Abstract
Previous research on inter-individual variation in the calls of corvids has largely been restricted to single call types, such as alarm or contact calls, and has rarely considered the effects of age on call structure. This study explores structural variation in a contextually diverse set of “caw” calls of the American crow (Corvus brachyrhynchos), including alarm, foraging recruitment and territorial calls, and searches for structural features that may be associated with behavioural context and caller sex, age, and identity. Automated pitch detection algorithms are used to generate 23 pitch-related and spectral parameters for a collection of caws from 18 wild, marked crows. Using principal component analysis and mixed models, we identify independent axes of acoustic variation associated with behavioural context and with caller sex, respectively. We also have moderate success predicting caller sex and identity from call structure. However, we do not find significant acoustic variation with respect to caller age.
Keywords: acoustic feature analysis, call classification, caller identity, sexual dimorphism, vocal ontogeny, PACS Code 43.80.Ka
Introduction
In species with complex social structures, it is often adaptive to exchange information on identity, sex, age and other attributes with conspecifics. Accurate signalling and recognition of identity may reduce the risk of inbreeding, decrease the intensity and frequency of aggressive interactions with conspecifics, and facilitate reciprocal altruism and kin selection (Tibbetts and Dale 2007). Not only contact calls and territorial advertisements, but even alarm signals may be more useful if they are individually distinctive, as they enable the receiver to selectively attend to signals produced by more reliable or informative senders (Blumstein et al. 2004, Pollard 2011). On the other hand, individual distinctiveness can be costly to the sender, as it makes deception more difficult, and allows receivers and eavesdroppers to consistently target the sender for harmful behaviour, e.g. predation attempts, social punishment, or rejection as a potential mate (Dale et al. 2001, Tibbetts and Dale 2007).
The corvids - crows, magpies, jays and their allies - are highly social songbirds, living in groups comprising individuals of different ages and sexes. Many species engage in cooperative breeding, foraging, and defence behaviours, and consistent relationships between individuals can last for many years (Kilham 1989; Ekman and Ericson 2006). Corvids are extremely vocal, employing calls in disputes over food and territory, collective defence against predators, and recruitment of conspecifics to food resources (Chamberlain and Cornwell 1971; Baeyens 1981; Heinrich 1988; Ha et al. 2003). Corvid species are therefore good candidates for the ability to recognize other individuals and small groups of conspecifics by their vocalizations, and indeed there is evidence of this ability in several species. Mexican jays (Aphelocoma ultramarina) can discriminate the “primary calls” of in-group birds from those of birds outside of their family groups (Hopp, Jablonski, and Brown 2001). Adult pinyon jays (Gymnorhinus cyanocephalus) recognize the calls of mates and of related nestlings, and nestlings preferentially beg in response to the calls of related adults (McArthur 1982). Juvenile rooks (Corvus frugilegus) can discriminate the calls of siblings from those of similarly aged non-siblings (Røskaft and Espmark 1984). Jungle crows (Corvus macrorhynchos) can be trained to show discrimination between the contact calls of familiar conspecifics (Kondo et al. 2010), while captive carrion crows (Corvus corone corone) spontaneously discriminate between the calls of familiar jackdaws (Corvus monedula) (Wascher et al. 2012). Jungle crows have even demonstrated the ability to recognize group members cross-modally, matching calls to visual representations (Kondo et al. 2012).
Although recognition of individuals and groups has thus been demonstrated, it is less clear what acoustic cues corvids rely on to perform such discrimination tasks. The studies that have explored this question have generally done so from a signal analysis perspective, by analysing the calls themselves and searching for properties that correlate significantly with caller identity. These studies, however, have for the most part confined themselves to a single call type, such as the “inflected alarm caw” of the American crow (Corvus brachyrhynchos), the “krah” call of the Hooded crow (Corvus corone cornix), the “ka” contact call of the jungle crow (Corvus macrorhynchos), and the food call of the common raven (Corvus corax) (Brown 1985; Allenbacher et al. 1995; Kondo et al. 2010; and Boeckle et al. 2012, respectively). This leaves open the question of whether corvids possess consistent signatures of individual identity across their entire repertoire of species-typical vocalizations. Such consistent signatures are not always found in other taxa, even if particular subsets of their calls are individually distinct. For instance, both alarm barks and contact barks in chacma baboons (Papio ursinus) show individually distinctive characteristics, but the pattern of individual variation is not consistent between bark types (Fischer et al. 2001). On the other hand, rhesus macaques (Macaca mulatta) and South Polar skuas (Catharacta maccormicki) each show individual characteristics in some call types and not others, but across all individually distinctive call types, the same parameters are relevant for individual identification (Rendall, Owren, and Rodman 1998; Charrier et al. 2001). Moreover, only a few studies of corvids have looked for acoustic correlates of demographic attributes above the individual level, such as sex and age (Laiolo, Palestrini, and Rolando 2000; Yorzinski et al. 2006). The American crow is a promising subject for research in this area, due to its complex social life and vocal behaviour (reviewed in Verbeek and Caffrey 2002). American crows are sexually monomorphic in appearance and most behaviour, although adult males are somewhat heavier on average than adult females. Crows are facultative cooperative breeders, with 0-10 nonbreeding auxiliaries residing on the territory of the breeding pair. Many of these auxiliaries are offspring from previous years, who may remain on their natal territory for up to six years before breeding themselves. Although both sexes of American crows reach physiological maturity by the end of their second year, most females are at least three years old before they establish independent breeding territories and take social mates, and most males are at least five (Verbeek and Caffrey 2002; Robinson, Jr. 2009).
Though not celebrated for their song, American crows produce a wide variety of vocalizations. One of the most common calls is the caw, produced in contexts ranging from territorial defence to food recruitment to predator mobbing (Tarter 2008), and general mild alarm (Yorzinski et al. 2006). Caws are harmonically rich and locally tonal, varying from a few hundred milliseconds to several seconds in length, and contain rapid variations in pitch and amplitude, which produce a “harsh” quality to the human ear (Laiolo and Rolando 2003). They are acoustically distinct from the rhythmic and largely atonal rattles, constant-frequency coos, and lower-pitched begging calls, which are also produced by American crows (Kilham 1989; Tarter 2008).
Caws vary considerably in pitch, duration, cadence and timbre, and observers have long sought to decode their behavioural significance. This task has proven quite challenging, due to the diversity of caws, the continuous distribution of their acoustic properties, and the difficulty of verifying age, sex and individual identity in the field for this gregarious, monomorphic species. The earliest attempts at comprehensive caw classification were based primarily on observations of large groups of unmarked birds, and defined most caws by their apparent collective function, e.g., assembly, dispersal, scolding and alert calls (Frings et al. 1958; Chamberlain and Cornwell 1971; Richards and Thompson 1978). Later researchers, such as Brown (1985), Parr (1997), and Tarter (2008), constructed classification systems that were based more purely on the acoustic properties of the caws. Parr in particular identifies approximately ten structural types of caw (short, medium and long; harsh, “ko”s, and “koaw”s; 2-syllable, doubled short, long-medium and medium-short). Parr draws many correspondences between her categories and those of earlier studies; for instance, her “ko” call matches Brown’s “inflected alarm caw” and Chamberlain and Cornwell’s “simple scolding call.” Nevertheless, most categories in one author’s classification system overlap with several in another, and it is not yet entirely clear how these systems will be reconciled.
No sex- or age-specific call types have been confirmed among birds past their first year, and Parr (1997) found that both sexes produced all types of “territorial” caws. However, the frequency of usage of certain call types may vary with sex and breeding status (Tarter 2008). Sexual dimorphism within caw types was reported by Davis (1958), although this was based on field observations without reliable verification of caller sex. The strongest evidence for individual distinctiveness and sex differences in caw structure has been produced by Yorzinski et al. (2006). Restricting their analysis to the “inflected alarm caw” (Brown 1985), Yorzinski et al. found that the caws of females tended to have a higher pitch, shorter duration, higher frequency, greater bandwidth, and more peaked pitch contours than those of the males, and linear classifiers were able to sort calls by sex and caller identity with rates well above chance.
In this study, we sought to extend the findings of Yorzinski et al. by searching for signatures of identity, sex, age, and behavioural context within a diverse set of caws that was not pre-sorted by type. The caws were recorded from a banded population of wild yearling and adult American crows in Ithaca, NY, which was also studied by Yorzinski et al. (2006), Tarter (2008) and Yorzinski & Vehrencamp (2009). Because caw pitch oscillates rapidly, caws are difficult to represent precisely using conventional Fourier analysis, which suffers from an inherent trade-off between time and frequency resolution (Cohen 1989). We therefore developed a pair of novel pitch detection algorithms with superior frequency resolution over short signal frames, and used them to represent the calls as locally periodic signals, similar to those generated by source-filter models of speech production. We then parameterized the calls according to fundamental frequency, amplitude and spectral properties. Finally, we explored the resulting distributions of call properties with respect to caller identity, sex, age and behavioural context. Specific hypotheses addressed were:
Individual Identity: The acoustic parameters of individual calls vary significantly with caller identity. An automated classifier can use these parameters to predict the identity of callers with accuracy above chance.
Sex and Age: The acoustic parameters of individual calls vary significantly with caller sex and age. Automated classifiers can use these parameters to predict the sex and age of callers with accuracy above chance. Interactions may exist, such that older birds show higher degrees of sexual dimorphism on acoustic parameters.
Behavioural context: The acoustic parameters of individual calls vary significantly with the observed behavioural context in which the call is produced (e.g. alarm/mobbing, territorial displays or recruitment to a food source). This variation is independent of that associated with identity/sex/age, allowing calls to provide simultaneous information about multiple caller attributes.
Materials & methods
Recording
Calls were recorded in Cayuga Heights, NY, June-August 2006, from wild crows marked as part of a long-term study of crows in the Ithaca (Tompkins Co.), NY area by the Ithaca Crow Research Group. The Ithaca Crow Research Group, made up of researchers from Binghamton and Cornell Universities, has marked nestlings and adult birds with leg bands and patagial tags annually since 1989. The calls analysed for the current study were recorded from a vehicle parked on known family territories. Territories of the birds in this study were all located in fairly quiet residential-suburban neighbourhoods or adjacent open managed habitats such as golf courses or cemeteries (McGowan 2001). If the resident family could not be located before the start of recording, peanuts were scattered to attract them, but there was no other interaction with the birds. Recordings were made in WAV format, at a 48 kHz sampling rate, using a Marantz PMD-670 solid-state recorder and a Sennheiser ME-67 directional microphone with wind shield. Call bouts were extracted in Amadeus II v3.8.7 (Hairersoft, 2007); all further processing was done in MATLAB R2009B and R2010B, using the Signal Processing and Statistics toolboxes, and in R 3.0.1, using the nlme and AICcmodavg package (Pinheiro et al. 2013, Mazerolle 2013).
Call selection
1,674 calls were selected for a high (>93%) signal to noise ratio, a visible caller identifiable by its leg bands and patagial tags, and a lack of overlap with other birds’ vocalizations. All calls used were tonal, harmonically rich “caws,” and were produced by 18 birds, 11 male and 7 female (Table 1). Birth years were known, and age from fledging was estimated using a fledging date of June 1. The typical fledging period for this population is late May through June, so ages are expected to be accurate to within approximately 30 days. Ages were 396-4833 days, with median 816; no birds younger than 1 year were included. We included 7-337 calls per bird; only three birds had <=15 calls each. A subset of 305 calls had behavioural contexts identified by observation. Contextual categories were: 1) food recruitment, 2) territorial “counter-cawing” (defined in Parr 1997), 3) beg rebuffs and 4) alarm calls. (Categories are further described in Table 4.) For the remaining calls, context was unclear or ambiguous in some way, including the focus of the caller’s attention being unknown, or the caller being accompanied by unidentified conspecifics.
Table 1.
Demographic and recording data for subjects of this study. Ages are given in years since fledging; all birds were at least one year old.
| Bird ID |
Sex | Age | Family | # of Calls Used |
# of Call Bouts Used |
# of Days Recorded |
Fraction of Total Call Weight |
|---|---|---|---|---|---|---|---|
| MH | f | 1 | NEEL | 145 | 41 | 3 | 6.82% |
| UB | f | 1 | CLAR | 15 | 5 | 2 | 3.71% |
| ZU | f | 1 | WWCK | 120 | 38 | 4 | 6.62% |
| AZ | f | 2 | NEEL | 39 | 12 | 6 | 5.12% |
| OY | f | 4 | WWCK | 337 | 87 | 10 | 7.61% |
| 8Z | f | 5 | SEPG | 117 | 25 | 7 | 6.30% |
| N1 | f | 6 | WKAY | 175 | 44 | 10 | 6.91% |
| AS | m | 1 | WWCK | 178 | 35 | 7 | 6.71% |
| IL | m | 1 | NEEL | 80 | 28 | 2 | 6.35% |
| KJ | m | 1 | NEEL | 29 | 12 | 3 | 5.09% |
| RE | m | 1 | CLAR | 24 | 6 | 3 | 4.01% |
| XW | m | 1 | WWCK | 169 | 40 | 6 | 6.83% |
| FT | m | 2 | CLAR | 58 | 10 | 3 | 5.07% |
| 33 | m | 3 | ORHA | 49 | 27 | 5 | 6.10% |
| 0E | m | 4 | NEEL | 25 | 6 | 2 | 4.10% |
| 0O | m | 4 | SEPG | 7 | 5 | 1 | 3.45% |
| BF | m | 11 | ROWA | 10 | 3 | 2 | 2.96% |
| AP | m | 13 | WKAY | 97 | 25 | 6 | 6.25% |
Table 4.
Observed behavioural contexts associated with a subset of calls (305 out of 1674).
|
Behavioural
Context |
Observational Criteria | #of Caws Included in Set |
#of Male Callers |
# of Female Callers |
|---|---|---|---|---|
| Food Recruitment |
Given by perched or standing birds on their territory, after bait was provided. Family members responded by approaching and foraging or begging. |
155 | 1 | 1 |
| Territorial Counter- Cawing |
Given by perched birds facing out of territory, while birds on neighbouring territories responded with a similar cadence. |
13 | 1 | 2 |
| Alarm Call | Given by perched birds watching a mammalian predator or potential predator (cat, human, skunk or squirrel) and calling with head downward. |
48 | 3 | 1 |
| Beg Rebuff | Given by adult birds in response to begging juveniles while on the ground. They did not feed the juveniles or coo at them, and almost always moved away while calling. Several times, they took flight and were pursued by juveniles at a low altitude. |
89 | 0 | 3 |
Representation of calls as locally periodic signals
Calls were represented as locally periodic signals with smoothly varying fundamental frequency (pitch). They were divided into overlapping frames, and within each frame they were represented as a sum of harmonics of a single fundamental frequency. The fundamental frequency of each frame was determined using an extension of Friedman’s Pseudo-Maximum Likelihood Estimator (PMLE), originally developed to estimate the pitch of human speech (Friedman 1977). We have developed two period indicator functions from the PMLE, which will be summarized here (E. A. Mates and J. C. Ha, unpublished data). The approximate pseudo-maximum likelihood estimator (APLE) approximates the PMLE with a weighted sum of autocorrelations that can be computed more efficiently. The extended pseudo-maximum likelihood estimator (EPLE) calculates the PMLE precisely, but allows for non-integer-valued periods and periods that vary over the span of a frame, and for arbitrarily chosen bandwidths. Additional details on these algorithms can be found in the supplementary materials.
To illustrate the resultant locally periodic representation, an example is displayed alongside a traditional spectrogram of the same call (Figure 1). For the calls used in this set, locally periodic approximations captured an average of 92.90% of each filtered frame’s energy. Measured fundamental frequency did indeed vary smoothly, with an average RMS fundamental frequency shift of 12.67 Hz between frames.
Figure 1.
Traditional and locally periodic representations of a crow call. The call is from AS, a yearling male. (A) Traditional spectrogram, using 512-sample Hamming-windowed frames and a 6-sample step size. (B) Locally periodic representation, using 240-sample frames and a 24-sample step size. Note that this representation assigns energy only to frequencies corresponding to harmonics of the estimated fundamental for each frame.
Parameterization of calls
Each clip of a single call was automatically partitioned into a “voiced” section preceded and followed by “silent” sections, based on the estimated call energy in each frame. Only the voiced section was used for further analysis.
Twenty-three parameters were extracted from each call (Table 2): 11 based on the pitch trace; six based on the power envelope; and six based on the spectral properties. Among the pitch trace parameters, the mean, 5th percentile and 95th percentile pitch were estimated from the pitch distribution. A cubic polynomial was fit to the pitch trace, in order to represent its gradual variation over the course of the call. The cubic curve was then subtracted from the pitch trace to leave a residual. The RMS magnitude of the residual was recorded as the “pitch instability.” The residual was expected to contain a “wobble” or high-frequency modulation in pitch, which is typically found in “caw”-type calls across the genus Corvus (Laiolo and Rolando 2003). This wobble’s fundamental frequency was estimated, and two alternative estimates of its magnitude were also computed.
Table 2.
Descriptive statistics of the acoustic call parameters used. The last column contains the coefficient of multiple determination for each parameter, when regressed on the 13 canonical variables used for call classification.
| Parameter | M | σ | γ1: Skew |
γ2: Excess Kurt. |
R2 by Canon. Vars. |
|
|---|---|---|---|---|---|---|
|
Pitch
Contour |
01. Mean F0 | 604.34 | 75.82 | −0.19 | −0.99 | 0.98 |
| 02. 95th Pctl F0 | 696.63 | 66.08 | −0.35 | −0.46 | 0.95 | |
| 03. 5th Pctl F0 | 476.60 | 84.26 | 0.58 | −0.81 | 0.84 | |
| 04. F0 Peak Location* | −0.13 | 0.19 | 0.44 | 1.38 | 0.86 | |
| 05. F0 Peak Value | 668.56 | 77.95 | −0.50 | −0.08 | 0.93 | |
| 06. F0 quadratic term | 187.71 | 83.46 | 0.00 | −0.60 | 0.90 | |
| 07. F0 cubic term* | 50.67 | 35.25 | −1.60 | 4.70 | 0.90 | |
| 08. Wobble Frequency | 50.15 | 12.04 | 0.04 | −0.85 | 0.99 | |
| 09. Wobble Periodicity 1* | 0.52 | 0.13 | 0.46 | 0.07 | 0.97 | |
| 10. Wobble Periodicity 2 | 0.38 | 0.15 | 0.67 | 0.31 | 0.98 | |
| 11. Pitch Instability | 28.16 | 12.88 | 0.80 | 1.57 | 0.72 | |
|
Power
Envelope |
12. Call Length* | −2.56 | 0.75 | 0.23 | −0.76 | 0.96 |
| 13. 2nd central moment* | −4.59 | 0.78 | 0.32 | −0.61 | 0.97 | |
| 14. 3rd central moment | 0.02 | 0.04 | 0.19 | 1.50 | 0.94 | |
| 15. 4th central moment* | −4.29 | 0.79 | 0.28 | −0.74 | 0.97 | |
| 16. Wobble Frequency | 47.97 | 15.67 | 0.55 | −0.84 | 0.99 | |
| 17. Wobble Magnitude | 0.38 | 0.17 | 0.26 | −0.45 | 0.97 | |
|
Spectral
Properties |
18. Time-averaged harmonic powers, 1st principal component |
0.60 | 0.99 | −0.46 | −1.13 | 0.89 |
| 19. Time-averaged harmonic powers, 2nd principal component |
−7.62 | 1.01 | 2.68 | 10.89 | 1.00 | |
| 20. Time-averaged harmonic powers, 3rd principal component |
4.36 | 1.00 | −0.11 | 12.80 | 0.98 | |
| 21. Linear trend in harmonic powers, 1st principal component |
0.19 | 0.98 | −1.19 | 3.50 | 0.99 | |
| 22. Linear trend in harmonic powers, 2nd principal component |
−0.29 | 1.03 | −1.69 | 9.59 | 0.99 | |
| 23. Linear trend in harmonic powers, 3rd principal component |
−0.11 | 0.99 | −0.48 | 31.28 | 0.98 |
Log- or power transformed. See text for details.
Among the signal power parameters, the duration of the voiced section of each clip was used as a measure of call length. The central moments of call energy with respect to time were also computed. As was the case for the pitch trace, there was expected to be a rapid “wobble” in signal power; its fundamental frequency and magnitude were estimated.
For the spectral parameters, we computed the relative energies of the first twelve harmonics in each frame. The time-averaged values and derivatives of these energies were then computed. Finally, we recorded the three largest principal components of the energy averages and the energy derivatives, respectively.
Six of the 23 parameters appeared to have distinctly non-normal distributions in terms of skewness and kurtosis, and were therefore transformed toward normality with logarithm or power transforms (Table 2).
Additional details on parameterization can be found in the supplementary materials.
Description & classification
Principal component analysis was used to reduce the number of variables from the original 23 parameters. Enough components were retained to account for >50% of the variance on each of the original parameters. Save for the largest component (which accounted for >3 times as much variance as any other component), the retained components were varimax-rotated so that each component would be more strongly correlated with one or more original parameters, improving its interpretability.
To investigate sex and age-related variation, we fit linear mixed-effect models to each component. Caller identity and call bout were specified as random effects. Fixed predictors included sex, linear and quadratic terms for log-transformed age. All distinct subsets of main effects and first order interactions were examined, under the usual constraint that no subset may contain a product or quadratic term unless it also contains the main or linear terms as well. This resulted in a total of 6 models. All models were fit with R 2.15.2, using version 3.1-108 of the “nlme” package (Pinheiro et al., 2010).
The explanatory power of models was judged by the Akaike Information Criterion with small-sample correction (AICc, Hurvich & Tsai, 1989). To extract predictions and effect estimates, we followed a model-averaging approach as described by Burnham and Anderson (2002). Model averaging was performed in R 2.15.2 with version 1.27 of the “AICcmodavg” package. Parameters were tested for significant difference from zero, using α = .0039, the Dunn-Šidák correction to α = .05 for 13 independently tested principal components. Only fixed effects were tested for significance, as AICcmodavg does not permit model-averaged estimation of random parameters; there exists relatively little literature on the latter question.
To investigate behavioural context-related variation, we again fit mixed-effect models to each component, specifying caller identity and call bout as random effects, and caller sex, age, and three orthogonal behavioural context variables as fixed predictors (Table 5). Eight models were compared for each component, and effects estimated using the model averaging approach described above.
Table 5.
Behavioural context-related variation in principal components of acoustic parameters, on a subset of 305 calls. β coefficients are averaged across mixed-effect models containing various subsets of sex, linear age and behavioural context as fixed effects. Context is coded with three orthogonal contrasts. Reported p-values correspond to t-tests that each coefficient differs from zero.
|
Principal
Component |
β: Sex | β: Age (Lin.) | β: C1 | β: C2 | β: C3 |
|---|---|---|---|---|---|
| 01 | 0.15 | −0.10 | −0.02 | −0.97** | 0.19 |
| 02 | 0.46 | 0.32 | −0.43 | −0.27 | 0.05 |
| 03 | 0.34 | 0.31 | 0.22 | 0.03 | 0.28 |
| 04 | −0.22 | −0.88 | 0.30 | −1.07** | −0.31 |
| 05 | 0.16 | 0.05 | −0.15 | 0.00 | 0.03 |
| 06 | 0.25 | 0.07 | 0.41 | −0.14 | 0.28 |
| 07 | −0.37 | 0.49 | −1.35 | 0.57 | 1.13* |
| 08 | 0.10 | 0.01 | 0.25 | 0.18 | −0.05 |
| 09 | 0.15 | −0.14 | −0.40 | −0.33** | 0.11 |
| 10 | 0.09 | 0.13 | −0.40 | 0.34** | −0.06 |
| 11 | −0.10 | 0.21 | −0.35 | −0.07 | −0.08 |
| 12 | −0.14 | 0.10 | −0.34 | −0.02 | −0.03 |
| 13 | −0.30 | 0.35 | −0.20 | 0.33* | 0.78* |
p < 0.0039
p < 0.00077
C1: Counter-Cawing vs. Food Recruitment, Alarm Call and Beg Rebuff
C2: Food Recruitment vs. Alarm Call and Beg Rebuff
C3: Alarm Call vs. Beg Rebuff
Calls were classified by sex, age, and caller identity, using linear discriminant analysis (LDA) on selected sets of principal components. Continuous dependent variables are not suitable for LDA, so we dichotomized age at a threshold of 2.0 years after hatching, thus separating subjects into “juvenile” and “adult” age groups. For the sex (age group) classifier, we used all components that were found to show a significant effect of sex (age group) in the mixed-effect models. If no components were found to show a significant effect, those with a marginally significant effect (corrected α = .0081) were used instead. For the identity classifier, we selected components differently, as identity was a random rather than fixed effect. We selected all components for which identity was estimated as accounting for at least 10% of their variance in the mixed-effect models.
Classifier accuracy was compared to the accuracy of three types of “chance” classifier: a classifier that randomly assigns classes to calls with uniform probability; a classifier that assigns all calls to the most common class; and a classifier of the same construction as our true classifiers, but applied to a data set with randomly permuted class memberships, following the recommendation of Mundry & Sommer (2007). The random permutation procedure is described in more detail in the supplementary materials. Classifier accuracy was also cross-validated using a “leave one out” procedure, performed at the level of the next nested grouping variable. That is, the sex and age-group classifiers were repeatedly trained on the calls of all but one individual, then tested on the calls of the remaining individual. The individual identity classifier was repeatedly trained on all but one call bout, then tested on the remaining call bout. Unless otherwise noted, all classifier accuracy values given in the text refer to cross-validated performance.
Additional detail on the construction and evaluation of mixed models and linear classifiers can be found in the supplemental materials.
Weighting
Because the number of calls and call bouts available for each bird varied considerably, it was necessary to weight the calls in a non-uniform fashion for data analysis. We chose to weight them following a rule for optimal least squares weighting of group means (Isaev 1979). The resultant weights tended to favour calls belonging to bouts and/or individuals that were represented by few other calls. The total weight assigned to the calls of each individual is shown in Table 1. It can be seen that individuals with more calls tend to receive more total weight, because their mean parameter values can be estimated more accurately; however, they do not receive as much weight as if all calls were weighted uniformly. Additional details can be found in the supplementary materials.
These weights were used for calculating all descriptive statistics, for transforming acoustic parameters, for principal component analysis and linear discriminant analysis, and for measuring the accuracies of our linear classifiers. They were not used in our mixed-effect models, as the algorithms used in nlme allow for unbalanced designs.
Results
Principal components
Thirteen principal components were sufficient to capture more than 50% of the variance of every parameter (Table S1, supplemental). Collectively, they captured 94.14% of the total variance. Principal Component 01 captured 39.58% of the total variance, over three times as much as the next largest component. The other 12 components each captured 3.3-7.4% of the total variance, after varimax rotation (or 2.5-9.2% before rotation). PC 01 was highly positively correlated (Pearson’s r > 0.71) with three parameters associated with call length, and highly negatively correlated with six parameters associated with call pitch, pitch wobble periodicity, pitch contour concavity and the first spectral component. In other words, calls with high PC 01 values were longer, lower-pitched, with flatter pitch contours that had less regular pitch wobble, and with more energy in the third harmonic as opposed to the second. The distribution of PC 01 was bimodal, but not discrete; no discrete call cluster could be identified via this or any other component (Figure 2).
Figure 2.
Distribution of call lengths and mean pitches among all calls, with first principal component of acoustic parameters projected onto this surface.
PC 02 was highly negatively correlated with the residual of various pitch parameters after subtraction of PC 01; that is, calls with high PC 02 values were unusually low-pitched, compared to other calls with similar PC 01 values but low PC 02 values (Table S1). PC 05 was associated with parameters measuring pitch contour symmetry, and PC 08 was associated with parameters measuring the magnitude of pitch wobble. All other principal components were highly correlated with one parameter each.
The percentage of residual variance (that is, the variance not ascribed to call bout or caller identity in a null model) varied between 8.96% (for PC 01) and 77.48% (for PC 010). For seven principal components, over 50% of the estimated variance was residual, suggesting that calls are not entirely stereotyped within bouts.
Individual identity
For all but two principal components (PC 01 and PC 03), caller identity accounted for less than 15% of their variance when a null model was used (Table S1). It accounted for 1.5-5.0% of the variance of PCs 05, 08, 09, 10, and 12, and 10.4-14.5% of the variance of PCs 02, 04, 06, 07, 11, and 13.
When all principal components with over 10% of variance accounted for by caller IDwere used in an LDA classifier, it correctly classified 35.36% of calls (24.24% cross-validated) by identity, significantly above chance (Table S2, supplemental).
Sex
Only PC 02 showed a significant main effect of sex, with females averaging lower values than males (Table 4). This implies that, for calls with a given value of PC 01, the calls of females are on average higher-pitched than the calls of males. Male and female PC 02 distributions overlap considerably, leading to a standardized β of 0.3; this predicts that female calls will be approximately 22 Hz higher in mean frequency, and 26 Hz higher in maximum pitch, than male calls with the same value of PC 01. The overlap is apparently due to variance between males, as well as within the call sets of individual males; three males (AS, BF and RE) had mean PC 02 values in the female region.
An LDA classifier using PC 02 correctly classified 66.65% of calls (66.19% cross-validated) by sex, significantly above all “chance” classifiers (Table S2). Two males (BF and RE) and one female (ZU) had calls that were categorized by sex with below-chance accuracy.
Age
No components showed significant main effects of age, although for four components (PCs 08, 11, 12 and 13), the models with linear and/or quadratic age terms had the lowest AICc value (Table 3). Marginal main effects (p < 0.0081, equivalent to α = 0.1 with Dunn-Šidák correction) were found for PCs 11 and 12, associated with harmonic linear trends and amplitude wobble frequency, respectively.
Table 3.
Sex and age related variation in the principal components of acoustic parameters, on the full set of 1674 calls. β coefficients are averaged across mixed-effect models containing various subsets of sex and linear/quadratic age as fixed effects. Reported p-values correspond to t-tests that each β coefficient differs from zero.
|
Principal
Component |
β: Sex | β: Age (Lin.) |
β: Age (Quad.) |
β: Sex:Age (Lin.) |
β: Sex:Age (Quad.) |
Lowest
AICc Model |
Akaike
Weight of Lowest Model |
|---|---|---|---|---|---|---|---|
| 01 | 0.13 | 0.03 | 0.14 | −0.15 | 0.19 | Null | 0.37 |
| 02 | 0.30** | 0.07 | 0.09 | −0.08 | −0.19 | Sex | 0.45 |
| 03 | 0.00 | −0.14 | 0.05 | −0.08 | 0.28 | Null | 0.31 |
| 04 | 0.00 | −0.11 | 0.15 | 0.01 | 0.07 | Null | 0.3 |
| 05 | 0.07 | −0.05 | 0.00 | −0.02 | 0.18 | Null | 0.3 |
| 06 | 0.01 | −0.06 | −0.10 | 0.01 | −0.01 | Null | 0.4 |
| 07 | 0.01 | 0.09 | 0.29 | −0.12 | −0.44† | Null | 0.28 |
| 08 | 0.04 | 0.08 | 0.02 | −0.07 | 0.02 | Age (Lin.) | 0.21 |
| 09 | 0.00 | 0.11 | 0.21† | −0.15* | −0.23** | Sex * Age (Quad.) |
0.61 |
| 10 | −0.02 | −0.02 | −0.01 | 0.08 | −0.07 | Null | 0.42 |
| 11 | 0.10 | 0.23† | −0.02 | 0.05 | 0.16 | Age (Lin.) | 0.35 |
| 12 | 0.01 | −0.07 | −0.15† | −0.03 | −0.05 | Age (Quad.) | 0.58 |
| 13 | 0.10 | −0.19† | −0.03 | 0.03 | −0.02 | Age (Lin.) | 0.31 |
p < 0.0081
p < 0.0039
p < 0.00077
An LDA classifier using PCs 11 and 12 correctly classified 60.35% of calls (57.52% cross-validated) by age group. This did not significantly exceed the accuracy of a “chance” classifier based on randomly permuted data (Table S2).
Interactions between sex and age
Significant age/sex interaction effects were found on PC 09, associated with energy wobble magnitude (Table 3). This interaction is difficult to interpret, as our individuals included males of ages 11 and 13 but no females older than 6. It appears to reflect the fact that PC 09 values were particularly low for females aged 2-5, relative to younger females and males of all ages.
Behavioural context
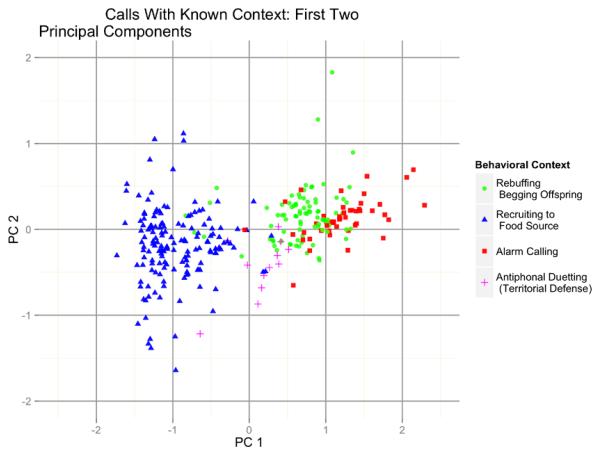
Six principal components varied significantly with at least one contextual contrast (Table 5). PC 01 in particular was almost parallel to the first linear discriminant for context (Pearson’s r = 0.96). It effectively separated food recruitment calls (which tended to be brief and high-pitched) from alarm calls and beg rebuffs (which tended to be longer and lower-pitched), with counter-cawing falling somewhere in the middle (Figure 3). Certain beg rebuffs were acoustically more similar to food recruitment calls, and may actually have been such calls, since they occurred in a context where a breeding female was near begging offspring and scattered food items simultaneously.
Figure 3.
Distribution of calls with known behavioural context, on two principal components.
PCs 04, 09, 10 and 13 also varied significantly with certain contrasts, although the effect sizes were not large enough to separate contextual classes of calls using these components. No significant effects of sex or age were observed in models that also included context; in particular, the sex effect on PC 02 did not reach significance (p = .017). However, this p-value was still smaller than the p-values for the context effects on PC 02 (p > .06 in all cases). Furthermore, the model-averaged, unstandardized effect of sex on PC 02 was not significantly affected by whether the models were fit to known-context or unknown-context calls (B = 0.46, SE = 0.19 for the known-context subset; B = 0.28, SE = 0.06 for the unknown-context subset; z = 0.91, p = 0.36). The random effect of identity accounted for <4% of estimated variance in PCs 01, 06, 09, 10, and 12, and 12-89% of estimated variance in all other PCs.
Discussion
The primary source of variation in the acoustic structure of “caw”-type calls appears to be behavioural context. A single principal component (PC 01) accounted for approximately 40% of the variance in the parameters we recorded, chiefly those measuring call length, mean pitch, and the wobble and peakedness of the pitch contour. The value of PC 01 did not vary significantly with age or sex, and the majority of its variance was not explained by caller identity. However, it did vary significantly with context, particularly the contrast between food recruitment calls on one hand, and alarm calls and beg rebuffs on the other. While this statistical relationship could only be tested for that subset of calls with known contexts (roughly 18% of the entire call set), context is a plausible explanatory factor for PC 01’s variation over the entire set as well, given the component’s statistical independence from other caller properties. Unfortunately, the four contextual categories that we were able to identify excluded common behaviours such as aerial mobbing, territorial fights, or calling in chorus, because birds engaging in these behaviours were more difficult to identify and record individually. It is probable that, if detailed contextual information were available for all calls, additional components of acoustic variation would turn out to be context-associated as well. This could be confirmed by studies similar in breadth to the current one, but conducted on calls that have been derived from other contexts, even if produced by unmarked birds.
The calls used for this study span multiple call types, as defined in other studies, where they were quantified mostly on length and the comparative intensities of various harmonics (Chamberlain and Cornwell 1971; Parr 1997; Tarter 2008). We found a pattern of continuous variation in all acoustic parameters (e.g. Figure 2), and hence did not attempt to classify our calls into discrete call types. Calls with low PC 01 values (short and high-pitched) are acoustically similar to Parr’s “regular short caws” and “double caws,” and to Tarter’s “short call” and “doubled short call.” Parr associates these calls with a variety of contexts and functions, including “generalized alarm” and “calls-to-arms for family members.” In our observations and those of Tarter (2008), they were given in the context of food provisioning on family territories, and family members responded by approaching the caller and foraging. Thus, it appears that these calls can be used for familial recruitment even outside of an “alarm” context.
Interestingly, although the short-duration “ko” or “inflected alarm caw” is commonly observed as an alarm call (Brown 1985; Parr 1997), none of the alarm caws in our known-context subset had low PC 01 values. This may support Brown’s contention that “inflected alarm caws” are only given toward a particular class of threat; in our subset, all of the potential predators scolded by crows were mammalian, moving across the ground at some distance from trees, and not obviously pursuing or watching the crows. Brown states that “inflected alarm caws” were given preferentially toward soaring raptors, but Parr objects that they are given toward climbing humans and perched raptors as well. Parr’s observations, and an experimental study on our population by Yorzinski & Vehrencamp (2009), suggest that inflected alarm caws do not denote a particular type of predator. Nevertheless, they could conceivably denote a particular predator location (airborne or arboreal) or threat level, which might explain why we did not record them. Alternatively, they may require body postures or movements (such as flight) which would have prevented us from reliably identifying the caller or establishing context.
Calls with high PC 01 values (long and low-pitched) resemble the “long caw” and “harsh caw” of Parr (1997), and the “rough call” and “scream call” of Tarter (2008). Such calls are typically associated in the literature with mobbing, conspecific aggression and predator alarm. However, as reported in Tarter (2008) and observed by us, some of these calls are also produced in the context of rebuffing begging offspring, and are not accompanied by obviously agonistic behaviour.
Calls with moderate PC 01 values were observed during “counter-cawing” between territories and are likely equivalent to Parr’s “medium caw” and Tarter’s “fading call,” calls of moderation duration and pitch that were also observed in that context. Parr found that crows responded strongly to and often approached playbacks of medium caws, providing additional evidence that they are associated with territorial advertisement.
The identity of American crows can be inferred from their calls, with accuracy significantly above chance (24% versus 6%), using a common set of parameters across all “caw”-type calls. This implies that callers can be identified even when the behavioural context of their calls is unknown, at least if the set of possible candidates is small. Crows may therefore be able to recognize conspecifics at a distance, without first approaching and assessing their behavioural state. This would be a valuable ability for a bird that must maintain long-term family bonds despite frequently traveling miles between home territories, foraging sites and communal roosts (Verbeek and Caffrey 2002). However, Yorzinski et al. (2006) were able to identify callers with considerably higher accuracy than we achieved, using alarm calls alone. This suggests that patterns of inter-individual variation are not very consistent between call types, and that contextual information can therefore significantly improve the accuracy of caller recognition. Individual variation in context usage (or in the chance of being recorded in a given context) may also increase the apparent distinctiveness of an individual’s calls. Our estimates of the variance explained by identity on the known-context call subset suggest that this is likely to be the case for certain of our acoustic components, such as PC 01.
Call properties, principally pitch, varied with the sex of the caller. Sex and behavioural context appeared to have independent effects on pitch, as expressed via PC 02 and PC 01; male calls tended to be lower-pitched regardless of context. Here a note of caution is warranted; since the analysis restricted to the known-context subset of calls did not return a significant sex effect on PC 02, it remains possible that the significant sex effect shown in the full call set was actually mediated by context. However, we think that this is unlikely to be the case, given the similarity in sex effect size and direction between the known-context and unknown-context call subsets, which suggests that the inclusion of context did not attenuate the effect of sex. The reduced significance of the sex effect in the known-context subset was probably due to the smaller size of that subset, which reduced our power for testing all fixed effects.
We are not the first to identify a sex effect on call pitch in crows, although this study provides new evidence that this difference persists across behavioural contexts. Sexual dimorphisms in call pitch and duration were also found by Yorzinski et al. (2006) when examining alarm calls alone, and pitch dimorphism was found by Laiolo et al. (2000) in another corvid, the red-billed chough (Pyrrhocorax pyrrhocorax). Males commonly produce lower-pitched calls in many avian species (Ballintijn and Cate 1997; Herting and Belthoff 2001).
Much of the acoustic variation we observed, particularly in pitch and frequency properties, may follow from variation in the dimensions of the syrinx and vocal tract. Syringeal size is usually correlated with overall body size, and in many avian species, larger individuals produce lower-pitched calls (Ballintijn and Cate 1997). Body size data were not available for our study subjects at adulthood, as they were banded in the nest, but American crows do generally show a slight sexual size dimorphism (Clark, James, and Morari 1991).
Thanks to this variation, the sex of an American crow can be inferred from a single call with above-chance accuracy: in our study, sexing accuracy was 66.65%, cross-validated. This level of sex classification accuracy was relatively low, compared to that achieved in other studies (65 - 100%) using a combination of body size metrics measured from birds in the hand (Clark, James, and Morari 1991; Yaremych et al. 2004; Ludwig, Begras-Poulin, and Lair 2009). It is also lower than that achieved by a prior study on this population (87% non-cross-validated, excluding one particularly atypical male), using the acoustic properties of single alarm caws (Yorzinski et al. 2006). This suggests that acoustic sexing, like caller identification, would be more accurate when applied to calls from a single behavioural context. Sexing error was largely due to inter-individual variance between males; three males had mean discriminant values that fell within the range spanned by the set of female means. Of these three, two were yearlings and one was a breeding male.
It should be noted that methods of sexing crows at a distance are generally unreliable. The sexes are not visually dimorphic, save for a brood patch in breeding females, and there are no known visually distinctive sex-specific behaviours except during copulation and egg-laying. Even the tail-quivering precopulatory display, reported from females of many corvid species, is performed by both sexes of American crow (Verbeek 1972; Kilham 1989). Using calls, therefore, may be the most effective way for both conspecifics and human researchers to sex crows at a distance, even if its accuracy is also limited.
Our attempts at age prediction met with little success. A linear classifier did not distinguish yearlings from older birds with sufficient accuracy to permit confidence that the classifier was not merely capitalizing on chance variation. Given that most yearling crows do not breed, it is notable and somewhat surprising that their vocal behaviour is not readily distinguishable from that of adults.
Thus, as we predicted, the sex and individual identity of a crow can be inferred from its call across multiple contexts; but, contrary to our prediction, its age cannot. This suggests that it may be adaptive for a crow to communicate sex and identity, but less so to communicate age, perhaps because younger birds make less desirable mates.
With the aid of a PMLE-based pitch estimator, we have demonstrated that American crow caws are locally periodic and can be therefore decomposed into fundamental frequency and spectral envelope components for subsequent analysis. The calls exhibit rapid oscillations in fundamental frequency and amplitude, which are relatively difficult to capture via traditional Fourier-based methods; we suggest that “pitch-first” analysis methods may be helpful for the calls of other corvid species as well. We have also shown that behavioural context, and the sex and identity of the caller, can be inferred from the structure of individual calls. This suggests that American crows could themselves make such inferences. Further improvements in automated classification along these lines may also point to practical methods for acoustic censusing of endangered and vulnerable corvid populations.
Supplementary Material
Acknowledgments
Financial support for this study comes principally from XXXXX, and from the National Institutes of Health (XXXXX).
Footnotes
Supplemental material for this article is available via the supplemental tab on the article’s online page at http://dx.doi.org/10.1080/09524622.2014.933446
Contributor Information
Exu Anton Mates, Department of Psychology, University of Washington, Seattle, WA, USA Mailing Address: Box 351525, Seattle, WA 98195 Phone: 510-295-3686 Fax: 206.685.3157 xamates@uw.edu.
Robin R. Tarter, Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, OH Mailing Address: 5600 University Way NE, Apt. 9, Seattle, WA 98105 Phone: 614-499-6781 cbrachy@gmail.com
James C. Ha, Department of Psychology, University of Washington, Seattle, WA, USA Mailing Address: Box 351525, Seattle, WA 98195 Phone: 206-543-2420, 206-543-7494 Fax: 206-685-3157 jcha@u.washington.edu
Anne B. Clark, Department of Biological Sciences, Binghamton University, Binghamton, NY, USA Mailing Address: PO Box 6000, Binghamton, NY 13902 Phone: 607-777-6228 Fax: (607) 777-6521 aclark@binghamton.edu
Kevin J. McGowan, Laboratory of Ornithology, Cornell University, Ithaca, NY, USA Mailing Address: 159 Sapsucker Woods Rd. Ithaca, NY 14850 Phone: 607-254-2452 kjm2@cornell.edu
References
- Allenbacher R, Böhner J, Hammerschmidt K. Individuelle Merkmale im “krah”-Ruf der Nebelkrähe Corvus corone cornix. Journal für Ornithologie. 1995;136(4):441–446. [Google Scholar]
- Baeyens G. The role of the sexes in territory defence in the magpie (Pica pica) Ardea. 1981;69:69–82. [Google Scholar]
- Ballintijn MR, Cate C ten. Sex differences in the vocalizations and syrinx of the collared dove (Streptopelia decaocto) The Auk. 1997;114:22–39. [Google Scholar]
- Blumstein DT, Verneyre L, Daniel JC. Reliability and the adaptive utility of discrimination among alarm callers. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271(1550):1851–1857. doi: 10.1098/rspb.2004.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckle M, Szipl G, Bugnyar T. Who wants food? Individual characteristics in raven yells. Animal Behaviour. 2012;84(5):1123–s1130. doi: 10.1016/j.anbehav.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ED. Functional interrelationships among the mobbing and alarm caws of Common Crows (Corvus brachyrhynchos) Zeitschrift für Tierpsychologie. 1985;67:17–33. [Google Scholar]
- Chamberlain DR, Cornwell GW. Selected vocalizations of the common crow. The Auk. 1971;88:613–634. [Google Scholar]
- Charrier I, Jouventin P, Mathevon N, Aubin T. Individual identity coding depends on call type in the South Polar skua Catharacta maccormicki. Polar Biology. 2001;24:378–382. [Google Scholar]
- Clark AB, Robinson DA, Jr., McGowan KJ. Effects of West Nile Virus Mortality on Social Structure of an American Crow (Corvus brachyrhynchos) Population in Upstate New York. Ornithological Monographs. 2006;60:65–78. [Google Scholar]
- Clark RG, James PC, Morari JB. Sexing adult and yearling American crows by external measurements and discriminant analysis. Journal of Field Ornithology. 1991;62:132–138. [Google Scholar]
- Cohen L. Time-frequency distributions--a review. Proceedings of the IEEE. 1989;77:941–981. DOI - 10.1109/5.30749. [Google Scholar]
- Dale J, Lank DB, Reeve HK. Signaling individual identity versus quality: a model and case studies with ruffs, queleas, and house finches. The American Naturalist. 2001;158(1):75–86. doi: 10.1086/320861. [DOI] [PubMed] [Google Scholar]
- Davis LI. Acoustic evidence of relationship in North American crows. The Wilson Bulletin. 1958;70(2):151–167. [Google Scholar]
- Ekman J, Ericson PGP. Out of Gondwanaland; the evolutionary history of cooperative breeding and social behaviour among crows, magpies, jays and allies. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1117–1125. doi: 10.1098/rspb.2005.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Hammerschmidt K, Cheney DL, Seyfarth RM. Acoustic features of female chacma baboon barks. Ethology. 2001;107:33–54. [Google Scholar]
- Frings H, Frings M, Jumber J, Busnel RG, Giban J, Gramet P. Reactions of American and French species of Corvus and Larus to recorded communication signals tested reciprocally. Ecology. 1958;39(1):126–131. [Google Scholar]
- Ha RR, Bentzen P, Marsh J, Ha JC. Kinship and association in social foraging northwestern crows (Corvus caurinus) Bird Behavior. 2003;15:65–75. [Google Scholar]
- Heinrich B. Winter foraging at carcasses by three sympatric corvids, with emphasis on recruitment by the raven, Corvus corax. Behavioral Ecology and Sociobiology. 1988;23:141–156. [Google Scholar]
- Herting BL, Belthoff JR. Bounce and double trill songs of male and female western screech-owls: Characterization and usefulness for classification of sex. The Auk. 2001;118:1095–1101. [Google Scholar]
- Hopp SL, Jablonski P, Brown JL. Recognition of group membership by voice in Mexican jays, Aphelocoma ultramarina. Animal Behaviour. 2001;62:297–303. [Google Scholar]
- Isaev AB. Applicability of the generalized least-squares method for processing correlated observations. Measurement Techniques. 1979;22(8):924–926. [Google Scholar]
- Kilham L. The American Crow and the Common Raven. 1st ed. Texas A&M University Press; College Station: 1989. Available from: http://books.google.com/books?id=GDYXjr2Pk-8C. [Google Scholar]
- Kondo N, Izawa EI, Watanabe S. Perceptual mechanism for vocal individual recognition in jungle crows (Corvus macrorhynchos): Contact call signature and discrimination. Behaviour. 2010;147:1051–1072. [Google Scholar]
- Kondo N, Izawa EI, Watanabe S. Crows cross-modally recognize group members but not non-group members. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1735):1937–1942. doi: 10.1098/rspb.2011.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiolo P, Palestrini C, Rolando A. A study of choughs’ vocal repertoire: variability related to individuals, sexes and ages. Journal für Ornithologie. 2000;141:168–179. [Google Scholar]
- Laiolo P, Rolando A. The evolution of vocalisations in the genus Corvus: effects of phylogeny, morphology and habitat. Evolutionary Ecology. 2003;17:111–123. [Google Scholar]
- Ludwig A, Begras-Poulin M, Lair S. Morphological description of American crow, Corvus brachyrhynchos, populations in southern Quebec. The Canadian Field-Naturalist. 2009;123:133–140. [Google Scholar]
- Mazerolle M. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c) R package version 3.0.1. 2013.
- McArthur PD. Mechanisms and development of parent-young vocal recognition in the piñon jay (Gymnorhinus cyanocephalus) Animal Behaviour. 1982;30:62–74. [Google Scholar]
- McGowan KJ. Reproductive and social behavior of two crow species in New York. U.S. Department of Agriculture; 1997. Available from: http://www.birds.cornell.edu/crows/hatchrep.html. [Google Scholar]
- McGowan KJ. Demographic and behavioral comparisons of suburban and rural American Crows. In: Marzluff JM, Bowman R, Donelly R, editors. Avian ecology and conservation in an urbanizing world. Kluwer Academic Press; Norwell, MA: 2001. pp. 365–381. [Google Scholar]
- Mundry R, Sommer C. Discriminant function analysis with nonindependent data: consequences and an alternative. Animal Behaviour. 2007;74(4):965–976. [Google Scholar]
- Parr CS. Social behavior and long-distance vocal communication in Eastern American crows [doctoral dissertation] University of Michigan; 1997. Available from http://mirlyn.lib.umich.edu/Record/003940533 by subscription. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.0.1. 2013.
- Pollard KA. Making the most of alarm signals: the adaptive value of individual discrimination in an alarm context. Behavioral Ecology. 2011;22(1):93–100. [Google Scholar]
- Rendall D, Owren MJ, Rodman PS. The role of vocal tract filtering in identity cueing in rhesus monkey (Macaca mulatta) vocalizations. J. Acoust. Soc. Am. 1998;103:602–614. doi: 10.1121/1.421104. [DOI] [PubMed] [Google Scholar]
- Richards DB, Thompson NS. Critical properties of the assembly call of the common American crow. Behaviour. 1978;64(3-4):184–203. [Google Scholar]
- Robinson DA., Jr. The relationship of nestling qualities to survival and breeding strategies of cooperatively breeding American crows in Ithaca, NY [dissertation] State University of New York at Binghamton; 2009. Available from: http://alephprod.binghamton.edu by subscription. [Google Scholar]
- Røskaft E, Espmark Y. Sibling recognition in the rook (Corvus frugilegus) Behavioural Processes. 1984;9:223–230. doi: 10.1016/0376-6357(84)90042-1. [DOI] [PubMed] [Google Scholar]
- Tarter RR. The vocal behavior of the American crow, Corvus brachyrhyncos [master’s thesis] Ohio State University; 2008. Available from: http://rave.ohiolink.edu/etdc/view?acc_num=osu1204876597. [Google Scholar]
- Thompson NS. A comparison of cawing in the European carrion crow (Corvus corone) and the American common crow (Corvus brachyrhynchos. Behaviour. 1982;80(1-2):106–117. [Google Scholar]
- Tibbetts EA, Dale J. Individual recognition: it is good to be different. Trends in Ecology & Evolution. 2007;22(10):529–537. doi: 10.1016/j.tree.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Verbeek NAM, Caffrey C. American Crow (Corvus brachyrhynchos), Issue No. 647. Birds of North America Online [Internet] 2002 Available from: http://bna.birds.cornell.edu/bna/species/647/articles/introduction.
- Verbeek NAM. Comparison of displays of the yellow-billed magpie (Pica nuttalli) and other corvids. Journal of Ornithology. 1972;113:297–314. [Google Scholar]
- Wascher CAF, Szipl G, Boeckle M, Wilkinson A. You sound familiar: carrion crows can differentiate between the calls of known and unknown heterospecifics. Animal Cognition. 2012;15(5):1015–1019. doi: 10.1007/s10071-012-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaremych SA, Levengood JM, Novak RJ, Mankin PC, Warner RE. Gender determination and lack of sex-specific West Nile virus mortality in American crows. Wildlife Society Bulletin. 2004;32:893–899. [Google Scholar]
- Yorzinski JL, Vehrencamp SL, Clark AB, McGowan KJ. The inflected alarm caw of the American crow: Differences in acoustic structure among individuals and sexes. The Condor. 2006;108:518–529. [Google Scholar]
- Yorzinski JL, Vehrencamp SL. The effect of predator type and danger level on the mob calls of the American crow. The Condor. 2009;111:159–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.