-protein targets (Figure 3a). This use of QDs in our system is very promising since it could enable detection of low-abundance proteins, which otherwise often need several fluorophore labels in order to be detectable. In addition to the fluorescence QDs, we chose one of the most commonly used enzymes incorporated with secondary antibodies, HRP, to detect proteins through enzymatic signal amplification. In our approach, HRP was conjugated to DNA and then hybridized to our UA to form UA-HRP. Similar to the previous approach for QDs, UA-HRP recognized the IgG-protein complexes on a WB membrane with a detection limit comparable to that of traditional WB using HRP-modified secondary antibody (Figure 3b). These results confirmed that our UA is a powerful platform molecule to link primary antibodies with a variety of reporters through DNA for protein detection.
Figure 3.
DNA-based protein detection system with different reporter molecules. (a) Protein detection using dot blot technique with quantum dots. Quantum dots were used to replace DNA nano-barcodes in our IgG nano-barcodes. HSF protein on PVDF membrane was recognized by its specific antibody and then labeled with UA-QDs. (b) Comparison of WB detection of HSF protein using DNA-modified HRP with IgG-UA (IgG-UA-HRP) (left) and traditional HRPmodified secondary antibody (right). Lanes 1–5, HSF protein detection using our IgG-UA-HRP at 0.25, 0.5, 0.75, 1, and 2 pmol; lanes 6–9, HSF protein detection using traditional WB method which utilized the HRP-modified secondary antibody at 0.25, 0.5, 0.75, and 1 pmol.
In conclusion, our DNA-based protein detection system is a versatile platform applicable to any methods that use IgG antibodies. By utilizing IgG nano-barcodes, we eliminated the need for secondary antibodies, thus avoiding undesirable interspecies cross-reactivity among secondary antibodies and overcoming the limited selection of primary and secondary antibody pairs. Moreover, the use of our universal adapter coupled with diverse DNA sequences allows us to link a library of different components, creating a modular capacity. This capacity is ideal for multiplexed detection; however, the noncovalent nature of the IgG-UA assembly also allows for the possibility of exchange reactions among IgG components in mixtures. Thus, we caution that, in using IgG nano-barcodes for multiplexed applications, conditions such as the time, temperature, and detergent concentrations should be optimized and controlled to minimize the potential for exchange reactions.
Furthermore, in addition to DNA nano-barcodes, our universal adapter can be hybridized to any DNA-modified material, such as fluorophores, quantum dots, enzymes, nanoparticles, or carbon nanotubes. Therefore, our DNAbased protein detection system is extensible to a wide range of readout modalities, including methods based on fluorescence, colorimetric, surface plasmon resonance, or Raman spectroscopy. By translating primary/secondary antibody recognition to DNA hybridization which is programmable, predictable, and precisely controllable, we have created a novel method platform for high-capacity, versatile, and multiplexed protein detection.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of Luo, March, and Lis laboratories for their help, advice, and input.
Footnotes
Supporting Information
Full experimental and characterization details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Renart J, Reiser J, Stark GR. Proc. Natl. Acad. Sci. U.S.A. 1979;76:3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Towbin H, Staehelin T, Gordon J. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Burnette WN. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 2.(a) Invitrogen. A guide to antibody labeling and detection. BioProbes 51. [accessed Aug 10, 2013]; http://www.invitrogen.com. [Google Scholar]; (b) Thermo Scientific. Choosing a secondary antibody: A guide to fragment specificity. [accessed Aug 10, 2013]; http://www.piercenet.com/files/TR0059-Choose-second-Ab.pdf. [Google Scholar]; (c) Labome.com. Western blot antibodies. [accessed Aug 10, 2013]; http://www.labome.com/review/westernblot-antibody.html. [Google Scholar]
- 3.(a) Sano T, Smith C, Cantor C. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]; (b) Jung Y, Lee JM, Jung H, Chung BH. Anal. Chem. 2007;79:6534–6541. doi: 10.1021/ac070484i. [DOI] [PubMed] [Google Scholar]; (c) Niemeyer CM. Angew. Chem. Int. Ed. 2010;49:1200–1216. doi: 10.1002/anie.200904930. [DOI] [PubMed] [Google Scholar]; (d) Niemeyer C. Chem. Soc. Rev. 2011;40:5910–5921. doi: 10.1039/c1cs15212b. [DOI] [PubMed] [Google Scholar]; (e) Morin I, Askin SP, Schaeffer PM. Analyst. 2011;136:4815–4821. doi: 10.1039/c1an15731k. [DOI] [PubMed] [Google Scholar]; (f) Saccà B, Meyer R, Erkelenz M, Kiko K, Arndt A, Schroeder H, Rabe KS, Niemeyer CM. Angew. Chem. Int. Ed. 2010;49:9378–9383. doi: 10.1002/anie.201005931. [DOI] [PubMed] [Google Scholar]; (g) Meyer R, Niemeyer CM. Small. 2011;7:3211–3218. doi: 10.1002/smll.201101365. [DOI] [PubMed] [Google Scholar]
- 4.(a) Nilsson B, Moks T, Jansson B, Abrahmsén L, Elmblad A, Holmgren E, Henrichson C, Jones TA, Uhlén M. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]; (b) Kuhara M, Takeyama H, Tanaka T, Matsunaga T. Anal. Chem. 2004;76:6207–6213. doi: 10.1021/ac0493727. [DOI] [PubMed] [Google Scholar]; (c) Jin T, Tiwari DK, Tanaka S, Inouye Y, Yoshizawa K, Watanabe TM. Mol. BioSyst. 2010;6:2325–2331. doi: 10.1039/c0mb00056f. [DOI] [PubMed] [Google Scholar]; (d) Mazzucchelli S, Colombo M, Palma CD, Salvadè A, Verderio P, Coghi MD, Clementi E, Tortora P, Corsi F, Prosperi D. ACS Nano. 2010;4:5693–5702. doi: 10.1021/nn101307r. [DOI] [PubMed] [Google Scholar]; (e) Thermo Scientific. Binding characteristics of antibody-binding proteins: Protein A, Protein G, Protein A/G and Protein L. [accessed Aug 10, 2013]; http://www.piercenet.com/files/TR0034-Ab-binding-proteins.pdf. [Google Scholar]
- 5.(a) Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. Nat. Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]; (b) Keppler A, Arrivoli C, Sironi L, Ellenberg J. BioTechniques. 2006;41:167–170ff. doi: 10.2144/000112216. [DOI] [PubMed] [Google Scholar]; (c) Jongsma MA, Litjens RH. Proteomics. 2006;6:2650–2655. doi: 10.1002/pmic.200500654. [DOI] [PubMed] [Google Scholar]; (d) Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, Trinquet E, Pin JP. Nat. Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Iversen L, Cherouati N, Berthing T, Stamou D, Martinez KL. Langmuir. 2008;24:6375–6381. doi: 10.1021/la7037075. [DOI] [PubMed] [Google Scholar]; (f) Engin S, Trouillet V, Franz CM, Welle A, Bruns M, Wedlich D. Langmuir. 2010;26:6097–6101. doi: 10.1021/la904829y. [DOI] [PubMed] [Google Scholar]; (g) Petershans A, Wedlich D, Fruk L. Chem. Commun. 2011;47:10671–10673. doi: 10.1039/c1cc12874d. [DOI] [PubMed] [Google Scholar]
- 6.(a) Li Y, Tseng YD, Kwon SY, d’Espaux L, Bunch JS, McEuen PL, Luo D. Nat. Mater. 2004;3:38–42. doi: 10.1038/nmat1045. [DOI] [PubMed] [Google Scholar]; (b) Um SH, Lee JB, Kwon SY, Li Y, Luo D. Nat. Protoc. 2006;1:995–1000. doi: 10.1038/nprot.2006.141. [DOI] [PubMed] [Google Scholar]; (c) Lee JB, Roh YH, Um SH, Luo D. Nat. Nanotechnol. 2009;4:430–436. doi: 10.1038/nnano.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li Y, Cu YT, Luo D. Nat. Biotechnol. 2005;23:885–889. doi: 10.1038/nbt1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Chan WC, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]; (b) Soman CP, Giorgio TD. Langmuir. 2008;24:4399–4404. doi: 10.1021/la704078u. [DOI] [PubMed] [Google Scholar]; (c) Zrazhevskiy P, Sena M, Gao X. Chem. Soc. Rev. 2010;39:4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Giri S, Sykes EA, Jennings TL, Chan WC. ACS Nano. 2011;5:1580–1587. doi: 10.1021/nn102873w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.