Abstract
Objective
To evaluate injection of microcrystalline sodium urate (MSU) for inducing articular pain in green-cheeked conures (Pyrrhura molinae) and the analgesic efficacy of liposome-encapsulated butorphanol tartrate (LEBT) by use of weight load data, behavioral scores, and fecal corticosterone concentration.
Animals
8 conures.
Procedures
In a crossover study, conures were randomly assigned to receive LEBT (15 mg/kg) or liposomal vehicle subsequent to experimental induction of arthritis or sham injection. The MSU was injected into 1 tibiotarsal-tarsometatarsal (intertarsal) joint to induce arthritis (time 0). Weight-bearing load and behavioral scores were determined at 0, 2, 6, 26, and 30 hours.
Results
MSU injection into 1 intertarsal joint caused a temporary decrease in weight bearing on the affected limb. Treatment of arthritic conures with LEBT resulted in significantly more weight bearing on the arthritic limb than treatment with vehicle. Administration of vehicle to arthritic conures caused a decrease in activity and feeding behaviors during the induction phase of arthritis, but as the arthritis resolved, there was a significant increase in voluntary activity at 30 hours and feeding behaviors at 26 and 30 hours, compared with results for LEBT treatment of arthritic birds. Treatment with LEBT or vehicle in conures without arthritis resulted in similar measurements for weight bearing and voluntary and motivated behaviors.
Conclusions and Clinical Relevance
Experimental induction of arthritis in conures was a good method for evaluating tonic pain. Weight-bearing load was the most sensitive measure of pain associated with induced arthritis. Pain associated with MSU-induced arthritis was alleviated by administration of LEBT.
Adequate pain management is important to the therapeutic treatment of all animals, including birds.1 The International Association for the Study of Pain has ascertained that pain and analgesia assessment varies considerably among species and has called for more studies that can outline species-specific differences in pain and pain-related behavior.1 Green-cheeked conures (Pyrrhura molinae) are a member of the family Psittacidae, but they are considerably smaller than commonly studied birds of this family, such as Amazon parrots (Amazona spp), cockatoos (Cacatua spp), and African grey parrots (Psittacus erithacus). Clinically, smaller parrots are treated similar to their larger counterparts, but extrapolations of results of studies are speculative at best, and it remains necessary to assess the needs and adaptations of these smaller birds to pain and analgesia. Administration of butorphanol (1 mg/ kg) has an isoflurane-sparing effect in anesthetized grey parrots and cockatoos; however, this effect was not detected in anesthetized Amazon parrots, perhaps indicating inherent differences within psittacine species and the need to quantify the analgesic requirements among its members.2,3
Butorphanol tartrate, a mixed opioid agonist-antagonist with µ-opioid receptor antagonism and κ-opioid receptor agonism, is currently considered the analgesic drug of choice for management of acute and chronic pain in birds.4 Pharmacodynamics and serum concentration data for butorphanol tartrate in African grey parrots and Hispaniolan parrots (Amazona ventralis), in addition to pharmacokinetic data for red-tailed hawks (Buteo jamaicensis) and great-horned owls (Bubo virginianus), suggest that the commercially available tartrate form administered IM may need to be repeated every 1 or 2 hours to maintain analgesic effects and serum concentrations.5–8 The liposomal formulation, although not commercially available, is suited for analgesia studies because a single treatment of 15 mg/kg provides analgesic effects in Hispaniolan parrots for a minimum of 72 hours.7
The MSU method for inducing articular pain has been applied in mammals, chickens, and pigeons and is considered appropriate for use in conures because it enables comparison with results for other psittacines with pain induced by use of this method.9–20 Injection of MSU into the tibiotarsal-tarsometatarsal (intertarsal) joint induces temporary, self-resolving arthritic pain.9 Pain and the effects of drugs with analgesic properties can be quantitatively assessed by weight load testing designed for use in perching avian species, by scoring voluntary and motivated behaviors with an ethogram developed for use in Hispaniolan parrots with arthritis, and by measuring fecal corticosterone concentrations.9
To our knowledge, the use of MSU to induce temporary arthritis in conures has not been investigated. Therefore, the objectives of the study reported here were to evaluate the use of MSU to experimentally induce tonic pain in conures, to evaluate analgesic properties of LEBT treatment, and to compare 3 methods (quantified weight load testing, scoring of voluntary and motivated behavior, and measurement of fecal corticosterone concentrations) for assessing pain-related physiologic and behavioral responses in conures.
Materials and Methods
Animals
A uniform population of 8 male green-cheeked conures (age, approx 5 years; mean ± SD body weight, 68.56 ± 3.55 g) was used in the study. The conures were bred in captivity and obtained from a commercial source.a Each conure had an aluminum identification band on the left tarsometatarsal region. Conures were considered healthy before and throughout the study.
Conures were maintained at a research facility at the School of Veterinary Medicine, University of Wisconsin, Madison. Conures were housed separately in adjacent stainless-steel laboratory cages (61 X 61 X 63.5 cm). Each cage contained a perch and a hanging toy. Conures were maintained on a cycle of 12 hours of light and 12 hours of darkness, fed a commercial pelleted diet formulated for psittacine birds,b and provided water ad libitum. The Institutional Animal Care and Use Committee of the School of Veterinary Medicine, University of Wisconsin, Madison, approved the experimental protocol.
Preparation of MSU
Preparation of the sodium urate monohydrate crystals has been described else where9 in accordance with a published method.21 Crystals were pipetted in 7-mL aliquots into sterile 2-dram vials. Each dose contained 2 mg of 8% uric acid crystals suspended in sterile saline (0.9% NaCl) solution. Prior to use, each aliquot was gently sonicated to reduce the size of the crystals so that they would be compatible with injection via a 25-gauge needle.
Preparation of LEBT
Powdered butorphanol tartratec (52 mg) was dissolved in 1 mL of 10mM sodium citrate buffer (pH, 4.0) and sterilized by use of a 0.22-µm filter.d A film of 80µM egg phosphatidylcholine was dried onto the walls of a 20-mm test tube by use of a rotating water bath (set at 45°C) connected to a suction device. Filtered t-butanol (1 mL) was added to the lipid film. The resulting lipid foam was maintained at 23°C for 1 hour; the solution was vortexed periodically. The lipid foam was frozen on dry ice for 30 minutes and then freeze-dried for at least 24 hours. The lipid foam was overlaid with 1 mL of the butorphanol tartrate solution. The drug solution–lipid mixture was maintained at 23°C for 1 hour with periodic vortexing. The mixture was transferred to dry ice for 20 minutes and then stored at −20°C. Liposomes were thawed at 22°C for 60 minutes. Saline-acetate buffer (10 mL of a 10mM solution) was added to the liposomes, and the suspension was gently vortexed. Liposomes were pelleted by ultracentrifugatione at 100,000 X g for 30 minutes at 4°C. Supernatant was removed, and the pellet was resuspended in 2 mL of fresh saline-acetate buffer.
As described in another study,9 liposomes were stored at 5°C until quantitated and used in experiments. Liposome preparations containing butorphanol tartrate were quantitated by suspending 200 µL of the liposome preparation in a solvent solution containing 600 µL of methanol and 200 µL of chloroform and agitating the solution gently on a test tube vortex. The resulting solution was placed in a cuvette, and absorbance was determined at 281 nm. Saline-acetate buffer suspended in the same solvent solution was used as a blank sample. Liposomes containing saline solution (ie, liposomal vehicle) were used as negative control treatments for in vivo experiments.
Experimental induction of arthritis
Each conure was anesthetized by mask induction with 5% isoflurane in 1 L of oxygen/min. Anesthesia was maintained by administration of 2% to 3% isoflurane in 1 L of oxygen/ min by use of a Bain circuit nonrebreathing system. Hot packs were used to aid in maintaining body temperature, and eye ointmentf was topically applied to provide corneal protection. Each conure was weighed, and the distal portion of the tibiotarsus and proximal portion of the tarsometatarsus region of the right (unbanded) pelvic limb were prepared by gentle plucking of feathers and application of chlorohexidine scrub and water.
To induce arthritis, a 25-gauge needle was inserted toward the plantar aspect of an intertarsal joint, and 0.05 mL of sterile 8% MSU suspension was injected. The joint was flexed and extended for 20 to 30 seconds to evenly distribute the MSU within the joint space. Sham treatment included anesthesia, preparation of the injection site, and flexion and extension of the joint, but not introduction of a needle or injection into the joint. Prior to cessation of anesthesia, the areas between the ventrolateral body wall and proximal medial femoral region were prepared with isopropol alcohol and the treatments (LEBT or liposomal vehicle) were injected SC by use of a 23-gauge needle. Isoflurane was then discontinued; when the righting reflex was detected, each conure was placed in a warm incubator for recovery.
Study design
The 8 conures were randomly allocated into 2 groups (4 conures/group). The total testing period was 12 weeks, with a minimum 2-week washout period between subsequent treatments.
The groups were initially used in a 2-period crossover experiment to evaluate the 2 treatments (LEBT or liposomal vehicle [control treatment]) for conures that underwent sham procedures for induction of arthritis (ie, no interarticular injection). After sham injection, 1 group received the LEBT first and the other group received the control treatment first; after a washout period, the sham injections were repeated but the order of the treatments was reversed. Time of sham injections and administration of treatments was designated as time 0. After another washout period, the groups then completed a second 2-period crossover experiment in which arthritis was induced and the 2 treatments were administered. After injection of MSU, conures in 1 group received the LEBT treatment (15 mg/kg, 0.6 mL, SC), whereas the other group received the control treatment (0.6 mL of liposome vehicle, SC); half of each dose was injected into the left side of each conure, and the other half of each dose was injected into the right side of each conure. After a washout period, the conures were again injected with MSU to induce arthritis, but the order of the treatments was reversed. Time of MSU injections and administration of treatments was designated as time 0. Thus, the 4 treatments of interest were sham injection followed by the control treatment (sham-control), sham injection followed by LEBT treatment (sham-LEBT), MSU-induced arthritis followed by the control treatment (arthritis-control), and MSU-induced arthritis followed by the LEBT treatment (arthritis-LEBT). This sequence of evaluation was selected because it was not known whether MSU-induced arthritis would have long-term effects in such small birds.
The MSU method used for this study was evaluated in conures receiving both analgesic and control treatments because, to our knowledge, this method had not been used in a small psittacine species; thus, it required a more thorough validation. Although the AVMA does not recommend inclusion of a placebo control group in a study such as this,22 this method has been used in a number of other species, including larger parrots, and the mild nature and duration of the pain were limited to a short period, which we believe made it appropriate to include the use of untreated control birds. In addition, the protocol included a requirement to remove any conure from the study when the bird had signs of excessive pain (such as loss of appetite or reluctance to move about the cage for periods of > 1 hour) following MSU injections to induce arthritis; conures removed because of excessive pain were to be provided additional analgesia and supportive care.
Assessment of pain and analgesia
During the 2 weeks preceding the onset of the study, the conures were conditioned to perch on an incapacitance meter perch (three 30-second intervals twice daily). Subsequently, each conure was placed in its home cage, and a food reward was hung on the front of the cage to habituate the bird to the motivated task of attempting to get the food reward. The response to treatment was evaluated by use of 3 methods.
Weight load testing
The weight load for each foot was obtained by use of an incapacitance meterg originally designed for force plate analysis in rodents. The rodent footpads were converted into 2 perching rods (1 for each foot). Conures were placed in a black rigid plastic box (11.5 X 23 X 27 cm) that had a clear front and a hinged door that fit over the perching rods to limit movement during measurements. The incapacitance meter used dual-channel weight averaging that enabled concurrent testing of both limbs. When a conure shifted from one foot to the other foot, the unit recorded the average weight load placed on each foot during a predetermined test period of 30 seconds. Weight load values were recorded for 3 consecutive 30-second intervals at 1 week before injections and at 0, 2, 6, 26, 30, 50, 54, 74, and 98 hours.
Voluntary and motivated behaviors
Behaviors found to be significantly affected in a study9 of MSU-induced arthritis in Hispaniolan parrots (a larger species of parrot) were evaluated in the conures. Additional behaviors in clinically normal conures housed individually in laboratory cage conditions were added to the ethogram. Eighteen behaviors were defined and scored or measured in terms of duration, intensity, or frequency by use of a scoring system described for use in Hispaniolan parrots.9 One additional behavior scored for the conures was the number of minutes spent in the proximity of the food dish (range, 0 to 15).
To avoid observer effects on behavior, a digital video camerah was used to remotely record the activity of each conure for 12 to 15 minutes at 0, 2, 4, 6, 26, 30, 50, 54, 74, and 98 hours. To optimize video imaging, cage doors were removed and a thin wire mesh (1 cm2) was placed over the front of each cage prior to video recording. A food reward was attached via a paperclip or cardboard tray to the front of each cage immediately prior to recording. Recordings were edited by use of a software programi and transferred to a DVD. Two investigators evaluated images and provided scores, and a mean value was calculated. Behaviors were compared among treatments at each time point, including scores at time 0.
Fecal corticosterone concentrations
Fecal samples were collected from each conure at each of the testing time points and frozen at −70°C until analysis. Fecal samples obtained from all birds at 0, 6, and 26 hours were analyzed to determine corticosterone concentrations by use of a radioimmunoassay kit at an endocrine laboratory.j
Statistical analysis
Data were calculated to reflect weight bearing of the MSU-injected or sham-injected limb by use of a simplified equation derived from one used in another study9 to account for the small size of the conures. The equation was as follows: change in weight bearing = (mean weight bearing of MSU-injected [or sham-injected] limb – mean weight bearing of MSU-injected [or sham-injected] limb at time 0). Weight load data and fecal corticosterone concentrations were analyzed by use of statistical software.k A repeated-measures ANOVA was used with fixed effects of treatment, time, and treatment-by-time interactions. A random effect of conure was used to account for correlations among observations on the same conure. Residuals resulting from the fitted model were verified to be normally distributed and had no evidence of heteroskedasticity. Pairwise comparisons of treatments, both within each time point and over all time points, were performed by use of the Tukey P value correction to account for multiple comparisons. Emphasis was placed on detecting changes in the dependent measures (drug-induced responses vs control responses) within and among treatments. Significance was inferred at values of P ≤ 0.05.
Data for voluntary and motivated behaviors were ordinal or binary and were analyzed by use of nonparametric analyses.l To reduce the number of behaviors for analysis, the Spearman rank order correlation was used to identify and remove ordinal behaviors that were substantially similar. For all remaining behaviors, treatments were compared on a pairwise basis for each time point by use of a 2-tailed Wilcoxon signed rank test. Significance for these analyses was also inferred at values of P ≤ 0.05.
Results
Weight-bearing load
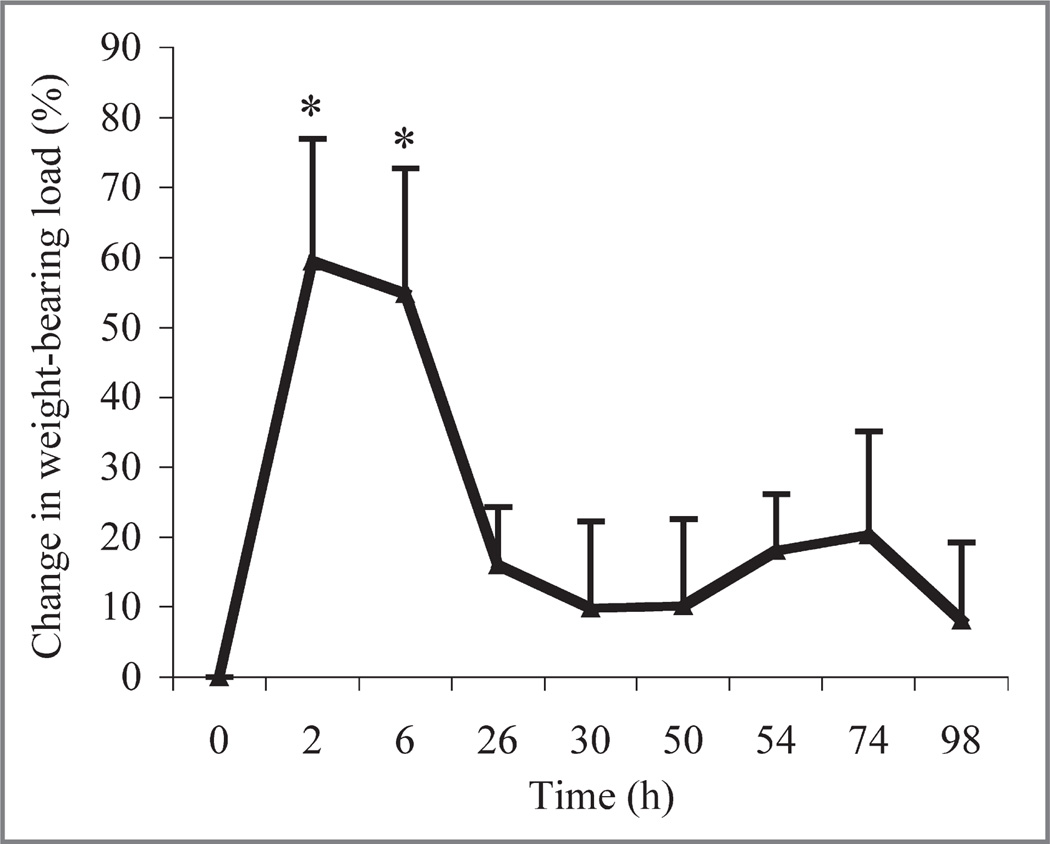
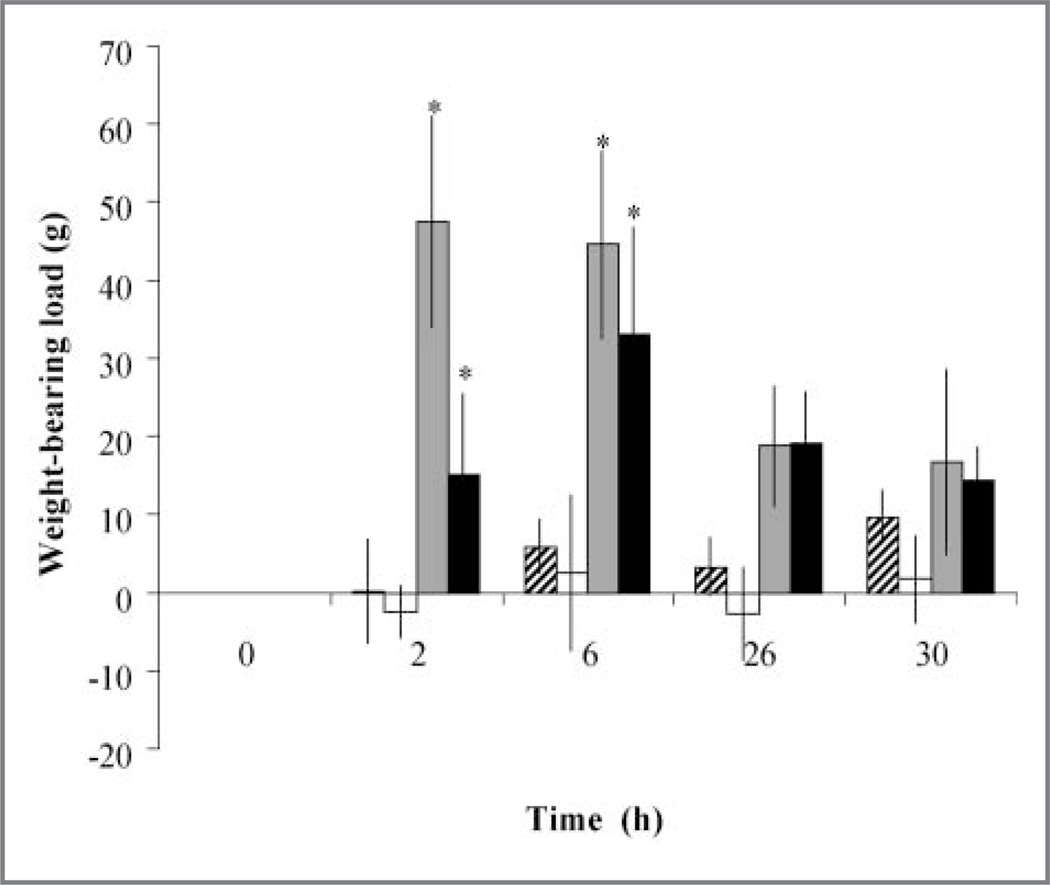
Interarticular injection of MSU into 1 intertarsal joint successfully induced a resolving arthritis in conures, as determined by a change in weight-bearing load (Figure 1). There was a significant (P = 0.01) overall treatment effect of the arthritis. The arthritis-control treatment resulted in significantly (P = 0.01) less weight bearing on the affected limb at 2 and 6 hours, compared with values obtained at 0 hours. Lameness resolved at 26 hours. The arthritis-control treatment resulted in significantly (P = 0.01) less weight bearing on the arthritic limb throughout the study, compared with results for arthritis-LEBT, sham-control, or sham-LEBT treatments (Figure 2). The sham-LEBT or arthritis-LEBT treatments resulted in similar results throughout the study. The arthritis-LEBT treatment resulted in significantly (P = 0.01) higher weight-bearing capacity on the arthritic limb throughout the study, compared with results for the arthritis-control treatment. At 6 hours, arthritis-control or arthritis-LEBT treatments had the lowest weight-bearing capacity on the arthritic limb, although arthritis-LEBT treatment enabled conures to bear significantly (P = 0.02) more weight on the arthritic limb, compared with the arthritis-control treatment. There was not a significant (P = 0.829) difference in weight bearing between the sham-LEBT and sham-control treatments.
Figure 1.
Mean ± SD percentage change in weight-bearing load determined at various time points for 8 green-cheeked conures (Pyrrhura molinae) after injection of MSU into a single tibiotarsal-tarsometatarsal (intertarsal) joint to induce arthritis followed by SC administration of 0.6 mL of liposome vehicle solution (control treatment). The equation used to determine percentage change in weight-bearing load at each time point was as follows: ([mean weight bearing of MSU-injected limb at 0 hours - mean weight bearing of MSU-injected limb at time point]/mean weight bearing of MSU-injected limb at 0 hours) X 100. Higher values indicate greatest change, decreasing the weight bearing on the limb with experimentally induced arthritis. *Value differs significantly (P≤ 0.05) from the value at 0 hours.
Figure 2.
Mean ± SD change in weight-bearing load in 8 conures during a 30-hour period after sham injection of an intertarsal joint followed by administration of the control treatment (diagonal-striped bars), sham injection of an intertarsal joint followed by administration of LEBT (15 mg/kg, SC [white bars]), injection of MSU into an intertarsal joint followed by administration of the control treatment (gray bars), and injection of MSU into an intersal joint followed by administration of LEBT (15 mg/kg, SC [black bars]). The sham injections or MSU injections were administered at time 0, and the treatments were administered immediately thereafter. Change in weight-bearing load is expressed as the change from 0 hours and was calculated for each testing point for each bird by use of the following equation: (mean weight bearing of noninjected limb at time point – mean weight bearing of noninjected limb at 0 hours)/(mean weight bearing of MSU-injected [or sham-injected] limb at time point – mean weight bearing of MSU-injected [or sham-injected] limb at 0 hours). Higher values indicate an increase in weight bearing on the noninjected limb and a decrease in weight bearing on the MSU-injected or sham-injected limb. Throughout the study, values for MSU injection followed by the control treatment resulted in a significantly (P ≤ 0.05) greater percentage change in weight-bearing load on the injected limb, compared with results for the other treatments. *Within a treatment, the value differed significantly (P ≤ 0.05) from the value at time 0. See Figure 1 for remainder of key.
Behavioral assessment
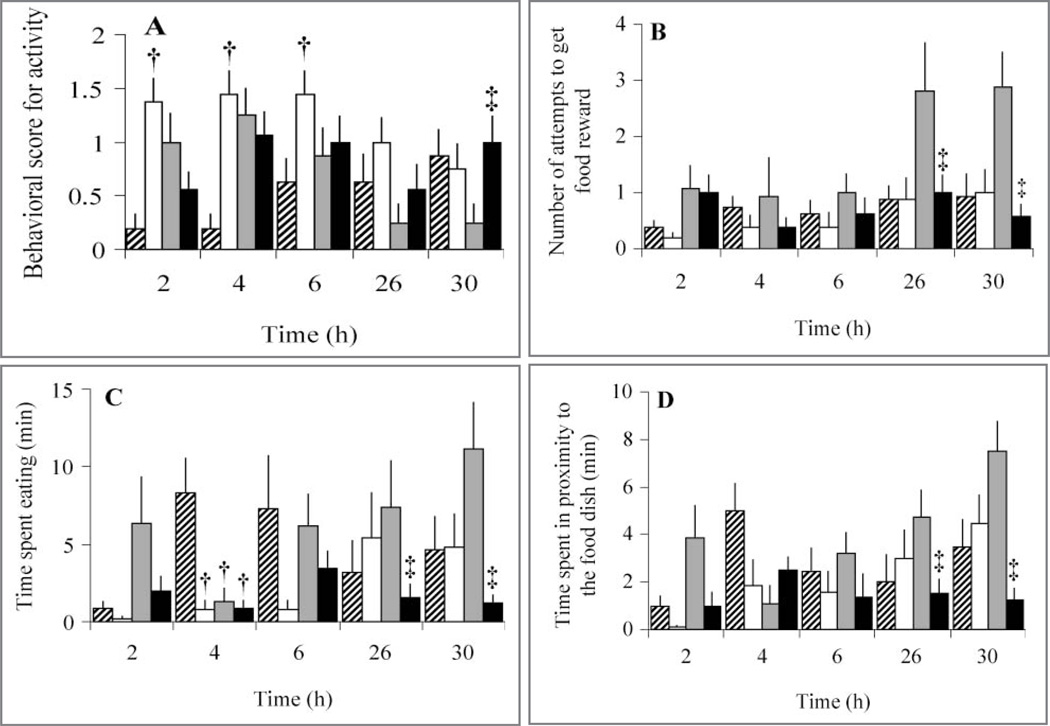
Behavioral scores were compared among all treatments at all time points in the study. Behavioral scores differed significantly among treatments during the first 30 hours of experimentally induced arthritis (Table 1). Behaviors differed significantly between LEBT and control treatments (with or without experimentally induced arthritis) at > 1 time point (Figure 3). Comparing sham-control and sham-LEBT treatments, most differences in behavior were at 2 to 6 hours after administration of the analgesic. Sham-LEBT treatment resulted in a decrease in motor activity behaviors, compared with results for the sham-control treatment, as indicated on the basis that activity scores increased because moving about the cage decreased significantly (P = 0.01) at 2, 4, and 6 hours and locomotion scores increased because use of both limbs decreased significantly (P = 0.01) at 2 hours. Similarly, inactivity time was significantly (P = 0.02) greater and time periods were significantly (P = 0.02) higher for the sham-LEBT treatment at 4 hours, and time hanging from the cage top was also significantly (P = 0.04) less at 2 hours for the sham-LEBT treatment. Feeding behaviors for the sham-LEBT treatment also differed significantly at 2 and 6 hours. Sham-LEBT treatment resulted in significantly less time spent eating at 2 (P = 0.03), 4 (P = 0.01), and 6 (P = 0.01) hours and significantly (P = 0.01) less time in the proximity of the food dish at 4 hours, compared with results for the sham-control treatment. In contrast, sham-LEBT treatment had a significantly faster response time to get the food reward (P = 0.01) and fewer attempts to get the food reward (P = 0.04) at 2 hours, compared with results for the sham-control treatment.
Table 1.
Differences in behavioral scores for 8 green-cheeked conures (Pyrrhura molinae) during a 30-hour period after sham injection of a tibiotarsal-tarsometatarsal (intertarsal) joint followed by administration of the control treatment (sham-control), sham injection of an intertarsal joint followed by administration of LEBT (15 mg/kg, SC; sham-LEBT), injection of MSU into an intertarsal joint followed by administration of the control treatment (arthritis-control), and injection of MSU into an intersal joint followed by administration of LEBT (15 mg/kg, SC; arthritis-LEBT). The sham injections or MSU injections were administered at time 0, and the treatments were administered immediately thereafter.
| Time (h) | Sham-control vs sham-LEBT |
Sham-control vs arthritis-control |
Sham-control vs arthritis LEBT |
Sham-LEBT vs arthritis-control |
Sham-LEBT vs arthritis LEBT |
Arthritis-control vs arthritis LEBT |
|---|---|---|---|---|---|---|
| 2 | Time from introduction of food reward to first contact with reward* (P = 0.004) |
— | — | Time from introduction of food reward to first contact with reward‡ (P = 0.043) |
Time from introduction of food reward to first contact with reward† (P = 0.004) |
— |
| Attempts made to get food reward* (P = 0.043) |
Picking at feathers on injected limb† (P = 0.007) |
Attempts made to get food reward§ (P = 0.026) |
||||
| Perching grasp‡ (P = 0.043) | ||||||
| Activity† (P < 0.001) | Hanging from top of cage‡ (P = 0.043) |
No. of attempts made to get food reward§ (P = 0.017) |
||||
| Locomotion† (P = 0.003) | ||||||
| Hanging from top of cage* (P = 0.043) |
Time spent eating‡ (P = 0.024) |
Picking at feathers on injected limb† (P = 0.007) |
||||
| Time spent eating* (P = 0.028) |
Use of injected limb to hold food while eating it‡ (P = 0.043) |
Activity† (P = 0.002) | ||||
| Time spent in proximity of food dish§ (P = 0.033) |
||||||
| Hanging from top of cage† (P = 0.043) |
||||||
| Time spent eating§ (P = 0.044) |
||||||
| Use of injected limb to hold food while eating it† (P = 0.043) |
||||||
| 4 | Activity† (P< 0.001) | Activity‡ (P = 0.008) | Activity§ (P = 0.005) | — | Grooming‡ (P = 0.026) | |
| Time spent in proximity of food dish* (P = 0.010) |
Time spent in proximity of food dish* (P = 0.001) |
Time spent in proximity of food dish* (P = 0.002) |
||||
| Time spent eating* (P = 0.001) |
Time spent eating* (P = 0.002) |
Time spent eating* (P = 0.002) |
||||
| Inactive† (P =0.002) | Locomotion‡ (P = 0.032) | |||||
| 6 | Activity‡ (P = 0.006) | — | — | No. of attempts to get food reward‡ (P = 0.005) |
Grooming† (P = 0.003) | — |
| Time spent eating* (P = 0.012) |
Grooming† (P = 0.001) | Picking at feathers on injected limb† (P = 0.003) |
||||
| Time spent in proximity of food dish‡ (P = 0.021) |
Hanging from top of cage§ (P = 0.043) |
|||||
| Hanging from top of cage‡ (P = 0.043) |
Time spent eating§ (P = 0.002) |
|||||
| Time spent eating‡ (P < 0.001) |
||||||
| 26 | — | Time spent in proximity of food dish‡(P = 0.004) |
— | No. of attempts to get food reward‡ (P = 0.032) |
— | Time spent in proximity of food dish§ (P = 0.004) |
| Activity† (P = 0.023) | ||||||
| Time spent eating‡ (P = 0.019) |
Time spent eating‡ (P = 0.015) |
|||||
| 30 | — | No. of attempts to get food reward‡ (P = 0.021) |
— | No. of attempts to get food reward‡ (P = 0.026) |
— | No. of climbs on wire front cage‡ |
| Inactive‡ (P = 0.030) | ||||||
| Time spent in proximity of food dish‡(P = 0.037) |
Activity§ (P = 0.043) |
|||||
| Inactive time§ (P = 0.020) |
||||||
| Time spent in proximity of food dish§ (P = 0.001) |
||||||
| Time spent eating‡ (P = 0.004) |
Behavioral scores were determined during a 15-minute period at 0, 2, 4, 6, 26, and 30 hours. Values were considered significant at P ≤ 0.05.
Within a comparison, the sham-control treatment had the higher mean score.
With in a comparison, the sham-LEBT treatment had the higher mean score.
With in a comparison, the arthritis-control treatment had the higher mean score.
With in a comparison, the arthritis-control treatment had the higher mean score.
Within a comparison, no significant differences in behavioral scores between treatments.
Figure 3.
Mean ± SD behavioral scores for activity (ie, moving about the cage; A), number of attempts to get a food reward (B), number of minutes spent eating (C), and number of minutes spent in the proximity of the food dish (D) during a 15-minute observation period in 8 conures after sham injection or injection of MSU into 1 intertarsal joint (time 0) and subsequent administration of LEBT or the control treatment. †Within a time point, value differs significantly (P ≤ 0.05) from the value for the sham injection followed by the control treatment. ‡Within a time point, value differs significantly (P ≤ 0.05) from the value for the MSU injection followed by the control treatment See Figures 1 and 2 for remainder of key.
Arthritis-LEBT or arthritis-control treatments did not result in significant differences in behavioral scores until the arthritis resolved at 26 and 30 hours. The arthritis-control treatment resulted in significant differences at 30 hours for activity score (a decrease because movement about the cage was increased [P = 0.04] and an increase in the number of climbs on the front of the cage [P = 0.01]), compared with results for the arthritis-LEBT treatment (Figure 3). Feeding behaviors were also affected between 26 and 30 hours, with the arthritis-control treatment resulting in significantly more time spent eating at 26 (P = 0.02) and 30 (P = 0.01) hours and more time in the proximity of the food dish at 26 (P = 0.01) and 30 (P = 0.01) hours, compared with results for the arthritis-LEBT treatment.
Few differences in behaviors were detected between the sham-control and arthritis-LEBT treatments. At 4 hours, arthritis-LEBT treatment resulted in significantly (P = 0.01) higher activity scores (which indicated little activity), less time spent eating, and less time spent in the proximity of the food dish, compared with results for the sham-control treatment. Conversely, at 2 hours, arthritis-LEBT treatment resulted in a significant increase in motor activity (reflected by an increased number of climbs on the front of the cage [P = 0.02] and lower scores for activity [P = 0.01] and locomotion [P = 0.05]), compared with results for the sham-LEBT treatment. The lower scores were indicative of near-normal amounts of activity and locomotion. Similarly, at 26 and 30 hours, arthritis-LEBT treatment resulted in more time spent eating and more time spent in the proximity of the food dish, compared with results for the sham-LEBT treatment. In addition, motivated behavior to obtain the food reward had a lower time from introduction of the food reward until first contact with the reward and more attempts to get the food reward for the arthritis-LEBT treatment, compared with results for the sham-LEBT treatment.
Fecal corticosterone concentration
Fecal corti-costerone concentrations were highly variable among conures, with median values of 291.45, 323.12, and 390.24 ng/g of dry feces at 0, 6, and 26 hours. There were no significant differences among treatments or time points.
Discussion
Green-cheeked conures were used as small psittacines for analgesic testing. Injection of MSU into a single intertarsal joint induced arthritis that was self-limiting and resolved at 26 hours, which was similar to results for larger Hispaniolan parrots.9 The greatest change in weight bearing was detected during the first 6 hours after intra-articular MSU injection when it was expected that the conures had the most pain in the injected limb. Weight load testing of the pelvic limb by use of an incapacitance meter adapted for use in parrots provided reliable and repeatable objective results. Compared with the larger Amazona species, the conures had a lighter body weight that resulted in lower weight loads and smaller changes in weight load. The smaller body weight may have also decreased the sensitivity of weight load data because a significant difference from 0 hours was only detected during the first 6 hours after MSU injection, whereas differences were detected for 26 hours in Amazon parrots.9 The equation for comparing changes in weight-bearing load in the conures used a simple calculation of the weight load on the arthritic pelvic limb. Although statistical outcomes were similar for a more complex equation that accounted for the differences between both pelvic limbs, the simple equation that used weight bearing on the arthritic limb at each data collection point (compared with values at time 0) provided the largest values for comparison among treatments. Alternatively, the rapid resolution and return to pretreatment weight bearing in conures may be attributable to faster resolution of experimentally induced arthritis, compared with resolution in the larger Hispaniolan parrots. It is doubtful that this can be attributed to the smaller volume of MSU (50% less) injected into the intertarsal joint of conures, compared with the volume injected in the Hispaniolan parrots, because the volume was proportionally larger when body size was compared (mean body weight of conures is 25% of the mean body weight of Hispaniolan parrots).
The study reported here is the first in which the effects of LEBT in perching in birds without arthritis have been evaluated. Weight bearing in conures was similar for sham-LEBT and sham-control treatments; therefore, butorphanol did not interfere with perching ability.
Differences in activity and eating behaviors associated with butorphanol effects were detected for sham injections followed by LEBT treatment, compared with sham injections followed by the control treatment (Figure 3). Differences were detected at early time points (typically 2 to 4 hours after injection of LEBT) and were consistent with mild sedative and appetite suppressant effects of opioid drugs (ie, the conures were less active and spent less time eating soon after administration of LEBT). Additionally, feeding behaviors and voluntary activity increased at 26 and 30 hours after sham injections followed by LEBT treatment, which are time points when concentrations of LEBT would be expected to be lower.9 However, differences in behavioral scores between arthritis-LEBT or the arthritis-control treatments could not be interpreted as easily and did not reflect the same degree of early sedative and appetite suppressant effects of LEBT evident for the sham injections (ie, when there was no experimentally induced arthritis). Except for grooming, which differed significantly between arthritis-LEBT and arthritis-control treatments at 4 hours, significant differences in voluntary motor activity and appetite were detected only at later time points (26 and 30 hours). The increase in motor activity and feeding behaviors for the arthritis-control treatment for later time points (ie, after the experimentally induced arthritis had presumably resolved) may have been compensatory behavior for pain during the first 26 hours, whereas the arthritis-LEBT treatment maintained lower but consistent behavioral scores throughout the study period. Compensatory behavior would be difficult to assess quantitatively, but the possibility has credence because of the observation that there were no differences in the same behavioral measures at 26 or 30 hours for the sham-LEBT or sham-control treatments. Additionally, the motor activity scores returned to normal at 26 hours, and there was higher than normal wire climbing activity at 26 and 30 hours. In contrast, there was a pattern for a gradual improvement in appetite, with low scores at 4 hours and a steady increase in the amounts of time spent eating and spent in the proximity of the food dish by 30 hours. This indicated the effect that pain can have on activity and appetite. The arthritis-LEBT treatment resulted in consistent activity and eating behaviors throughout the study.
Another interpretation could attribute these observations to sedative and appetite-suppressant effects of the long-acting liposomal preparation being evident in conures for up to 30 hours. Serum concentrations of LEBT have been characterized in Hispaniolan parrots7; a single SC injection can provide therapeutic concentrations of butorphanol in serum for at least 5 days. In that study,7 frank sedation was only detected in the Hispaniolan parrots during the first hour after the butorphanol injection. Although sedation scores were not recorded in the study reported here, the results were consistent with the gross observations of conures by the researchers. Appetite-suppressant effects may be subtle and may persist longer than sedative effects.
Rebound hyperalgesia can develop in humans and other animal subjects after opioid analgesics have been discontinued but there is still a pain stimulus (eg, residual pain from a surgical incision) or a pain stimulus are provided after the discontinuation of pain medication (eg, a second surgical procedure).23 The possibility that the results in the study reported here were attributable to late sedative effects or to rebound hyperalgesia is relatively remote. Expected blood concentrations of butorphanol in the conures, as determined on the basis of another study7 conducted by our laboratory group, would not be expected to cause sedation and appetite suppression at 24 to 30 hours after administration. Conversely, the conures should have had adequate serum drug concentrations at that time to prevent development of rebound hyperalgesia. Pharmacokinetic experiments with the LEBT preparation in conures would be necessary to definitively determine the blood concentration of butorphanol in this species, but use of similar liposomal opioid drug preparations in mammals has yielded results similar to those obtained in our pharmacologic studies7,24,25 in Hispaniolan parrots. Also, the MSU-induced arthritis resolved by 26 hours after injection (as indicated by the results of the weight loading experiments) in the Hispaniolan parrots9 and conures; therefore, the birds would not be expected to have pain at later time points.
Results for this study in conures, compared with results for the study9 in Hispaniolan parrots, illustrated the variability between species within the same taxonomic family regarding behavioral response to arthritic pain and analgesic treatment. A general observation made during the course of this study was that behavioral measures serving as reliable predictors of the response to experimentally induced arthritis in Hispaniolan parrots were not as reliable in conures. This was particularly true of motivated behaviors such as the number of attempts to get a food reward and time from introduction of a food reward to first contact with the reward. Conures were not highly motivated by the food reward offered, and more time may have been needed to condition them to the food reward. Behaviors not directly associated with appetite or motor activity were more difficult to identify in conures with arthritic pain. The conures used in this study were obtained from a different source than the source from which we obtained the Hispaniolan parrots, and the conures may have had less exposure to handling and habituation with humans. These small parrots may require a longer period to adapt to solitary living, a new environment, new stimuli, and a laboratory setting, compared with the period needed by the Hispaniolan parrots used in another study.9 Extending the training and acclimation period that preceded the study could have allowed more time to condition the conures to a unique food reward that would have stimulated a higher degree of voluntary movement during behavioral testing.
Green-cheeked conures are considerably smaller and lighter than Hispaniolan parrots. Hispaniolan parrots typically weigh 240 to 260 g, whereas green-cheeked conures typically weigh 55 to 75 g. It is possible that the conures used in this study did not respond with signs of pain because they did not bear as much weight on the affected limb when moving about freely in their home cage and that the difference in weight bearing was only apparent in the more rarefied environment of the test chamber for the weight load testing.
Finally, the 2 species are geographically isolated in their native habitat. Hispaniolan parrots are restricted to the island of Hispaniola, whereas green-cheeked conures are native to mainland South America, principally to Bolivia and western Brazil.26 Geographic isolation can cause profound differences in behavior among species of rodents within the same genus. The desert species of deer mouse (Peromyscus californicus) is monogamous and territorial, and the males participate in caring for the young. The temperate deer mouse (Peromyscus leucopus) is polygamous and does not defend its territory, and the males do not exhibit paternal care behavior.2,27 Hispaniolan parrots and green-cheeked conures are both genetically and geographically separated more completely than North American Peromyscus spp; as such, they may have extremely different behavioral repertoires. Ethograms have been generated for a number of laboratory and companion animals and should reflect what is known about the behavior of a specific species. Additional observations may be necessary to establish an ethogram that is reflective of the behavioral repertoire of green-cheeked conures.
Green-cheeked conures were acceptable for use in analgesic testing. Butorphanol in its liposomal formulation was an efficacious analgesic in these conures, as measured by weight bearing after unilateral injection of MSU into the right intertarsal joint. Voluntary and motivated behaviors were not extremely predictive of analgesic efficacy, and fecal corticosterone concentration was only affected by handling. More studies are necessary to better define behavioral and physiologic end points for analgesiometric evaluations in this species.
Acknowledgments
Supported in part by the National Institutes of Health (grant No. R01RR018802-02).
ABBREVIATIONS
- LEBT
Liposomal encapsulated butorphanol
- MSU
Microcrystalline sodium urate
Footnotes
Kaytee Products Inc, Chilton, Wis.
Exact, Kaytee Products Inc, Chilton, Wis.
Butorphanol tartrate, Sigma Chemical Co, St Louis, Mo.
Gelman filters, Pall Corp, Ann Arbor, Mich.
Beckman model L8-M ultracentrifuge, Beckman Coulter Inc, Fullerton, Calif.
Paralube, Fougera, Melville, NY.
IITC model 600 incapacitance meter, IITC Life Science, Woodland, Calif.
Canon Optura 40, Canon USA Inc, Lake Success, NY.
Windows Movie Maker, version 2.1, Microsoft Corp, Redmond, Wash.
Saint Louis Zoo Endocrinology Lab, Saint Louis Zoo, St Louis, Mo.
SAS, version 9.1.3, SAS Institute Inc, Cary, NC.
SPSS, version 11.1, SPSS Inc, Chicago, Ill.
References
- 1.Paul-Murphy J, Ludders JW, Robertson SA, et al. The need for a cross-species approach to the study of pain in animals. J Am Vet Med Assoc. 2004;224:692–697. doi: 10.2460/javma.2004.224.692. [DOI] [PubMed] [Google Scholar]
- 2.Curro TG, Brunson DB, Paul-Murphy J. Determination of the ED50 of isoflurane and evaluation of the isoflurane-sparing effect of butorphanol in cockatoos (Cacatua spp.) Vet Surg. 1994;23:429–433. doi: 10.1111/j.1532-950x.1994.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 3.Curro TG. Evaluation of the isoflurane-sparing effects of butorphanol and flunixin in Psittaciformes, in Proceedings . Annu Conf Assoc Avian Vet. 1994:17–19. [Google Scholar]
- 4.Paul-Murphy J. Pain management for the pet bird. In: Gaynor JS, Muir WW, editors. Handbook of veterinary pain management. 2nd ed. St Louis: Elsevier Saunders; 2008. pp. 267–280. [Google Scholar]
- 5.Riggs SM, Hawkins MG, Craigmill AL, et al. Pharmacokinetics of butorphanol tartrate in red-tailed hawks (Buteo jamaicensis) and great horned owls (Bubo virginianus) Am J Vet Res. 2008;69:596–603. doi: 10.2460/ajvr.69.5.596. [DOI] [PubMed] [Google Scholar]
- 6.Paul-Murphy JR, Brunson DB, Miletic V. Analgesic effects of butorphanol and buprenorphine in conscious African grey parrots (Psittacus erithacus erithacus and Psittacus erithacus timneh) Am J Vet Res. 1999;60:1218–1221. [PubMed] [Google Scholar]
- 7.Sladky KK, Krugner-Higby L, Meek-Walker E, et al. Serum concentrations and analgesic effects of liposome-encapsulated and standard butorphanol tartrate in parrots. Am J Vet Res. 2006;67:775–781. doi: 10.2460/ajvr.67.5.775. [DOI] [PubMed] [Google Scholar]
- 8.Paul-Murphy J. In: Pain management. Clinical avian medicine. Harrison GJ, Lightfoot TL, editors. I. Palm Beach, Fla: Spix Publishing Inc; 2006. 2006. pp. 233–239. [Google Scholar]
- 9.Paul-Murphy JR, Sladky KK, Krugner-Higby LA, et al. Analgesic effects of carprofen and liposome-encapsulated butorphanol tartrate in Hispaniolan parrots (Amazona ventralis) with experimentally induced arthritis. Am J Vet Res. 2009;70:1201–1210. doi: 10.2460/ajvr.70.10.1201. [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Nakahama H, Miyakawa H, et al. Arthritis induced in cat by sodium urate: apossible animal model for tonic pain. Pain. 1984;18:287–297. doi: 10.1016/0304-3959(84)90823-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Burton-Wurster N, Glant TT, et al. Spontaneous and experimental osteoarthritis in dog: similarities and differences in proteoglycan levels. J Orthop Res. 2003;21:730–737. doi: 10.1016/S0736-0266(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura A, Akahoshi T, Takahashi M, et al. Attenuation of monosodium urate crystal-induced arthritis in rabbits by a neutralizing antibody against interleukin-8. J Leukoc Biol. 1997;62:444–449. doi: 10.1002/jlb.62.4.444. [DOI] [PubMed] [Google Scholar]
- 13.Gentle MJ, Hocking PM, Bernard R, et al. Evaluation of intraarticular opioid analgesia for the relief of articular pain in the domestic fowl. Pharmacol Biochem Behav. 1999;63:339–343. doi: 10.1016/s0091-3057(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 14.Gentle MJ, Thorp BH. Sensory properties of ankle joint capsule mechanoreceptors in acute monoarthritic chickens. Pain. 1994;57:361–374. doi: 10.1016/0304-3959(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Hocking PM, Gentle MJ, Bernard R, et al. Evaluation of a protocol for determining the effectiveness of pretreatment with local analgesics for reducing experimentally induced articular pain in domestic fowl. Res Vet Sci. 1997;63:263–267. doi: 10.1016/s0034-5288(97)90031-x. [DOI] [PubMed] [Google Scholar]
- 16.Hocking PM, Robertson GW, Gentle MJ. Effects of anti-inflammatory steroid drugs on pain coping behaviours in a model of articular pain in the domestic fowl. Res Vet Sci. 2001;71:161–166. doi: 10.1053/rvsc.2001.0510. [DOI] [PubMed] [Google Scholar]
- 17.Anhut H, Brune K, Frölich JC, et al. Prostaglandin D2 is the prevailing prostaglandin in the acute inflammatory exudate of urate arthritis in the chicken. Br J Pharmacol. 1979;65:357–359. doi: 10.1111/j.1476-5381.1979.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiltz C, Lioté F, Prudhommeaux F, et al. Monosodium urate monohydrate crystal-induced inflammation in vivo: quantitative histomorphometric analysis of cellular events. Arthritis Rheum. 2002;46:1643–1650. doi: 10.1002/art.10326. [DOI] [PubMed] [Google Scholar]
- 19.Lunam CA, Gentle MJ. Substance P immunoreactive nerve fibres in the domestic chick ankle joint before and after acute urate arthritis. Neurosci Lett. 2004;354:87–90. doi: 10.1016/s0304-3940(03)00575-5. [DOI] [PubMed] [Google Scholar]
- 20.Brune K, Walz D, Bucher K. The avian microcrystal arthritis I. Simultaneous recording of nociception and temperature effect in the inflamed joint. Inflamm Res. 1974;4:21–26. [Google Scholar]
- 21.Mandel NS. Structural changes in sodium urate crystals on heating. Arthritis Rheum. 1980;23:772–776. doi: 10.1002/art.1780230610. [DOI] [PubMed] [Google Scholar]
- 22.AVMA. [Accessed Aug 31, 2009];Use of placebo controls in assessment of new therapies for alleviation of acute pain in client-owned animals. Available at: www.ma.org/issues/policy/placebocontrols.asp.
- 23.Ekblom M, Hammarlund-Udenaes M, Paalzow L. Modeling of tolerance development and rebound effect during different intravenous administrations of morphine to rats. J Pharmacol Exp Ther. 1993;266:244–252. [PubMed] [Google Scholar]
- 24.Smith LJ, KuKanich B, Hogan BK, et al. Pharmacokinetics of a controlled-release liposome-encapsulated hydromorphone administered to healthy dogs. J Vet Pharmacol Ther. 2008;31:415–422. doi: 10.1111/j.1365-2885.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LJ, Krugner-Higby L, Clark M, et al. A single dose of liposome-encapsulated oxymorphone or morphine provides long-term analgesia in an animal model of neuropathic pain. Comp Med. 2003;53:280–287. [PubMed] [Google Scholar]
- 26.Collar NJ. Psittacidae. In: del Hoyo J, Elliott A, Sargatal J, editors. Handbook of the birds of the world: sandgrouse to cuckoos. Vol. 4. Barcelona, Spain: Lynx Publishing; 1997. pp. 280–479. [Google Scholar]
- 27.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]