Abstract
Importance
Mutations in the GNAL gene have recently been shown to cause primary torsion dystonia. The GNAL-encoded protein (Gαolf) is important for dopamine D1 receptor function and odorant signal transduction. We sequenced all 12 exons of GNAL in 461 patients from Germany, Serbia, and Japan, including 318 patients with dystonia (190 with cervical dystonia), 51 with hyposmia and Parkinson disease, and 92 with tardive dyskinesia or acute dystonic reactions.
Observations
We identified the following two novel heterozygous putative mutations in GNAL: p.Gly213Ser in a German patient and p.Ala353Thr in a Japanese patient. These variants were predicted to be pathogenic in silico, were absent in ethnically matched control individuals, and impaired Gαolf coupling to D1 receptors in a bioluminescence energy transfer (BRET) assay. Two additional variants appeared to be benign because they behaved like wild-type samples in the BRET assay (p.Ala311Thr) or were detected in ethnically matched controls (p.Thr92Ala). Both patients with likely pathogenic mutations had craniocervical dystonia with onset in the fifth decade of life. No pathogenic mutations were detected in the patients with hyposmia and Parkinson disease, tardive dyskinesias, or acute dystonic reactions.
Conclusions and Relevance
Mutations in GNAL can cause craniocervical dystonia in different ethnicities. The BRET assay may be a useful tool to support the pathogenicity of identified variants in the GNAL gene.
Introduction
In two independent studies,1,2 exome sequencing of the GNAL gene (RefSeq NM_001142339) was used recently to study GNAL mutations as a cause of autosomal dominant primary torsion dystonia in patients of European and African American ancestry. The GNAL gene is located on the short arm of chromosome 18p11, and the absence of GNAL may contribute to dystonia in patients with the 18p deletion syndrome.3 Onset among mutation carriers occurred mainly in the neck (82%) at a mean age at onset of 31.3 (range, 7-54) years. On examination, almost all patients had cervical dystonia (93%), but cranial (57%) and speech involvement (44%) were also quite common.1
The GNAL gene encodes the stimulatory α subunit, Gαolf, that links G protein–coupled receptors to downstream effector molecules and functions as a heterotrimer composed of α, β, and γ subunits. The Gαolf subunit is expressed prominently in the brain, especially in the striatum, where it may couple dopamine D1 receptors and adenosine A2A receptors to the activation of adenylyl cyclase type 5.1,4 In fact, efficiency of heterotrimer formation or coupling to D1 receptors was previously shown to be impaired in GNAL mutation carriers using a bioluminescence energy transfer (BRET) assay.1 The Gαolf subunit is also involved in odorant signal transduction.5 Notably, Gnal-null mice are anosmic,6 raising the possibility that mutations in GNAL may also cause hyposmia in humans.2 Because Gαolf plays a role in dopamine signal transduction, mutations in GNAL potentially increase susceptibility to movement disorders induced by dopamine antagonists. Differential methylation of CpG islands in the vicinity of exon 1 also exists, suggestive of genomic imprinting of the GNAL gene.7 We screened for GNAL mutations in a multiethnic sample with different dystonia phenotypes and other clinical phenotypes linked to the putative function of the gene.
Methods
The study was approved by the respective institutional review boards, and all patients gave written informed consent. Patients were recruited from movement disorder clinics in Germany, Serbia, and Japan. The sample populations (Table) consisted of patients with different dystonia phenotypes, including cervical, segmental, and generalized dystonia; patients with idiopathic PD with low scores (<15th percentile) on the University of Pennsylvania Smell Identification Test; and patients with tardive dyskinesias or acute dystonic reactions to dopamine receptor–blocking agents. The diagnoses were based on accepted clinical criteria. A detailed neurological assessment was performed on mutation carriers, including a videotaped neurological examination and assessment of dystonia severity (Burke-Fahn-Marsden Dystonia Rating Scale8) and cognition (Montreal Cognitive Assessment9). Olfaction was assessed using either the Brief Smell Identification Test (BSIT; Sensonics, Inc) or by intravenous administration of thiamine propyldisulfide (Alinamin) as previously described.10 The GAG deletion in TOR1A (RefSeq NM_000113) and mutations in THAP1 (RefSeq NM_018105) had previously been excluded in the German and Serbian patients with dystonia.11
We extracted DNA from blood samples using standard methods. Sanger sequencing was performed for all 12 exons of GNAL and exon-intron junctions (Supplement [eTable 1]). When a potentially pathogenic variant was identified, we screened for this variant in an ethnically matched control sample. Quantitative polymerase chain reaction of exon 9 was performed on a commercially available system (LightCycler 480; Roche Diagnostics) using an asymmetrical cyanine dye (SYBR Green; Life Technologies) to test for whole gene deletions/duplications (Supplement [eMethods]). To screen for GNAL expression in mutation carriers, RNA was extracted from peripheral blood leukocytes using a commercially available kit (PAXgene blood kit; Qiagen). After reverse transcription–polymerase chain reaction, sequencing was performed using primers located within exons 4 and 12 (Supplement [eTable 1]). Functional consequences of mutations were investigated using a BRET cell-based assay, whereby stimulation of D1 receptors by dopamine results in the dissociation of Gαolf from the heterotrimer, releasing Gβγ subunits tagged with venus fluorescent protein that become available for interaction with a reporter fragment derived from G protein receptor kinase 3 tagged with NanoLuc luciferase, thereby producing the BRET signal (described in detail in the Supplement [eMethods] and elsewhere1).
Results
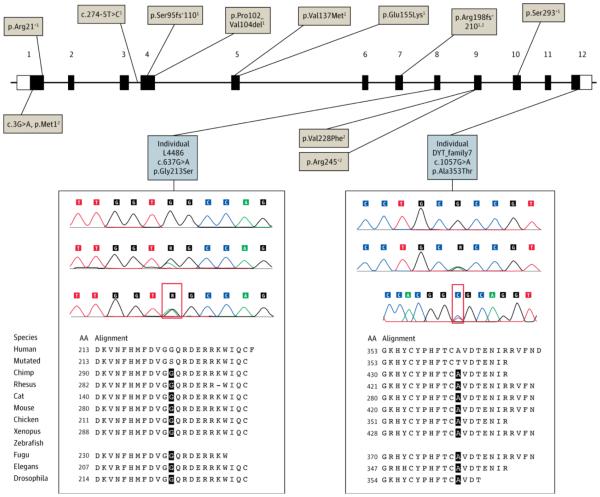
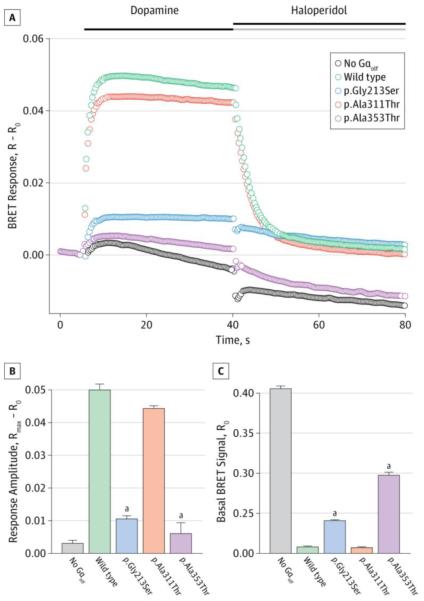
Four hundred sixty-one patients underwent screening, including 318 with dystonia, 51 with PD and hyposmia, and 92 with tardive dyskinesia or acute dystonic reactions. Approximately 18% of patients with cervical dystonia had a known positive family history. We identified the following two putatively pathogenic heterozygous missense variants in the GNAL gene (Figure 1): p.Gly213Ser in a German patient and p.Ala353Thr in a Japanese patient. Mutations were predicted to be damaging using three different software tools (Mutation Taster [http://www.mutationtaster.org], PolyPhen-2 [http://genetics.bwh.harvard.edu/pph2], and SIFT [http://sift.jcvi.org]) (Supplement [eTable 2]) and were absent in the exome variant server database (http://evs.gs.washington.edu/EVS) and in ethnically matched (538 German or 192 Japanese) control chromosomes. Furthermore, the BRET assay found that both variants disturbed Gαolf function markedly, with increased basal BRET ratios (R0) and a severely attenuated signal after application of dopamine (Rmax − R0) reflecting impairments in Gαolf-Gβγ heterotrimer formation and functional coupling to D1 receptors, respectively (Figure 2). A p.Ala311Thr variant was found in a German patient with sporadic dystonia confounded by the diagnosis of relapsing-remitting multiple sclerosis. The variant was absent in the German controls and predicted to be pathogenic on Mutation Taster and SIFT but was benign on PolyPhen-2 and indistinguishable from the wild type on the BRET assay. A p.Thr92Ala variant was detected in a patient of Filipino descent with cervical dystonia who was recruited from Germany; however, this variant was also present in 19 of 570 Filipino control chromosomes. In addition, we found the novel synonymous variants p.Ile222Ile and p.Cys352Cys in a German patient and a Japanese patient, respectively, and nonsynonymous variants in 2 German controls (p.Ala303Thr and p.Ile272Phe) and 1 Japanese control (p.Met373Ile).
Figure 1. Schematic Representation of Mutations in the GNAL Gene.
Tan boxes indicate previously reported mutations1,2; gray boxes, putatively pathogenic mutations detected in this study. Electropherograms show the wild-type sequence (above), the mutation (middle) at DNA level, and the complementary DNA (cDNA) sequence (below). Forward and reverse cDNA strands are shown for individuals L4486 and DYT_family7, indicating equal expression of the wild-type and mutant alleles (red boxes). The cross-species sequence alignment of stimulatory α subunit Gαolf obtained from Mutation Taster software (http://www.mutationtaster.org) is also shown.
Figure 2. Functional Effects of GNAL Mutations in a Cell-Based Bioluminescence Energy Transfer (BRET) Assay.
A, Time course of changes in BRET signal (R) after stimulation of cells expressing dopamine D1 receptor with dopamine and subsequent deactivation by haloperidol. B, Change in the BRET ratio from basal (R0) to maximal response (Rmax), reflecting the extent of the stimulatory α subunit Gαolf activation. C, Basal BRET ratios calculated before the application of dopamine, reflecting the extent of Gαolf association with the Gβγ subunits. Two of the mutations (p.Gly213Ser and p.Ala353Thr) had greatly diminished amplitudes of the BRET response after dopamine application (shown in parts A and B), with responses virtually indistinguishable from the random baseline fluctuations seen in the absence of Gαolf, suggesting that these mutations lead to a complete loss of Gαolf function. In contrast, the p.Ala311Thr variant performed similarly to the wild type. Error bars indicate SEM values. One-way analysis of variance followed by the Holm-Sidak method was performed to determine statistically significant differences relative to wild-type control.
aP < .001.
Sequencing of complementary DNA revealed comparable levels of mutant and wild-type messenger RNA (ie, equal expression of both alleles) in peripheral blood leukocytes from the two patients with likely pathogenic mutations (p.Gly213Ser and p.Ala353Thr). Both mutation carriers had onset of dystonia in the cervical region. No pathogenic mutations were identified in patients with PD and hyposmia, tardive dyskinesias, or acute dystonic reactions. No patients were found to have exon 9 deletions or duplications. A follow-up clinical evaluation was performed on mutation carriers. No other family members were available for clinical or genetic assessment.
Report of cases
The carrier of the p.Gly213Ser mutation was a man in his 50s (individual L4486) who had onset of cervical dystonia at 40 years of age. On examination he had severe retrocollis, laterocollis to the left, torticollis to the right, head tremor, left shoulder elevation, oromandibular dystonia, and blepharospasm (Video). The family history was negative for cervical dystonia, and results of assessment of olfaction (ie, sense of smell on the Brief Smell Identification Test) and cognition (29 of 30 on the Montreal Cognitive Assessment) were normal.
The Japanese patient carrying the p.Ala353Thr variant was a woman in her 50s with isolated cervical dystonia and an age at onset of 44 years. No evidence of hyposmia (normal latency and duration for the thiamine propyldisulfide test) or cognitive dysfunction (30 of 30 on the Montreal Cognitive Assessment) was found. Her father, who died in a motor vehicle crash at age 73 years, was also affected by cervical dystonia, with onset in the fifth decade of life after a traumatic head injury.
Discussion
Mutations in GNAL have been identified in families of European and African American descent with multi-incident dystonia.1,2 In this screening study, we detected likely pathogenic GNAL mutations in a German and a Japanese patient. The predominant phenotype appears to be dystonia, with onset in the neck and progression to other sites, particularly the cranial region. The family history in both GNAL mutation carriers was difficult to confirm given that no family members were available for assessment, and the father of the Japanese patient developed cervical dystonia after a head injury.
Putatively pathogenic mutations in GNAL were found in approximately 1% of patients with cervical dystonia in this sample. Most of the patients in this sample had sporadic cervical dystonia (82.1% without a known family history vs 17.9% with a known family history). This result extends the original study that found GNAL mutations in 19% of multiplex families with primary torsion dystonia.1 The GNAL gene is now one of several implicated as a cause of primary torsion dystonia, including TOR1A (DYT1), THAP1 (DYT6), and more recently CIZ1,12ANO3,13 and TUBB4.14 Of these genes, THAP1, CIZ1, ANO3, TUBB4, and GNAL are considered to have prominent craniocervical involvement, although the pathophysiological basis for this anatomical predilection is not clear. In the present study, neither carrier of confirmed mutations had evidence of hyposmia, so olfactory dysfunction may not be a useful biomarker for GNAL mutations. Moreover, no mutations were found in patients with PD and hyposmia, tardive dyskinesias, or acute dystonic reactions, so these phenotypes are less likely to be linked to mutations in GNAL.
Although GNAL has been suggested to be an imprinted gene, we demonstrated equal expression of mutant and wild-type alleles in peripheral blood leukocytes. This finding argues against allele-specific expression dependent on the parental origin of the allele. Furthermore, the BRET assay might serve as a valuable tool to support the pathogenicity of detected variants in the GNAL gene. Although dopamine receptor pathways are clearly implicated in the etiology of dystonia, potential Gαolf interactions with adenosine receptors might also be affected, or mutations could influence other unknown binding partners or activities. Therefore, if a variant exhibits wild-type behavior on the BRET assay, a dystonia-causing mutation is not necessarily excluded. Also of note, the ethnically matched control populations in this study were relatively small.
We have identified likely pathogenic GNAL mutations in patients of German and Japanese descent with craniocervical dystonia. Further studies of the pathophysiological mechanisms underlying GNAL mutations are now required.
Supplementary Material
A Patient With Severe Cervical Dystonia
A man with a GNAL mutation carrier (individual L4486 with a p.Gly213Ser mutation) with severe cervical dystonia (retrocollis, laterocollis to the left, and torticollis to the right), slight dystonic head tremor, and mild involvement of the oromandibular and ocular regions (slight involuntary muscle contractions). Proximal brachial dystonia with obvious elevation of the left shoulder is also observed.
Acknowledgments
Funding/Support: This study was supported by a grant-in-aid for exploratory research and grants-in-aid from the Research Committee of Central Nervous System Degenerative Diseases from the Japanese Ministry of Health, Labor, and Welfare (Dr Kaji); and by grants NS081282, DA021743, and DA026405 from the National Institutes of Health (Dr Martemyanov).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr Kumar receives a scholarship from the National Health and Medical Research Council (NHMRC). Dr Kostic is supported by a research grant from the Serbian Ministry of Education and Science (project grant 175090). Dr Sue receives grants from the Australian Brain Foundation and the NHMRC. Dr Westenberger is supported by a research grant from the Fritz Thyssen Foundation and by the Jake’s Ride for Dystonia research grant through the Bachmann-Strauss Dystonia & Parkinson Foundation. Dr Klein is supported by grants from the Bachmann Strauss Dystonia and Parkinson Foundation, intramural funds from the University of Luebeck, and the Hermann and Lilly Schilling Foundation.
Footnotes
Conflict of Interest Disclosures: No other disclosures were reported.
References
- 1.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45(1):88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vemula SR, Puschmann A, Xiao J, et al. Role of Gα(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet. 2013;22(12):2510–2519. doi: 10.1093/hmg/ddt102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasir J, Frima N, Pickard B, Malloy MP, Zhan L, Grünewald R. Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov Disord. 2006;21(6):859–863. doi: 10.1002/mds.20846. [DOI] [PubMed] [Google Scholar]
- 4.Corvol JC, Studler JM, Schonn JS, Girault JA, Hervé D. Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76(5):1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244(4906):790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 6.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20(1):69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 7.Corradi JP, Ravyn V, Robbins AK, et al. Alternative transcripts and evidence of imprinting of GNAL on 18p11.2. Mol Psychiatry. 2005;10(11):1017–1025. doi: 10.1038/sj.mp.4001713. [DOI] [PubMed] [Google Scholar]
- 8.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa M, Kamide M, Miwa T, Umeda R. Significance of intravenous olfaction test using thiamine propyldisulfide (Alinamin) in olfactometry. Auris Nasus Larynx. 1988;15(1):25–31. doi: 10.1016/s0385-8146(88)80006-3. [DOI] [PubMed] [Google Scholar]
- 11.Lohmann K, Uflacker N, Erogullari A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20(2):171–175. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao J, Uitti RJ, Zhao Y, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71(4):458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlesworth G, Plagnol V, Holmström KM, et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91(6):1041–1050. doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann K, Wilcox WR, Winkler S, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. doi: 10.1002/ana.23829. [published online December 13, 2012] doi:10.1002/ana.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Patient With Severe Cervical Dystonia
A man with a GNAL mutation carrier (individual L4486 with a p.Gly213Ser mutation) with severe cervical dystonia (retrocollis, laterocollis to the left, and torticollis to the right), slight dystonic head tremor, and mild involvement of the oromandibular and ocular regions (slight involuntary muscle contractions). Proximal brachial dystonia with obvious elevation of the left shoulder is also observed.