Abstract
Background
The clinical effect of cerebral microbleeds (CMBs) on cognition has been receiving much research attention, but results are often inconsistent.
Material/Methods
We searched PubMed, Embase, Web of Science, and some Chinese electronic databases. A total of 15 studies were included.
Results
Patients with CMBs had higher incidence of cognitive dysfunction (OR 3.14; 95% CI 1.66–5.92) and lower scores of cognitive function (SMD was −0.36 [−0.55, −0.18] in the MMSE group and −0.65 [−0.99, −0.32] in the MoCA [Montreal Cognitive Assessment] group). The results also indicated that a higher number of CMB lesions led to more severe cognitive dysfunction (SMD was −2.41 [−5.04, −0.21] in the mild group and −2.75 [−3.50, −2.01] in the severe group). We also found that cognitive performance was significantly impaired when CMBs were located in deep (−0.4 [−0.69, −0.11]), lobar regions (−0.50 [−0.92, −0.09]), basal ganglia (−0.72 [−1.03, −0.41]), and thalamus brain regions (−0.65 [−0.98, −0.32]).
Conclusions
This meta-analysis showed that CMBs were associated with cognitive dysfunction according to higher number and different locations of CMBs. Future work should focus on long-term prognosis of continuing cognitive decline and specific treatments to reduce the formation of CMBs.
MeSH Keywords: Cerebral Hemorrhage, Cognition, Meta-Analysis
Background
Cerebral microbleeds (CMBs) are radiological entities that appear as small, rounded, homogeneous, hypointense lesions on T2*-weighted gradient-recalled echo (T2*-GRE) and susceptibility-weighted imaging (SWI) [1]. CMBs have emerged as an important new manifestation and diagnostic marker of small vessel pathology. Although CMBs are generally considered to be clinically silent, a study on a neurovascular clinical population has shown a relation between CMBs and cognitive impairment [2]. Currently, the clinical effect of CMBs on cognition remains an active field of research [3]. Abnormal small vessels associated with CMBs are mainly affected by sporadic cerebral small vessel diseases, including hypertensive arteriopathy and cerebral amyloid angiopathy (CAA). Hypertensive arteriopathy and CAA, which are highly prevalent in the elderly, play critical roles in vascular cognitive impairment [4–6]. Several studies have shown that these 2 disorders are characterized by different patterns of CMB distribution in the brain. Hypertensive arteriopathy is associated with CMBs in deep brain regions (basal ganglia, thalamus, and brainstem), whereas CAA is characterized by CMBs in a lobar distribution [7]. Thus, recognizing the presence of CMBs may aid in the detection, quantification, and mapping of the effects of small vessel disease and amyloid deposition in patients with cognitive impairment. CMBs may be more specific, particularly if their anatomical distributions are mapped, for the underlying pathology than some other imaging manifestations of small vessel diseases [8].
Research on the effect of CMBs on cognition has been conducted in different populations, and different conclusions have been drawn. Some studies have indicated that CMBs are associated with lower scores on the Mini-Mental State Examination (MMSE) [9–11]. Another study has shown that executive dysfunction is more common in people with CMBs and is related to CMB location in the frontal lobes or basal ganglia [2]. Multiple CMBs are reportedly associated with lower scores on tests that are sensitive to processing speed and executive function. These associations are stronger in patients with multiple CMBs located in deep or infratentorial regions [12]. By contrast, no association between CMBs and cognitive impairment has been found by other researchers [13–15]. The Rotterdam Scan Study has recently investigated the association between the number of CMBs and cognitive dysfunction [16].
Results of the aforementioned studies are inconsistent, partially because they had relatively small sample sizes. Accordingly, this meta-analysis was conducted to address important clinical questions about CMBs and cognitive impairment. We also aimed to quantify the strength of the association between CMBs and cognitive dysfunction, as well as to quantify and map CMBs, which are correlated with detailed cognitive evaluation.
Material and Methods
Search methods
The relevant publications included in this meta-analysis were acquired from the electronic databases of PubMed, Embase, Web of Science, China National Knowledge Infrastructure, Wanfang Data, and China Biology Medicine. The search for information involved the key words: “cognitive dysfunction,” “cognitive impairment and microbleeds,” and “small vessel disease.” The data sources were searched from inception to 31 January 2014. The reference sections of all primary studies were explored for additional references.
Selection criteria
All potentially relevant studies were reviewed if they met the inclusion criteria presented below. The titles and abstracts of the studies were first screened to determine if the studies met the selection criteria. When the study passed the initial screening, the entire article text was retrieved. Published studies with no language or race restrictions were included to avoid any publication bias. Then, citations related to each eligible study were examined, and all references in the retrieved articles were evaluated to acquire all relevant studies.
Inclusion and exclusion criteria
For inclusion, all studies had to be case-control, with participants divided into CMB and non-CMB groups. Studies on CMBs and cognitive function that included patients of any age and sex were considered eligible. CMBs were defined as small, rounded, or ovoid (rather than linear or curvilinear), blooming, homogeneous, hypointense lesions with diameters less than 10 mm on T2*-GRE or SWI [17]. Outcome measures included the presence of CMB lesions that met certain criteria of the Microbleed Anatomical Rating Scale. CMBs were classified into deep areas, lobar regions, or infratentorial categories. Studies that described CMB lesions in a more detailed manner were also included when possible. Deep regions contained the basal ganglia, thalamus, internal capsule, external capsule, corpus callosum, and deep and periventricular white matter; lobar regions included the frontal, parietal, temporal, occipital, and insula; and infratentorial regions comprised the brainstem and cerebellum [8]. Studies that presented the number of CMB lesions were also included in the meta-analysis.
For studies to be eligible, they had to separately evaluate either the global cognitive function in the aggregate or the domains of cognitive function. Studies that diagnosed cognitive dysfunction in various ways, including the MMSE and Montreal Cognitive Assessment (MoCA), were incorporated. MMSE and MoCA were used to test the global cognitive function and covering domains, including abstraction, attention, delayed recall, executive language, memory, naming, and orientation [18]. Studies that measured cognitive function through the neuropsychological test were also included. The neuropsychological test was designed to assess global cognitive functioning with a specific focus on memory and executive function [19].
Case studies, reviews, and articles that were insufficient in CMB quantification or measurements of cognitive function based on their titles and abstracts were excluded from the meta-analysis. Studies with a more complete description of the data were considered. We also excluded studies with a more complete description of the data and contemporaneously published studies that had the same first author and data acquisition methods and similar patient characteristics, data analysis, and results.
Data extraction and quality assessment
Relevant data in each eligible study were collected and recorded in an Excel spreadsheet. A list of extraction items was then developed, and included: 1) study characteristics, 2) CMB outcomes, and 3) cognitive function outcomes. The principal outcomes of the studies were scores obtained from MMSE, MoCA, and neuropsychological tests. The Newcastle-Ottawa Scale was used to assess the quality of the eligible included studies, which were all case-control.
Statistical analyses
Two reviewers independently screened the abstracts of all eligible studies for primary selection. Screening was based on the inclusion criteria. When the study was indeterminable from the abstract, the paper was included in the full-text screening conducted by the same 2 reviewers. Disagreements were resolved through consultations with a third reviewer. Collected data were analyzed using R software. The odds ratio (OR) was measured, along with a 95% confidence interval (CI) for dichotomous variables. A 95% CI, excluding 1 or P<0.05, was considered statistically significant. Standardized mean difference (SMD) was measured for continuous variables, in which a 95% CI, excluding 0 or P<0.05, was considered statistically significant. Heterogeneity was assessed by Cochran’s Q test. P>0.05 was judged as non-significant heterogeneity, and the fixed-effect model was used. P<0.05 was interpreted as evidence of heterogeneity, and the random-effect model was used. The I2 index was calculated to estimate total variation across the studies. A random-effect model was used when I2>50%. A fixed-effect model was used when I2<50%. Risk of publication bias was evaluated by visual inspection of funnel plots. To be more reliable, Egger’s and Begg’s tests were also conducted to quantify publication bias.
Results
Overview of studies
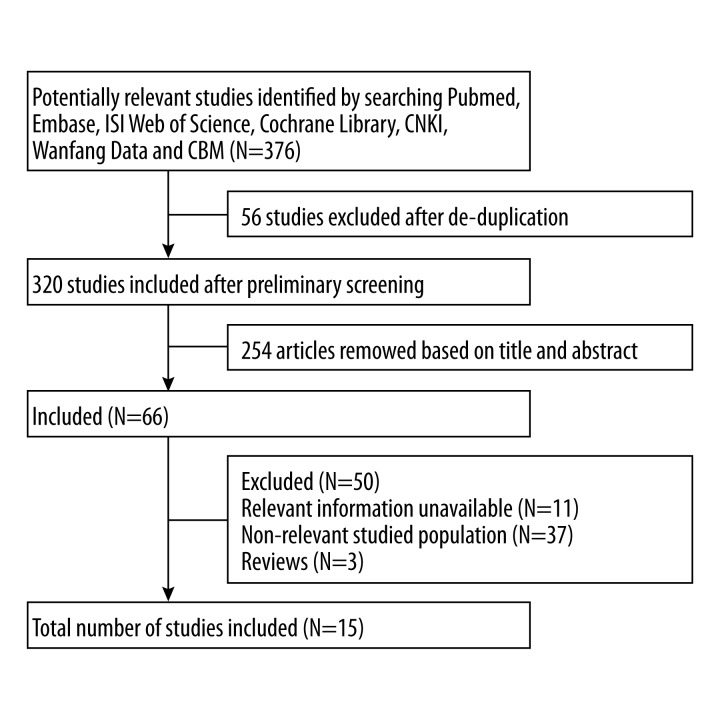
We identified 376 articles in the primary search 376. Fifty-six studies were excluded after de-duplication, and 320 studies were included after preliminary screening. A total of 254 articles were removed based on their titles and abstracts. Eleven articles lacked relevant information. We excluded 37 articles because the studied population was irrelevant, and 3 review articles were excluded. A total of 15 articles met the inclusion criteria after duplicate removal and full-text review. The process and result of literature screening are shown in Figure 1. The basic characteristics of the included studies are summarized in Table 1. The 15 studies included in the meta-analysis were all case-control studies. Seven studies [2,7,12] received a quality score of 8 out of 9. Four studies [4–6,8] received a quality score of 7, and the remaining studies [1–3] received a quality score of 6 (Table 1).
Figure 1.
Flow diagram of search strategy and study selection.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Reference | Study design | Country | MRI Magnet | Cognitive Measurement | NOS Score | CMBs | Non-CMBs | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample size, n | Mean age, years | Sample size, n | Mean age, years | ||||||
| Fan 2011 | Case-control | China | 1.5T | MOCA | 6 | 30 | 75.0±9.4 | 130 | 66.4±5.8 |
| Zhang 2013 | Case-control | China | 3.0T | MOCA | 6 | 80 | 72.06±5.59 | 89 | 67.01±7.15 |
| Shi 2013 | Case-control | China | 1.5T | MOCA/MMSE | 6 | 46 | 64.12±2.51 | 50 | 62.31±2.26 |
| chen 2010 | Case-control | China | 1.5T | MOCA | 7 | 47 | 69±11 | 47 | 6.85±5.21 |
| David J 2004 | Case-control | UK | 1.5T. | Neuropsychological | 7 | 25 | 67.6±11.9 | 30 | 67.2±10.4 |
| Saima 2013 | Case-control | Singapore | 1.5T | MMSE | 7 | 91 | 70.1±6.4 | 191 | 71.2±5.9 |
| A.C.G.M 2011 | Case-control | Netherland | 1.5T | MMSE | 8 | 106 | 77±3 | 333 | |
| S.M.Gregoire 2012 | Case-control | UK | – | Neuropsychological | 7 | 9 | 65 (44–86) | 17 | 62 (35–75) |
| Raffaele 2011 | Case-control | Austria | 1.5T | MMSE | 6 | 13 | 69.7±5.7 | 15 | 68.9±6.4 |
| Min 2013 | Case-control | China | 3.0T | MOCA/MMSE | 8 | 41 | 70.6±5.2 | 46 | 70.9±6.4 |
| Yusuke 2008 | Case-control | Japan | 1.5-T | MMSE | 8 | 35 | 57.6 (52.3–63.6) | 483 | 56.8 (50.1–63.8) |
| Yusuke 2012 | Case-control | Japan | 1.5-T | MMSE | 8 | 98 | 63 (58–67) | 1181 | 58 (50–65) |
| Zhanga 2013 | Case-control | china | 3.0 T | MOCA | 8 | 35 | Number of aged ≥65=9 | 50 | Number of aged ≥65=30 |
| Sophie 2014 | Case-control | Netherland | 3.0 T | MMSE | 8 | 26 | 80.7±6.9 | 41 | 76.4±7.3 |
| Hang 2013 | Case-control | china | 3.0 T | MOCA | 8 | 30 | – | 40 | – |
CMBs – cerebral microbleeds; MMSE – Mini-Mental State Examination; MoCA – Montreal cognitive assessment; NOS, Newcastle-Ottawa Scale.
Publication bias
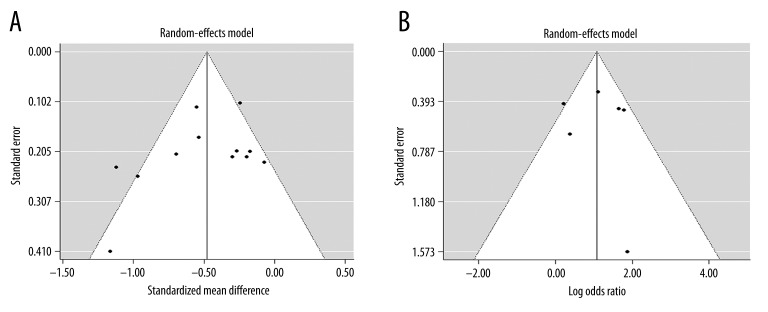
The funnel plot for studies on the incidence of cognitive impairment was symmetrical. Both Egger’s (P=0.11) and Begg’s (P=0.233) tests showed that publication bias for these studies was not significant. Similar results were obtained for studies on cognition score. The funnel plots indicated an absence of publication bias, as illustrated by both Egger’s (P=0.88) and Begg’s (P=0.719) tests (Table 2). However, the effect size heterogeneity and the small number of studies in this meta-analysis required us to be cautious in interpreting these results.
Table 2.
Results of publication bias according to Egger’s and Begg’s tests.
| Cognitive function | Egger’s test | Begg’s test | ||
|---|---|---|---|---|
| t | p | t | P | |
| Incidence | −2.24 | 0.11 | −0.60 | 0.233 |
| Score | −0.168 | 0.88 | −0.20 | 0.719 |
Heterogeneity test and synthesized efficacy
Incidence of cognitive impairment in CMB and non-CMB patients
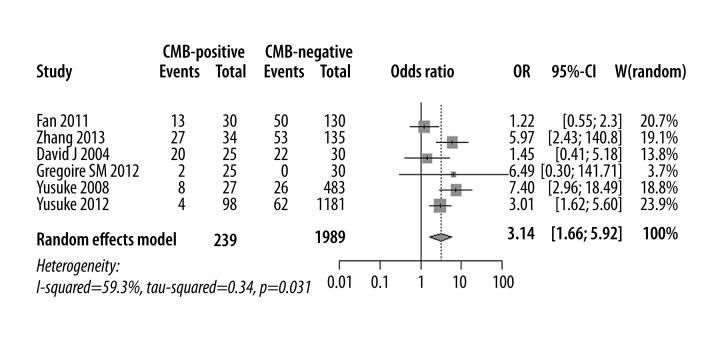
Six studies were eligible for comparing the incidence of cognitive impairment in CMB versus non-CMB patients. The total number of participants was 2228, among which 239 patients served as the case group and 1989 patients served as the control group. The synthesized efficacy (OR) of efficient probability between groups was 3.14 [1.66, 5.92] (P<0.01). This value indicated that the incidence of cognitive impairment was higher in CMB than in non-CMB patients (Figure 2).
Figure 2.
Funnel plots demonstrating that the heterogeneity was not due to publication bias. (A) Incidence of cognitive impairment in CMBs versus non-CMBs patients. (B) Cognitive assessment score of CMBs versus non-CMBs patients.
Comparison of cognitive status assessment between CMB versus non-CMB
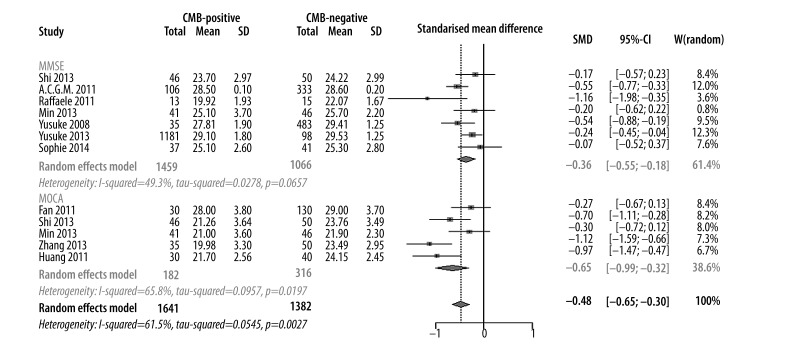
A total of 3023 participants (1641 in case group and 1382 in control group) from 11 studies were eligible. A random-effect model was used for meta-analysis with heterogeneity I2 of 62.5% (P=0.12). The synthesized effect (SMD) was −0.48 [−0.65, −0.30] (P<0.01). This finding suggested that CMB patients had an impaired cognitive function compared with non-CMB patients. In the subtype analysis based on cognitive measurements, SMD was −0.36 [−0.55, −0.18] (P<0.01) in the MMSE group and −0.65 [−0.99, −0.32] (P<0.01) in the MoCA group. Both values suggested a lower cognitive function of CMB patients (Figures 3 and 4).
Figure 3.
Meta-analysis of incidence of cognitive impairment in CMBs versus non-CMBs.
Figure 4.
Meta-analysis of cognitive assessment score in CMBs versus non-CMBs based on MMSE and MoCA.
Number of CMBs affecting cognitive impairment
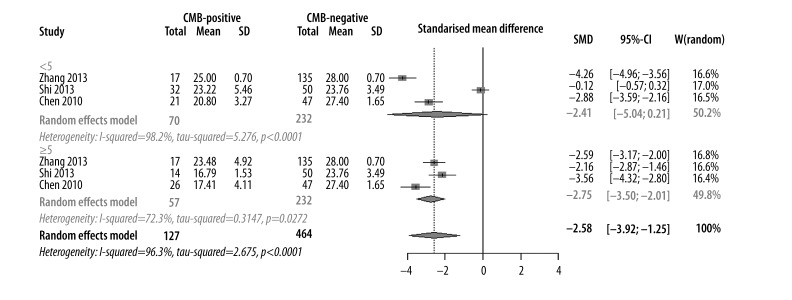
The severity of CMBs was determined by the number of CMB lesions. CMBs were considered “mild” when the lesions were less than 5 and “severe” when lesions were 5 or more. Nine studies that included 591 participants (123 in case group and 464 in control group) were eligible for analysis. SMD was −2.58 [−3.92, −1.25] (P<0.01), which suggested that cognitive function was impaired in CMB patients compared with non-CMB patients. In the subgroup analysis based on the number of CMBs, SMD was −2.41 [−5.04, −0.21] (P>0.05) in the mild group and −2.75 [−3.50, −2.01] (P<0.01) in the severe group. These values indicated that a higher number of CMB lesions led to more severe cognitive dysfunction (Figure 5).
Figure 5.
Meta-analysis of number of CMBs effecting the cognitive impairment.
Evaluation of cognitive performance in CMBs based on various locations
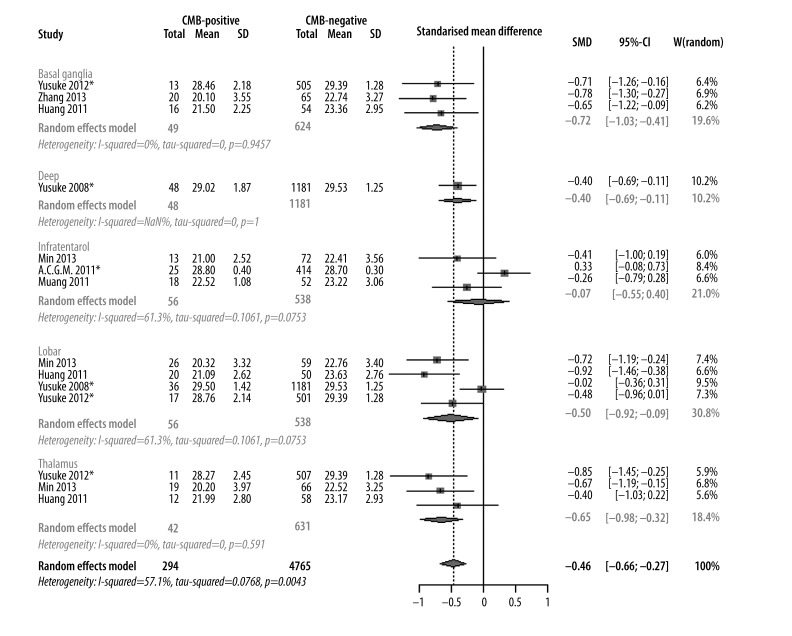
Four studies were included in the meta-analysis. The cognitive performance impairment of CMBs was evaluated based on 5 different CMB locations: lobar, infratentorial, deep, basal ganglia, and thalamus. Results indicated that cognitive performance was significantly impaired when CMBs were located in deep (−0.4 [−0.69, −0.11]), lobar (−0.50 [−0.92, −0.09]), basal ganglia (−0.72 [−1.03, −0.41]), and thalamus (−0.65 [−0.98, −0.32]). CMBs in the infratentorial location were not significantly different in cognitive performance compared with non-CMBs (0.07 [−0.55, −0.40]) (Figure 6).
Figure 6.
Meta-analysis of cognitive assessment score based on different locations of CMBs.
Evaluation of cognitive domains based on various locations of CMBs
Compared with the control group, patients with CMBs in labor had impaired executive (−0.69 [−1.16; −0.22]) and orientation (−0.25 [−0.48; 0.01]) functions. Impairment of attention (−0.85 [−0.48; −1.23]) and executive (−0.69 [−0.17; −1.20]) functions was observed in the basal ganglia group. Patients with CMBs at the thalamus had lower scores in attention (−0.65 [−0.23; −1.08]), language (−0.49 [0.07; 0.92]), and orientation (−0.83 [−1.26; −0.41]). However, patients with infratentorial CMBs did not present impaired cognitive function in any of the 7 performances of abstraction, attention, executive, language, memory, naming, or orientation (Table 3).
Table 3.
Meta-analysis of cognitive assessment score of different cognitive domains based on different locations of CMBs.
| k | Model | SMD [95%CI] | Z | P | Q | P | I2 | |
|---|---|---|---|---|---|---|---|---|
| Labor | ||||||||
| Abstraction | 1 | – | −0.20 [−0.66; 0.27] | −0.83 | 0.41 | – | – | – |
| Attention | 3 | Random model | −0.40 [−0.86;0.06] | −1.69 | 0.091 | 7.03 | 0.030 | 71.50% |
| Delayed recall | 2 | Fixed model | −0.021 [−0.29;0.26] | −0.15 | 0.88 | 0.22 | 0.64 | 0% |
| Executive | 1 | – | −0.69 [−1.16;−0.22] | −0.22 | 0.004 | – | – | – |
| Language | 3 | Fixed model | −0.05 [−0.29;0.19] | −0.42 | 0.68 | 0.31 | 0.86 | 0% |
| Memory | 1 | – | −0.26 [−0.73;0.20] | −1.11 | 0.27 | – | – | – |
| Naming | 1 | – | −0.17 [−0.63; 0.29] | −0.71 | 0.48 | – | – | – |
| Orientation | 3 | Fixed model | −0.25[−0.48;0.01] | −2.04 | 0.04 | 0.62 | 0.73 | 0% |
| Basal ganglia | ||||||||
| Abstraction | 1 | – | −0.48 [−0.99;0.025] | −1.86 | 0.06 | – | – | – |
| Attention | 2 | Fixed model | −0.85 [−0.48; −1.23] | −4.44 | 0.00 | 0.14 | 0.71 | 0% |
| Delayed recall | 1 | – | 0.10 [−0.65;0.45] | −0.34 | 0.73 | – | – | – |
| Executive | 1 | – | −0.69 [−0.17; −1.20] | −2.62 | 0.01 | – | – | – |
| Language | 2 | Fixed model | −0.21 [−0.59; 0.16] | −1.13 | 0.26 | 1.08 | 0.30 | 7.10% |
| Memory | 1 | – | 0.27 [−0.77; 0.24] | −1.03 | 0.30 | – | – | – |
| Naming | 1 | – | −0.06 [−0.44; 0.56] | −0.23 | 0.82 | – | – | – |
| Orientation | 2 | Fixed model | −0.36 [−0.73; 0.01] | −1.89 | 0.06 | 0.02 | 0.89 | 0% |
| Thalamus | ||||||||
| Abstraction | 1 | – | −0.42 [−1.01;0.18] | −1.36 | 0.17 | – | – | – |
| Attention | 2 | Fixed model | −0.65 [−0.23; −1.08] | −3.01 | 0.003 | 0 | 0.99 | 0% |
| Delayed recall | 1 | – | −0.34 [−0.94;0.25] | −1.13 | 0.26 | – | – | – |
| Executive | 1 | – | −0.44 [−1.04;0.16] | −1.45 | 0.15 | – | – | – |
| Language | 2 | Fixed model | −0.49 [0.07; 0.92] | −2.29 | 0.02 | 0.92 | 0.34 | 0% |
| Memory | 1 | – | −0.12 [−0.72; 0.47] | −0.40 | 0.69 | – | – | – |
| Naming | 1 | – | −0.10 [−0.69;0.50] | −0.32 | 0.75 | – | – | – |
| Orientation | 2 | Fixed model | −0.83 [−1.26;−0.41] | −3.83 | 0.00 | 0.63 | 0.43 | 0% |
| Infratentorial | ||||||||
| Abstraction | 1 | – | −0.27 [−0.86;0.33] | −0.88 | 0.38 | – | – | – |
| Attention | 1 | – | −0.21 [−0.80; 0.38] | −0.69 | 0.49 | – | – | – |
| Executive | 3 | Random model | 0.13 [−1.43; 1.69] | 0.16 | 0.87 | 164.96 | 0.00 | 98.80% |
| Language | 1 | – | −0.32 [−0.91;0.27] | −1.06 | 0.29 | – | – | – |
| Memory | 3 | Random model | −1.51 [3.81;−0.79] | −1.29 | 0.20 | 267.67 | 0.00 | 99.30% |
| Naming | 1 | – | 0.00 [−0.59; 0.59] | 0 | 1 | – | – | – |
| Orientation | 1 | – | 0.07 [−0.67; 0.53] | −0.53 | 0.83 | – | – | – |
K – number of studies included; SMD – standardized mean difference.
Discussion
This meta-analysis shows that patients with CMBs had higher incidence of cognitive dysfunction and lower scores of cognitive function. Evidence from the studies included in the analysis also suggests that a higher number of CMB lesions and CMBs located in lobar regions, deep areas, basal ganglia, and thalamus regions were correlated with cognitive impairment. However, infratentorial CMBs did not significantly affect cognitive impairment. Different locations of CMBs were also associated with different cognitive domains. We believe that this meta-analysis is the first to quantify and compare the strength of association between CMBs and cognitive impairment in terms of incidence rate, cognition score, number and location of CMBs, and specific domains of cognitive function. Moreover, this meta-analysis considered studies that used MMSE, MoCA, and neuropsychological tests to evaluate overall cognitive capacities.
CMBs are histologically characterized by hemosiderin around abnormal small vessels, with necrosis or infarction of the surrounding tissue [20]. CMBs are thus expected to cause cognitive impairment if they disrupt strategically important white matter tracts or cortical areas [2]. The precise pathophysiological mechanisms responsible for the associations between CAA and cognition are yet to be established. One possibility is that vascular amyloid-β deposition progressively affects the function of the neurovascular unit, which is a key player in microvascular and neurodegenerative processes [21,22]. CAA also causes impaired vascular reactivity that may result in chronic ischemia [23] or acute focal ischemic lesions, which can potentially contribute to clinical impairment. In parallel, small vessel damage (including hypertensive arteriopathy) can lead to impaired clearance of amyloid-β, which results in its further deposition in the vessel wall [3]. Apart from this disorder, evidence also shows that CMBs contribute to tissue damage. Histological research has revealed that hemosiderin deposits, which are characteristic of CMBs, may be surrounded by gliosis, infarction, and necrosis [20]. If positioned at strategic locations, such changes could contribute to cognitive decline [19].
Results of the meta-analysis showed that cognitive performance was significantly affected when CMBs were located in lobar areas, basal ganglia, and the thalamus. CMBs in the infratentorial did not affect cognitive performance. This finding is consistent with the fact that frontal lobes, basal ganglia, and thalamus participate in frontal-subcortical circuits, which are involved in cognitive function. CMBs in these regions are associated with tissue necrosis that inflicts damage on the frontal-subcortical circuits or white matter tracts, thereby inducing cognitive impairment [24]. Executive function impairment was related to CMBs in lobar regions and basal ganglia. This finding is consistent with the possible direct effect of CMBs on frontal-subcortical circuits, which have important executive functions [2]. Attention impairment was related to CMBs in basal ganglia and thalamus. Attention reflects widely distributed cognitive skills, which are also related to the integrity of frontal-subcortical circuits [2]. Orientation impairment was related to CMBs in lobar regions and the thalamus, and language impairment was related to CMBs in the thalamus. Since the thalamus is also considered part of the neuronal network, it is also integrally involved in cognitive function [25]. However, more studies on specific cognitive domains must be conducted in the future because very few such studies have been conducted.
The effect of CMBs on cognition may also depend on the number of CMB lesions. The RUN DMC Study, which examines non-demented subjects with cerebral small vessel disease, reported significant associations of the presence and number of CMBs with global cognitive function, as measured by the cognitive index, psychomotor speed, and attention. However, no association was found with the MMSE [26]. By contrast, data from the Rotterdam Study (n=3979) suggest that the number of CMBs, especially the presence of 5 or more CMBs, is associated with several non-memory-related cognitive domains, independent of other imaging markers of cerebral small vessel disease. This association suggests an independent role for microbleed-associated vasculopathy in cognitive impairment [16]. Other studies in white populations have failed to identify an independent association between CMBs and cognitive decline [27]. A modest and significant graded relationship was observed between the number of CMBs and lower score of cognitive impairment. This finding suggests that an increasing CMB load may have a more general effect on cognitive function. The effect on general cognitive function is likely to reflect the cumulative effects of CMBs that are widely anatomically distributed in the brain [2]. High counts of CMBs may reflect a worse disease state, which leads to poor cognitive function [28].
This meta-analysis was limited by the heterogeneity of the results, possibly due to the various methodologies of the studies and the quality of included studies. The population of case and control groups could not be unified because the patients were from hospitals or the community, which may cause selection bias. In addition, MMSE and MoCA scores were easily affected by age and educational level, such that higher education may lead to false-negatives and lower education to false-positives among the elderly. Another limitation of this meta-analysis was that different studies identified CMB lesions using different MRI protocols, which may lead to heterogeneity in the numbers of detected CMB lesions. Unfortunately, most of the studies did not include a detailed analysis of CMB locations and cognitive function domains. Thus, CMB location in lobar regions, basal ganglia, thalamus, and infratentorial locations were included in this meta-analysis, along with 3 studies to measure specific cognitive function domains.
Conclusions
The finding that CMBs are associated with cognitive deficits has potential diagnostic and therapeutic implications [2]. CMBs may also help objectively monitor the progression of small vessel disease in observational or therapeutic studies. Information on the long-term prognosis of continuing cognitive decline with CMBs is insufficient. Future work should focus on determining whether specific treatments (e.g., aggressive hypertension treatment, statin use, and glycemic control) can reduce the formation of CMBs, and on identifying the relation of CMB accumulation to long-term clinical outcomes [29].
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China under Grant No. 81371212 and Scientific Program of Science and Technology Committee of Shanghai under Grant No. 11411950303
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–75. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 3.Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci. 2012;322:50–55. doi: 10.1016/j.jns.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuropathology Group, Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 6.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 7.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 8.Gregoire SM, Chaudhary UJ, Brown MM, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 9.Yakushiji Y, Nishiyama M, Yakushiji S, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–28. doi: 10.1161/STROKEAHA.108.516112. [DOI] [PubMed] [Google Scholar]
- 10.Goos JD, Kester MI, Barkhof F, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40:3455–60. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan A, Patel P, Rahman R, et al. Tissue microstructural changes are independently associated with cognitive impairment in cerebral amyloid angiopathy. Stroke. 2008;39:1988–92. doi: 10.1161/STROKEAHA.107.509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75:2221–28. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordonnier C, van der Flier WM, Sluimer JD, et al. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–60. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- 14.Hanyu H, Tanaka Y, Shimizu S, et al. Cerebral microbleeds in Alzheimer’s disease. J Neurol. 2003;250:1496–97. doi: 10.1007/s00415-003-0245-7. [DOI] [PubMed] [Google Scholar]
- 15.Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–95. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 16.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–33. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 17.Kidwell CS, Greenberg SM. Red meets white: do microbleeds link hemorrhagic and ischemic cerebrovascular disease? Neurology. 2009;73:1614–15. doi: 10.1212/WNL.0b013e3181c17fa1. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2005;76:1140–45. doi: 10.1136/jnnp.2004.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Es AC, van der Grond J, de Craen AJ, et al. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77:1446–52. doi: 10.1212/WNL.0b013e318232ab1d. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A, Ueno Y, Nakayama Y, et al. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke. 1999;30:1637–42. doi: 10.1161/01.str.30.8.1637. [DOI] [PubMed] [Google Scholar]
- 21.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–96. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez Garcia PL, Rodriguez Garcia D. Letter by Rodriguez-Garcia and Rodriguez-Garcia [corrected] regarding article, “Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association”. Stroke. 2011;42:e584. doi: 10.1161/STROKEAHA.111.634279. [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–6. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei C, Lin S, Tao W, et al. Association between cerebral microbleeds and cognitive function: a systematic review. J Neurol Neurosurg Psychiatry. 2013;84:693–97. doi: 10.1136/jnnp-2012-303948. [DOI] [PubMed] [Google Scholar]
- 25.Karussis D, Leker RR, Abramsky O. Cognitive dysfunction following thalamic stroke: a study of 16 cases and review of the literature. J Neurol Sci. 2000;172:25–29. doi: 10.1016/s0022-510x(99)00267-1. [DOI] [PubMed] [Google Scholar]
- 26.van Norden AG, van den Berg HA, de Laat KF, et al. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke. 2011;42:3382–86. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JA. Brain microbleeds and cognitive function. Stroke. 2007;38:1730–31. doi: 10.1161/STROKEAHA.107.487173. [DOI] [PubMed] [Google Scholar]
- 28.Patel B, Lawrence AJ, Chung AW, et al. Cerebral microbleeds and cognition in patients with symptomatic small vessel disease. Stroke. 2013;44:356–61. doi: 10.1161/STROKEAHA.112.670216. [DOI] [PubMed] [Google Scholar]
- 29.Arauz A. Cerebral microbleeds and cognitive function. J Neurol Neurosurg Psychiatry. 2013;84:592. doi: 10.1136/jnnp-2012-304437. [DOI] [PubMed] [Google Scholar]