Abstract
Background
Assessing patient-reported health behaviors is a critical first step to prioritizing prevention in primary care. We assessed the feasibility of point-of-care behavioral health assessment in nine diverse primary care practices, including four federally-qualified health centers (FQHCs), four Practice-based Research Network (PBRN) practices, and a Department of Veterans Affairs (VA) practice.
Methods
In this prospective mixed-methods study, practices were asked to integrate a standardized paper-based health behavior and mental health assessment into their workflow for 50 or more patients. We used three data sources to examine the implementation process: 1) patient responses to the health assessment, 2) patient feedback surveys about how assessments were used during encounters, and 3) post-implementation interviews.
Results
Most (71%) non-urgent patients visiting the participating practices during the implementation period completed the health assessment, but reach varied by practice (range: 59-88%). Unhealthy diet, sedentary lifestyle, and stress were the most common patient problems with similar frequencies observed across practices. The median number of “positive screens” per patient was similar across FQHCs (3.7-positives, SD=1.8), PBRN practices (3.8-positives, SD=1.9), and the VA clinic (4.1-positives, SD=2.0). Primary care clinicians discussed assessment results with patients about half of the time (54%), with considerable between practice variation (range: 13%-66% with lowest use among FQHC clinicians). Although clinicians were interested in routinely implementing assessments, many reported not feeling confident of having resources or support to address all patients’ behavioral health needs.
Conclusions
Primary care practices will need to revamp their patient-reported data collection processes in order to integrate routine health behavior assessments. Implementation support will be required if health assessments are to be actively used as part of routine primary care.
Background
Prioritizing prevention within the context of primary care is a key tenet of the Affordable Care Act and is central to the adoption of a patient-centered medical home model. The development of methods to more accurately assess patient-reported health behaviors in primary care is a critical first step. Primary care clinicians, however, are faced with many challenges in addressing adult patients’ multiple behavioral health issues during traditional 15-minute office encounters (Bodenheimer and Laing 2007; Fiscella and Epstein 2008). Previous research on the implementation and impact of point-of-care behavioral health assessment has been primarily conducted in practices affiliated with primary care practice-based research networks (PBRNs) (Fernald et al. 2012), but limited information exists about the implementation of behavioral health assessment in federally qualified health centers (FQHCs) that primarily serve low-income patients.
The National Institutes of Health (NIH), in partnership with the Society of Behavioral Medicine (SBM), recently led an initiative to identify a brief, practical, standardized set of items to collect patient-reported data on health behaviors, behavioral health and psychosocial issues, appropriate for inclusion in the electronic health record (EHR), with the potential to enhance patient-centered care and public health. A three-phase national expert panel process of consensus building resulted in the identification of core behavioral health measures relevant for primary care: anxiety, depression, stress, sleep quality, smoking, smokeless tobacco use, risky drinking of alcohol, substance use, sugar-sweetened beverage consumption, fruit and vegetable consumption, fast food consumption, physical activity (Coleman et al. 2012), and self-rated health (Estabrooks et al. 2012). The expert panel's measure selection considerations included the extent to which evidence-based primary care interventions were available to address the problem health behavior, the value of the information in providing a nuanced understanding of patient health behaviors and clinical data, and their relevance for improving patient-centered outcomes of care.
We conducted a feasibility study to administer the instrument assessing the 13 selected behavioral health measures among non-urgent patients in diverse primary care practices. Each practice implemented the health assessment during a brief intervention period. We assessed the acceptability of the health assessment among diverse patients, examined the extent to which primary care clinicians and/or other team members discussed the assessment results with patients, whether the patients discussed setting goals for improving health behaviors, and whether patients intended to follow-up with their clinician about their concerns.
Methods
Study Setting
The participating practices were recruited by investigators from four research centers located in different geographic areas around the U.S. The four participating FQHC sites (Sites A-D) are in located in the greater Los Angeles, California region, and each serve low-income patients from different racial and ethnic backgrounds. Site A primarily serves low-income Chinese-American patients, Site B primarily serves low-income Mexican-American patients, Site C primarily serves low-income Filipino-American and Mexican-American patients, and Site D primarily serves low-income Mexican-American and Central-American patients.
Two of the four PBRN-affiliated primary care practice sites (sites G and H) are located in urban Richmond, Virginia region. The other two PBRN practices (Site E and F) are located in northeastern Vermont and rural Appalachia Virginia, respectively. The rural PBRN practices primarily serve White patients and the urban PBRN practices primarily serve African-American patients. The participating VA practice (Site I) is located in eastern Massachusetts and primarily serves an older, White lower-middle class male Veteran patient population. Table 1 summarizes the primary care practice location, primary populations served, history of implementing behavioral health assessments, electronic health record use, length of each practices’ intervention period and the number of health assessment and feedback surveys received.
Table 1.
Participating Primary Care Practice Characteristics
| Practice site | Location | Practice Type | Primary patient demographic | History of implementing behavioral health assessments | Electronic health record | Duration | Total assessments completed (% reach) | % providing feedback |
|---|---|---|---|---|---|---|---|---|
| Site A | Los Angeles, CA | FQHC | Low-income, Chinese-American (85%) | Registered nurse administered behavioral health assessment during initial intake appointments. | Yes | 6 days | 66 (79%)* | 94.0 |
| Site B | Santa Ana, CA | FQHC | Low-income, Mexican-American (76%) | Prior use of depression screening (PHQ-9) | No | 5 days | 59 (74%)* | 59.3 |
| Site C | Eagle Rock, CA | FQHC | Low-income, Filipino-American (58%) and Mexican-American (27%) | No routine collection of behavioral health data | Yes | 10 days | 65 (65%)* | 93.8 |
| Site D | Los Angeles, CA | FQHC | Low-income, Mexican-Americans (51%) and Central-Americans (31%) | No routine collection of behavioral health data | Yes | 4 days | 93 (85%)* | 95.7 |
| Site E | Montpelier, Vermont | PBRN | Lower-middle income, White (90%) | Intermittent use of depression (PHQ-9), anxiety (GAD), and alcohol use (Audit-C) | Yes | 3 days | 22 (64%)* | 95.5 |
| Site F | Front Royal, VA | PBRN | Lower-middle income, White (85%) | Routinely assessed multiple health behaviors through personal health record | Yes | 3 days | 45 (73%)** | 88.9 |
| Site G | Bon Secours, Virginia | PBRN | Lower-middle income, African-American (85%) | Routinely assessed multiple health behaviors through personal health record | Yes | 2 days | 20 (88%)** | 85.0 |
| Site H | Richmond, Virginia | PBRN | Lower-middle income, African-American (60%) and White (30%) | Routinely assessed smoking status, but no other behavioral or mental health assessment | Yes | 4 days | 35 (59%)** | 100 |
| Site I | Bedford, MA | VA | Lower-middle income, White Veterans (90%) | Intermittent collection of behavioral health data, but no comprehensive behavioral health assessment in place. | Yes | 4 days | 57 (60%)* | 91.2 |
Note: FQHC= federally-qualified health center, PBRN= practice-based research network, VA= Veterans Health Administration
For 6 practices, reach was calculated using administrative reports (# of completed surveys / # number of non-urgent patient visits).
For 4 practices, reach was calculated using tallies by the research team (# of completed surveys / # of non-urgent patients offered survey).
The Intervention
In this prospective mixed-methods study, practices were asked to integrate a standardized paper-based health behavior and mental health assessment into their workflow for 50 or more patients. The health assessment was administered by existing primary care staff at the participating practices to 463 adult patients receiving non-urgent care, e.g., return visit or routine/wellness visit, during a 2-10 day intervention period (during June-September 2012) across the nine participating practice sites. The assessment was administered in English (Sites A-J), Spanish (Sites A-D), and Chinese (Site A) and was primarily self-administered, although primary care staff helped patients who needed assistance, similar to the practice utilized for other forms completed at the point of registration. The assessment consisted of the 13 brief items assessing health behavior and mental health identified by the national expert panel process as relevant for primary care (Estabrooks et al. 2012) and demographic questions. Primary care clinicians and staff were encouraged to discuss the health assessment results with patients during the clinical encounter. Clinicians had access to binders with written guidelines and handouts focused on treatment and referral options for addressing each of the behavioral health and mental health measures covered in the assessment.
Data Collection
We used three primary data sources to examine the implementation and impact of point-of-care behavioral health and mental health assessment: 1) behavioral health assessment responses collected through administration of the instrument to clarify the number and nature of behavioral health issues among patients at each practices, 2) patient feedback surveys to assess how the health assessment was used during their clinical encounter, and 3) post-intervention interviews of primary care clinicians, staff, and practice stakeholders to assess their perspective on the utility of the health assessment in helping patients.
Patient Feedback Surveys
After each non-urgent encounter, research staff offered patients that completed the health assessment an opportunity to provide their feedback by completing an 8-item survey about the health assessment completion process and the use of the health assessment survey during the clinical encounter, including the extent to which patients felt comfortable completing the questions and whether clinicians discussed the results with the patient, discussed goals related to health behavior change, and whether patients planned to follow up with their provider about health concerns from the health assessment. Participants (n=408; 92% of health assessment completers) received a $5 bill for providing their feedback.
Post-Intervention Interviews of Primary Care Stakeholders
We conducted post-intervention interviews of a convenience sample of primary care clinicians, staff, and clinical leaders at each practice site (n=20) approximately 1-2 weeks after the completion of the intervention period in their practice. Interviews assessed the participants’ perception of the utility of the health assessment in helping patients with their behavioral health problems at the point-of-care, barriers and facilitators of implementing the assessment, and debrief on their experiences of using the health assessment or similar assessment as part of routine care in the future. Interviews lasted 30-45 minutes and were all recorded and transcribed with the permission of the participants. A $25 gift card was provided to each participant after the completion of the interview.
Analyses
First, we calculated the reach of the health assessment. Percent (%) “reach” was calculated in two different ways because of practice record keeping, documentation, and workflow differences across the participating practices. For 6 practices, reach was calculated using administrative reports (# of completed surveys / # number of non-urgent patient visits). For 3 practices, reach was calculated using tallies by the research team (# of completed surveys / # of non-urgent patients offered survey). Next, we assessed the extent to which patient sociodemographic characteristics and health status characteristics differed across the nine practices so that results of the feasibility trial are understood in context. We used chi-square statistics to examine differences for categorical patient variables and t-tests to estimate differences for continuous patient variables between the four FQHCs practices, the five PBRN practices, and the VA practice.
Using published cut points for “positive screens” or values that would warrant further discussion, (Cella et al. 2012; Coleman et al. 2012; Kroenke et al. 2009; Paxton et al. 2011; Rohrer et al. 2009; Smith et al. 2009, 2010; Snowden et al. 2011; Wiener 2013), we calculated the proportion of respondents who would qualify for primary care intervention for each health behavior and mental health measure. We calculated the frequency of each positive screen and total number of positive screens per patient for each practice and for subgroups of practices (FQHC vs. PBRN vs. VA). Next, we specified multivariable regression models to clarify the extent to which unadjusted differences in positive screens based on the primary care practice type were explained by the sociodemographic characteristics and health profile of their patients. We used linear regression to examine the relation of primary care practice type and the total number of positive screens per patient and logistic regression to examine the relation of primary care practice type and screening positive for each of the health behavior and mental health measures. These multivariable models accounted for patient clustering within practices using random practice effects and also controlled for patient age, sex, marital status, educational attainment, employment, and U.S. nativity.
We used the patient feedback survey data to examine differences in the use of the health assessment on clinical discussions. To clarify experiences of implementing health assessments at each practice, we analyzed data from interviews of clinicians and staff of the participating practices conducted 1-2 weeks following the practice's intervention period. We used a combination of deductive and inductive approaches to analyze the interview data (Fereday and Muir-Cochrane 2006). We based the initial codebook on the interview guide, as well as independent open coding of four transcripts by two researchers. Coding was compared for consistency, and after consensus was reached, the codebook was revised. Each researcher then coded half the transcripts (or interview notes for unrecorded interviews) using ATLAS.ti software (2009). We analyzed the content of frequently used codes and identified the most consistent themes and patterns of health assessment use across the primary care practice types.
Results
Reach of the Health Assessment to Non-Urgent Patients
An estimated 71% of eligible non-urgent patients visiting during implementation period returned the health assessment. The reach of the health assessment differed across the participating practices (range: 59-88%) (Table 1). The main reasons for not reaching patients noted by research staff and reported during practice key informant interviews included: 1) patients left the practice before the survey could be collected (most common), 2) practice staff forgot to hand out the assessment to some non-urgent patients, especially when clinic staffing was low and/or patient demands were high, 3) patients did not want to complete the survey, and 4) the use of researchers to administer survey in one practice (Site I) may have made patients less inclined to participate compared to when primary care team members asked the patients to complete the survey.
Respondent Characteristics
FQHC respondents were more likely than PBRN or VA patients to be female, have less than a high school education, be non-White, be foreign-born, complete the survey in a language other than English, and need an interpreter for health care encounters (Table 2).
Table 2.
Respondent Characteristics, by Primary Care Setting
| Patient Characteristics | Overall | FQHCs | PBRNs | VA | p-value |
|---|---|---|---|---|---|
| N | 463 | 284 | 122 | 57 | |
| Female (%) | 61.2 | 70.2 | 65.3 | 3.8 | *** |
| Age | *** | ||||
| <30 (%) | 5.0 | 5.4 | 4.3 | 3.9 | |
| 30-39 (%) | 7.66 | 7.6 | 10.3 | 1.9 | |
| 40-49 (%) | 15.8 | 17.4 | 15.5 | 7.7 | |
| 50-59 (%) | 32.2 | 37.7 | 24.1 | 21.2 | |
| 60-69 (%) | 28.4 | 26.5 | 29.3 | 36.5 | |
| 70-79(%) | 7.4 | 5.1 | 11.2 | 11.5 | |
| 80+ (%) | 3.6 | 0.4 | 5.2 | 17.3 | |
| Education | *** | ||||
| Less than high school (%) | 34.6 | 50.4 | 11.2 | 3.9 | |
| High school graduate or GED (%) | 25.3 | 21.7 | 33.6 | 25.5 | |
| Some college (%) | 14.8 | 11.0 | 19.0 | 25.5 | |
| Associates degree / technical training (%) | 10.7 | 8.1 | 12.1 | 21.6 | |
| 4 year college degree + (%) | 14.6 | 8.8 | 24.1 | 23.5 | |
| Race/Ethnicity | *** | ||||
| Non-Hispanic White (%) | 29.0 | 6.7 | 69.6 | 82.9 | |
| Black/African American (%) | 7.8 | 1.9 | 23.9 | 9.8 | |
| Mexican-American (%) | 24.8 | 36.3 | 2.2 | 0.0 | |
| Other Hispanic (%) | 13.8 | 19.5 | 2.2 | 2.4 | |
| Chinese (%) | 14.3 | 21.4 | 0.0 | 0.0 | |
| Filipino (%) | 6.8 | 10.1 | 0.0 | 0.0 | |
| Other (%) | 3.8 | 4.1 | 2.2 | 4.9 | |
| US Born (%) | 45.3 | 14.2 | 94.6 | 95.7 | *** |
| Survey Language | *** | ||||
| English (%) | 56.4 | 28.9 | 100.0 | 100.0 | |
| Spanish (%) | 31.1 | 50.7 | 0.0 | 0.0 | |
| Chinese (%) | 12.5 | 20.4 | 0.0 | 0.0 | |
| English Literacy | *** | ||||
| Very well/well (%) | 56.8 | 31.8 | 99.1 | 100.0 | |
| Not well (%) | 20.9 | 33.2 | 0.0 | 0.0 | |
| Not at all (%) | 22.3 | 35.0 | 0.9 | 0.0 | |
| Interpreter Needs | *** | ||||
| No (%) | 64.7 | 44.8 | 97.4 | 100.0 | |
| Yes (%) | 22.0 | 34.3 | 1.8 | 0.0 | |
| Sometimes (%) | 13.4 | 20.9 | 0.9 | 0.0 | |
| Employment | ** | ||||
| Full-time (%) | 21.1 | 14.4 | 37.7 | 19.2 | |
| Part-time (%) | 12.8 | 18.1 | 3.5 | 5.8 | |
| Unemployed (%) | 16.7 | 21.0 | 9.7 | 9.6 | |
| Homemaker (%) | 15.3 | 23.3 | 3.5 | 0.0 | |
| Disabled (%) | 10.1 | 5.5 | 18.4 | 15.4 | |
| Other (%) | 24.0 | 17.7 | 27.2 | 50.0 | |
| Marital Status | N/S | ||||
| Married (%) | 48.5 | 50.0 | 49.6 | 38.5 | |
| Single, never married (%) | 17.3 | 16.9 | 18.3 | 17.3 | |
| Divorced (%) | 12.4 | 10.4 | 10.4 | 26.9 | |
| Other (%) | 21.8 | 22.7 | 21.7 | 17.3 |
* p <.05
p <.01
p <.001
N/S= no statistically significant differences between primary care practice type.
Positive Screens per Measure
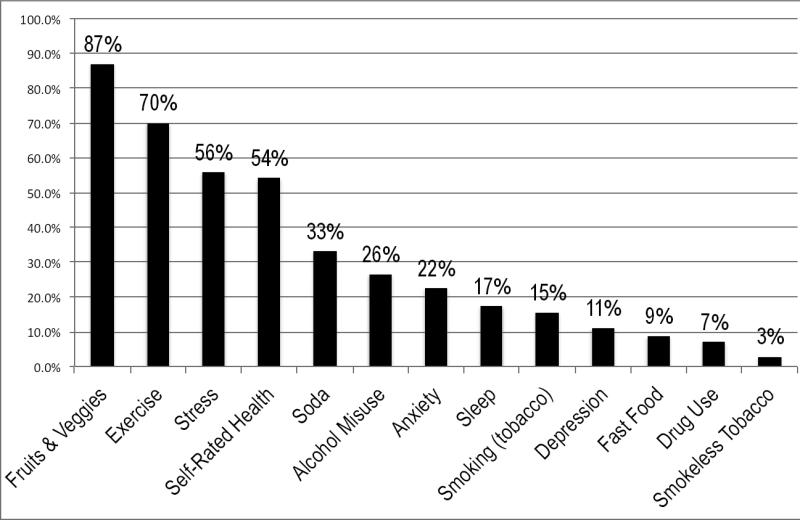
Fruit and vegetable consumption was the most common “positive screen” for patients because most (87%) did not endorse eating five or more fruits and vegetables per day. The next most prevalent positive screens were for physical activity (70%), poor or fair overall self-rated health (54%), and stress (60%) (Figure 1). A key finding was that more than a quarter (26%) of patients reported recent problem drinking. The most common seven problem health behaviors were similarly ranked across settings, but a higher proportion of VA patients reported problem alcohol use, anxiety, and high-stress compared to FQHC and PBRN patients. FQHC patients were more likely to report “fair” or “poor” health status compared to PBRN and VA patients (data not shown).
Figure 1.
Proportion of respondents screening “positive” for intervention for each health behavior and mental health measure
Total Positive Screens per Respondent
The median patient had four positive screens across the domains and total positive screens were similar across FQHCs (3.7-positives per patient, SD=1.8), PBRN practices (3.8-positives per patient, SD=1.9), and the VA practice (4.1-positives per patient, SD=2.0). In multivariate linear regression models accounting for patient sociodemographic characteristics and patient clustering, FQHC patients were more likely to screen positive for low fruit and vegetable consumption (OR=8.8; p<0.05) and risky alcohol use (OR=5.0; p<0.05), but less likely to screen positive for fast food consumption (OR=0.16; p<0.05) compared to PBRN patients (Table 3). In adjusted analyses, VA patients were more likely to screen positive for drug use (OR=7.34; p<0.05) compared to PBRN patients. There were no statistically significant differences in the total number of “positive screens” per patient across primary care practice settings (Table 3).
Table 3.
The Relation of Primary Care Practice Type and Screening Positive, Unadjusted vs. Adjusted Analyses
| Health Behavior Measure | FQHC (vs. PBRN) | VA (vs. PBRN) | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | p-value | Adjusted OR | p-value | Unadjusted OR | p-value | Adjusted OR | p-value | |
| Fast Food | 0.22 | *** | 0.16 | * | 0.44 | 1.06 | ||
| Fruits/Veg | 0.77 | 8.8 | * | 0.93 | 1.26 | |||
| Soda | 0.74 | 0.76 | 0.8 | 1.87 | ||||
| Exercise | 1.78 | 1.14 | 0.89 | 0.54 | ||||
| Stress | 1.45 | * | 1.25 | 1.66 | 2.1 | |||
| Anxiety/Worry | 0.86 | 0.65 | 1.04 | 1.46 | ||||
| Depression/Interest | 1.06 | 1.18 | 1.89 | 1.14 | ||||
| Sleep | 0.64 | 0.82 | 1.01 | 1.19 | ||||
| Smoking | 0.36 | ** | 0.52 | 1.7 | 0.87 | |||
| Smokeless Tobacco | 0.31 | * | 47.8 | 1.64 | 1.75 | |||
| Alcohol | 1.1 | 5.01 | * | 1.81 | * | 2.47 | ||
| Drug Use | 1.59 | 0.68 | 8.44 | ** | 7.34 | * | ||
| Self-rated Health | 1.69 | * | 1.66 | 0.77 | 0.77 | |||
| Total Positive Screens+ | −0.05 | 0.2 | 0.37 | 0.6 | ||||
Note: + Coefficient is interpreted as the odds of primary care practice type (FQHC or VA) patient screening positive for the measure compared to PBRN patients
p<0.05
p<0.01
p<0.001 compared to PBRN practices
Adjusted analyses control for patient sex, age, education, race/ethnicity, nativity, employment status, and marital status.
The Health Assessment and Clinical Discussions
Primary care clinicians discussed the health assessment results with patients about half (54%) the time (Table 4), with considerable variation across the nine practices (range: 13-66%) (Table 3). Nevertheless, 72% of patients indicated that their primary care clinician helped identify specific steps for improving their health. Also, most patients were comfortable answering the health assessment questions (86%) and most (83%) indicated that they planned to follow-up with their provider about concerns from the health assessment.
Table 4.
The Use of the Behavioral Health Assessment during the Clinical Encounter, by Primary Care Practice Type
| Overall | FQHC | PBRN | VA | p-value | |
|---|---|---|---|---|---|
| Sample size | 408 | 241 | 115 | 52 | |
| Felt comfortable answer the questions (%) | 85.6 | 91.7 | 76.3 | 77.5 | *** |
| Provider showed results from the behavioral health assessment (%) | 58.5 | 53.7 | 67.7 | -- | * |
| Received copy of results to take home (%) | 36.0 | 28.4 | 50.0 | 41.0 | ** |
| Provider asked patient about concerns about the results (%) | 54.1 | 46.7 | 67.0 | 64.3 | ** |
| Provider asked which health concerns patient would like to work on (%) | 60.2 | 48.7 | 81.3 | 71.4 | *** |
| Provider helped identify specific steps patient can take to address concerns (%) | 72.1 | 64.6 | 85.6 | 81.5 | ** |
| Plan to follow up with provider about health concerns from the behavioral health assessment (%) | 83.2 | 77.5 | 91.1 | 93.2 | ** |
p <.05
p <.01
p <.001 for overall differences across primary care practice types.
Perceived Utility of the Assessment
Duplication of effort was often raised as an issue because the practice's health plans (payers) required their own health assessments that were often much longer, did not use validated questions, and were considered by participants as less actionable compared to the health assessment implemented as part of the feasibility study. For example, one health plan's intake form included over 100 questions and patients often needed assistance with reading the form content. Clinicians and staff consistently reported that the project's assessment was much shorter, easier to use, and asks more specific and actionable questions compared to the health assessments used by the practice. Several PBRN stakeholders, however, noted that their patients do not have time to complete even brief health assessments. As one PBRN PCP said, “I don't feel like patients really take the time and attention to complete surveys...I feel like it's viewed as more of an aggravation...We have more of an urgent care setting lately, or atmosphere, where patients just want to come in and out...they don't want to be bothered with surveys.”
Preferences for Routinely Administering the Health Assessment
Key informants of PBRN and FQHC practices noted similar preferences for how the health assessment should be administered, i.e., before the clinical encounter, patient self-administered in the patients’ preferred language, scored and ready to use by the PCP. PBRN and FQHC clinicians and staff differed in their expectations for patient completion. PBRN key informants preferred that patients complete the health assessment before the date of the clinical encounter. For example, the health assessment could be administered through a secure web-portal, and for the responses to be automatically scored, incorporated into the EHR and the results used during the clinical encounter. The VA and PBRN practices generally do not serve large numbers of non-English speaking patients and VA and PBRN key informants expressed hesitance about providing the health assessment in other languages because of the time and resources that would be necessary (i.e., online or telephone translation services). As a VA clinician said, “I think it would be just the family member that they brought with them or a telephone translator, but the latter takes time to arrange and there is often no time to do that, even in the regular visits.”
FQHC PCPs often identified staff members who could help patients fill out the assessment in the patient's preferred language, hand score the assessment, and then provide the results back to them for their use in the clinical encounter. In spite of strong preferences for staff support to assist patients as they complete and interpret the assessment results, FQHC stakeholders were often skeptical that routine staff-supported completion would be possible. As one PCP noted, “At safety net institutions...because of pay rates for medical assistants, it can be challenging...The number of MAs that we have and their skill level make it challenging to raise expectations...I think a lot of places like ours, the ability to spread out and reassign those tasks are a little more challenged compared to other practices...”
Resources to Address Patients’ Behavioral Health Needs
Key informants across the practices indicated that referring patients to appropriate care to address positive screens for mental health, substance abuse, nutrition, and physical activity promotion were limited by the few referral sources available in the community and the extent to which patients’ have health insurance coverage that includes these supportive services. Key stakeholders across the practices indicated that health behavior interventions are often “crowded out” by more immediate clinical concerns during PCP-patient encounters. As one PCP noted, “...what ends up happening is just the reality of the situation is that the medical issues are addressed ...and then when it comes to patient health promotion, that part falls to the bottom of the list.”
Discussion
Our feasibility study of routine health behavior assessment in diverse primary care settings demonstrates that practices can implement point-of-care behavioral health assessments and patients are generally comfortable answering the questions. The reach of the health assessment was good (71%), but some challenges to reach were observed including practice staff forgetting to hand out the assessment to some non-urgent patients, especially when clinic staffing was low and/or patient demands were high. Clinicians, however, encountered many challenges in using the health assessment to assist patients during clinical encounters. The mean number of “positive screens” per patient was high (median=4) across diverse primary care settings, underscoring that routine point-of-care health assessments may increase the number of issues that may need to be addressed during a visit. The nature of behavioral health and mental health problems and number of problems per patient were similar across diverse primary care settings. Primary care clinicians’ use of the behavioral health assessment, however, varied considerably across practices. Although practice interview participants expressed that routine behavioral health assessment would be ideal for patient care, many believed that their practice did not have sufficient internal or referral resources to address all patients’ behavioral health and mental health needs. If routine assessment were to be implemented, better decision-support for primary care teams, fostering community linkages, and technical assistance will be necessary to aid primary care teams in helping patients prioritize their health behavior improvement goals and monitor their progress. Importantly, efforts are now underway to examine the impact of decision-support to aid clinicians and patients in using electronic PRO data of health behavior and mental health when discussing health behaviors change, setting goals, and monitoring behavior change (Krist et al. 2013).
Our study has some important limitations. While our study has the advantage of studying implementation of point-of-care health assessments in diverse practices, all practices volunteered to participate. Even with volunteer practices and a short intervention period (2 -10 days), three practices faced major challenges using the health assessment information during clinical encounters with patients. As a result, we believe that our study captured a diverse range of experiences of point-of-care implementation of behavioral health assessments. Most of the participating practices serve low to lower-middle income patients that may have more need for behavioral health support. The fact that the number of positive screens did not differ by practice type suggests that most primary care practices are likely to have significant need for behavioral health support. Also, we were unable to assess the extent to which behavioral health issues would be discussed during clinical encounters without the health assessment. Previous research, however, suggests that clinical discussions about behavioral health and mental health (Makoul et al. 2006) occur much less frequently than the level of clinician-patient discussions reported by patients in the current study. Finally, a chart audit was not conducted to assess the concordance of self-reports and clinical data. Chart audits, however, have their own limitations because of incomplete and inconsistent documentation of behavioral health discussions.
Facilitating primary care clinician-patient discussions about health behaviors and mental health issues appears to be challenging in some practices because of time constraints, challenges of increasing responsibilities of medical assistants that need to provide on-site support for patients to complete the assessment, and limited referral resources for mental health, substance abuse, nutrition, and physical activity promotion. If EHR meaningful use requirements extend to collecting, reporting, and using comprehensive behavioral health assessments (Glasgow and Emmons 2011; Glasgow et al. 2012), primary care practices are likely to face major challenges in using behavioral health data to improve clinical care. As health reform unfolds and primary care practices are expected to integrate routine health behavior assessment, practices will be faced with a need to revamp their patient-reported data collection processes. Technical assistance in the form of training on the use of behavioral health assessments, practice facilitation (Nutting et al. 2010), structured rapid cycle quality improvement support (Rubenstein et al. 2010), and interorganizational learning opportunities (Nembhard 2012) may aid practices in using PRO data and disseminating best practices in supporting patient health behavior change in low-resource settings. Unless implementation support is provided to practices, the routine collection and meaningful use of behavioral health data will likely flounder, particularly in low resource practices that serve the most vulnerable patient populations. Payment reform and integration of behavioral health measures into EHRs will be important for accelerating the use of PRO data to aid patients in their health behavior change efforts, as duplicative data collection and clinical information systems were noted as barriers to the meaningful use of PRO data.
Acknowledgments
The research was conducted while Dr. Rodriguez was in the Department of Health Policy and Management, UCLA Fielding School of Public Health. The research described was supported by the NIH/National Cancer Institute (#U48DP001946) and NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124. The UCLA South General Campus Institutional Review Board (IRB#12-000297), the subcommittee on Human Studies of the VA Department of Affairs, and Virginia Commonwealth University Institutional Review Board (IRB# HM14523) approved of the research study. We thank Julie Volkman, Suzi Spear, PhD, Dylan Roby, PhD, Mark Kelly, and Melissa Hayes for their implementation support and assistance to the research project. We are especially grateful for the steadfast leadership of Russell Glasgow, PhD, for his oversight of the collaborative process, and critical feedback. Finally, we thank the patients, clinicians, and staff from the nine practices for their active participation in the project.
Footnotes
Conflicts of interest: None
References
- ATLAS.ti (version 6) (release: Scientific Software Development. 2009 [Google Scholar]
- Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5(5):457–61. doi: 10.1370/afm.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, Reder AT, Sufit R, Simuni T, Holmes GL, Siderowf A, Wojna V, Bode R, McKinney N, Podrabsky T, Wortman K, Choi S, Gershon R, Rothrock N, Moy C. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–7. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, Sternfeld B, Sallis RE. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–6. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- Estabrooks PA, Boyle M, Emmons KM, Glasgow RE, Hesse BW, Kaplan RM, Krist AH, Moser RP, Taylor MV. Harmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factors. J Am Med Inform Assoc. 2012 doi: 10.1136/amiajnl-2011-000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. International Journal of Qualitative Methods. 2006;5(1):80–92. [Google Scholar]
- Fernald DH, Dickinson LM, Froshaug DB, Balasubramanian BA, Holtrop JS, Krist AH, Glasgow RE, Green LA. Improving multiple health risk behaviors in primary care: lessons from the Prescription for Health Common Measures, Better Outcomes (COMBO) study. J Am Board Fam Med. 2012;25(5):701–11. doi: 10.3122/jabfm.2012.03.110057. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Epstein RM. So much to do, so little time: care for the socially disadvantaged and the 15-minute visit. Arch Intern Med. 2008;168(17):1843–52. doi: 10.1001/archinte.168.17.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R, Emmons KM. The public health need for patient-reported measures and health behaviors in electronic health records: a policy statement of the Society of Behavioral Medicine. Transl Behav Med. 2011;1(1):108–9. doi: 10.1007/s13142-011-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Kaplan RM, Ockene JK, Fisher EB, Emmons KM. Patient- reported measures of psychosocial issues and health behavior should be added to electronic health records. Health Aff (Millwood) 2012;31(3):497–504. doi: 10.1377/hlthaff.2010.1295. [DOI] [PubMed] [Google Scholar]
- Krist AH, Glenn BA, Glasgow RE, Balasubramanian BA, Chambers DA, Fernandez ME, Heurtin-Roberts S, Kessler R, Ory MG, Phillips SM, Ritzwoller DP, Roby DH, Rodriguez HP, Sabo RT, Sheinfeld Gorin SN, Stange KC. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implement Sci. 2013;8:73. doi: 10.1186/1748-5908-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–21. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- Makoul G, Dhurandhar A, Goel MS, Scholtens D, Rubin AS. Communication about behavioral health risks: a study of videotaped encounters in 2 internal medicine practices. J Gen Intern Med. 2006;21(7):698–703. doi: 10.1111/j.1525-1497.2006.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nembhard IM. All teach, all learn, all improve?: the role of interorganizational learning in quality improvement collaboratives. Health Care Manage Rev. 2012;37(2):154–64. doi: 10.1097/HMR.0b013e31822af831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutting PA, Crabtree BF, Stewart EE, Miller WL, Palmer RF, Stange KC, Jaen CR. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Ann Fam Med 8 Suppl. 2010;1:S33–44. doi: 10.1370/afm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton AE, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med. 2011;40(1):67–71. doi: 10.1016/j.amepre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rohrer JE, Herman DC, Merry SP, Naessens JM, Houston MS. Validity of overall self-rated health as an outcome measure in small samples: a pilot study involving a case series. J Eval Clin Pract. 2009;15(2):366–9. doi: 10.1111/j.1365-2753.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein LV, Chaney EF, Ober S, Felker B, Sherman SE, Lanto A, Vivell S. Using evidence-based quality improvement methods for translating depression collaborative care research into practice. Fam Syst Health. 2010;28(2):91–113. doi: 10.1037/a0020302. [DOI] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24(7):783–8. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155–60. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden A, White CA, Christie Z, Murray E, McGowan C, Scott R. The clinical utility of the distress thermometer: a review. Br J Nurs. 2011;20(4):220–7. doi: 10.12968/bjon.2011.20.4.220. [DOI] [PubMed] [Google Scholar]
- Wiener RC. Association of smokeless tobacco use and smoking in adolescents in the United States: An analysis of data from the Youth Risk Behavior Surveillance System survey, 2011. J Am Dent Assoc. 2013;144(8):930–8. doi: 10.14219/jada.archive.2013.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]