Abstract
Background
Maternal asthma has been associated with adverse pregnancy outcomes. Little is known about the influence of other atopic diseases on pregnancy outcomes. We assessed how various maternal atopic diseases might affect preterm birth, stillbirth, and neonatal death.
Methods
By linking Norwegian national registries, we acquired information on maternal health, socio-demographic factors, pregnancy, birth, and neonatal outcome on all births in Norway from 1967 through 2003.
Results
A total of 1,974,226 births were included. Of these, 1.8% had a record of maternal asthma, 3.4% of maternal atopic dermatitis, and 0.4% of maternal allergic rhinoconjunctivitis. Overall rates of preterm birth, stillbirth, and neonatal death were 6.0%, 0.6%, and 0.5%, respectively. After adjustments for possible confounders, maternal asthma was associated with increased risk of preterm birth (RR 1.15, [95% confidence interval (CI) 1.10, 1.21]). In contrast, maternal atopic dermatitis was associated with decreased risk of preterm birth (RR 0.90, [95% CI 0.86, 0.93]), stillbirth (RR 0.70, [95% CI 0.62, 0.79]), and neonatal death (RR 0.76, [95% CI 0.65, 0.90]). Similarly, maternal allergic rhinoconjunctivitis was associated with decreased risk of preterm birth (RR 0.84, [95% CI 0.76, 0.94]) and stillbirth (RR 0.40, [95% CI 0.25, 0.66]).
Conclusions
We confirmed the previously reported association of maternal asthma with increased risk for preterm birth. Unexpectedly, maternal atopic dermatitis and allergic rhinoconjunctivitis were associated with decreased risk of preterm birth and stillbirth. Mechanisms for these protective associations are unclear, and our findings require confirmation in further studies.
Introduction
Atopic diseases (including asthma, atopic dermatitis, and allergic rhinoconjunctivitis) are among the most common chronic disorders in pregnant women. These conditions share a number of risk factors, and persons with atopic dermatitis in childhood are at increased risk for developing asthma and allergic rhinoconjunctivitis in later life.1
Previous studies have shown that mothers with asthma have an increased risk of adverse pregnancy outcomes.2, 3 Little is known about pregnancy outcomes of mothers with other atopic disorders. Since both atopy and pregnancy involve immunological adaptations,4, 5 we hypothesized that other atopic diseases also influence pregnancy outcomes. The aim of this study was to explore associations of maternal atopic diseases with preterm birth, stillbirth, and neonatal death in a national cohort of Norwegian births.
Methods
We identified all births in Norway from 1967 through 2003 registered in the Medical Birth Registry of Norway.6 Data on birth, pregnancy, and maternal health were provided by the birth registry and information on parental education by the national registry of education. The two registries were linked using the unique personal identification number of Norwegian citizens. During prenatal care, maternal health conditions are registered on a standardized pregnancy health chart by general practitioners and midwives. This health chart is brought to the maternity unit at admission for delivery, and information from the chart and the hospital records is reported to the birth registry. Diagnoses were identified in the birth registry using the International Classification of Diseases (ICD) codes, version 8 before December 1998 and version 10 thereafter. Maternal asthma was identified as ICD-8 code 493 and ICD-10 code J45, maternal atopic dermatitis as ICD-8 codes 691 and 692 and ICD-10 code L20, and maternal allergic rhinoconjunctivitis as ICD-8 code 507 and ICD-10 codes J30.1–J30.4 and H10.1.
Gestational age (GA) was calculated from the first day of the last menstrual period. Preterm birth was defined as birth before 37 weeks’ gestation, and divided into two categories: 23–31 weeks and 32–36 weeks. Term birth was defined as birth at 37–41 weeks’ gestation, and postterm birth as birth at 42–44 weeks. Neonatal death was defined as death within 28 days after live birth. Small for gestational age was defined as birthweight below 2 standard deviations from the mean.7
We included maternal age, parity, season of birth, parental education, and single mother at birth as possible confounders, and we also adjusted for year of birth. Parity was based on previous live births and stillbirths. Season of birth was divided into winter (December – February), spring (March – May), summer (June – August), and autumn (September – November). Since stillbirths are not assigned a personal identification number, we were unable to link these with data on parents’ education.
The study was approved by the Western Regional Ethics Committee, Norway.
Statistical analysis
We examined characteristics of mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and none of these diseases. Mothers with a record of more than one of the three diseases were included in the analysis of each.
In the main analyses, we explored how atopic diseases of the mother were related to preterm birth, stillbirth, and neonatal death of her offspring. First, we estimated the association between each atopic disease and the outcomes in separate models. For these models, the reference groups varied slightly and consisted of mothers without each specific atopic disease. Second, we performed adjusted analyses with mothers with none of the atopic diseases as the reference group. In the analysis of preterm birth, relative risks were compared with risks of not giving birth in the specific GA category. Mothers born preterm have an increased risk of delivering preterm,8 and being born preterm may influence risk of later atopic disease.9, 10 We therefore repeated the analyses restricted to mothers born at term. This analysis required a subset of mother-child pairs with complete data on GA at birth for both mother and child.
Most preterm deliveries, stillbirths, and neonatal deaths are known at the time when information on maternal health is reported to the birth registry. We therefore considered whether recording of maternal diseases could have been affected by the outcome by analyzing the relations between two other maternal conditions presumably unrelated to infant outcome (psoriasis and cystitis in pregnancy) and preterm birth, stillbirth, and neonatal death.
Prevalences and rates were assessed using SPSS (Version 21.0, Armonk, NY, USA) and adjusted analyses were performed with log-binomial regression models in STATA (version 12.1, College Station, TX, USA). Since a considerable proportion of mothers delivered more than one child during the study period, all births were not entirely independent observations. Therefore, we used the robust estimation of variance option in STATA in the regression analyses to account for the correlation between births of the same mother. The main analyses were also repeated within more narrow time strata (1967–1978, 1979–1990, and 1991–2003).
Results
A total of 2,159,445 births from 1967 through 2003 were identified through the birth registry. We excluded 137,059 (6.3%) births with missing data on GA, 25,846 (1.2%) with unlikely GA (birthweight outside 3 standard deviations from the sex- and GA-specific mean),7 6,646 (0.3%) with less than 23 weeks’ gestation, 14,595 (0.7%) with more than 44 weeks’ gestation, and 1,073 (<0.1%) with missing identification number of mother. This exclusion left 1,974,226 births (91%) in the study cohort. Data on socio-demographic factors and other potential confounders were complete for 96%. A total of 35,771 (1.8%) mothers had a record of asthma, 66,535 (3.4%) of atopic dermatitis, and 8,505 (0.4%) of allergic rhinoconjunctivitis. Characteristics of mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and none of these diseases are shown in Table 1.
Table 1.
Socio-demographic and pregnancy characteristics of mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births
| Mothers with asthma | Mothers with atopic dermatitis | Mothers with allergic rhinoconjunctivitis | Mothers with no atopic disease | |
|---|---|---|---|---|
| No.a | 35,771 | 66,535 | 8,505 | 1,876,211 |
| Maternal age at birth (years, mean) | 27.2 | 27.2 | 28.0 | 27.1 |
| Educationb | ||||
| <11 years | 25 % | 19 % | 14 % | 24 % |
| 11–14 years | 42 % | 43 % | 38 % | 45 % |
| >14 years | 32 % | 37 % | 47 % | 29 % |
| Single mother at birthb | ||||
| Yes | 13 % | 11 % | 8 % | 9 % |
| No | 87 % | 89 % | 92 % | 90 % |
| Parityc | ||||
| 0 | 46 % | 47 % | 47 % | 41 % |
| 1 | 32 % | 34 % | 35 % | 35 % |
| ≥2 | 22 % | 19 % | 19 % | 24 % |
| Season of birth | ||||
| Winter | 23.7 % | 23.5 % | 22.7 % | 23.9 % |
| Spring | 26.4 % | 26.9 % | 24.3 % | 27.2 % |
| Summer | 25.8 % | 25.3 % | 26.5 % | 25.2 % |
| Autumn | 24.2 % | 24.3 % | 26.5 % | 23.7 % |
| Pregnancy length (days, mean) | 280.1 | 281.0 | 281.3 | 280.7 |
| Gestational age of offspring | ||||
| 23–31 weeks | 1.2 % | 0.8 % | 0.6 % | 1.0 % |
| 32–36 weeks | 5.8 % | 4.6 % | 4.3 % | 4.9 % |
| 37–41 weeks | 78.5 % | 80.9 % | 81.4 % | 80.4 % |
| 42–43 weeks | 14.5 % | 13.7 % | 13.7 % | 13.7 % |
| Pregnancy complications | 13.3 % | 10.2 % | 12.3 % | 9.3 % |
| Chorioamnionitis | 0.4 % | 0.3 % | 0.4 % | 0.3 % |
| Placental abruption | 0.7 % | 0.6 % | 0.4 % | 0.6 % |
| Placenta previa | 0.2 % | 0.2 % | 0.2 % | 0.2 % |
| Prolonged rupture of membranes | 2.2 % | 0.7 % | 2.6 % | 1.2 % |
| Unspecified bleeding | 3.6 % | 3.1 % | 3.9 % | 2.5 % |
| Pre-eclampsia | 4.5 % | 3.7 % | 3.5 % | 2.9 % |
| Caesarean section | 13.8 % | 11.4 % | 10.9 % | 8.7 % |
| Birthweight (gram, mean) | 3,473 | 3,523 | 3,556 | 3,504 |
| Small for gestational aged | 3.2 % | 2.5 % | 2.3 % | 2.8 % |
| Apgar 1 min <7e | 5.3 % | 4.4 % | 4.4 % | 4.5 % |
| Apgar 5 min <7e | 1.3 % | 1.1 % | 0.8 % | 1.2 % |
| Stillbirth | 0.5 % | 0.4 % | 0.2 % | 0.6 % |
| Neonatal deathf | 0.3 % | 0.3 % | 0.2 % | 0.5 % |
Mothers with a record of more than one disease is registered in each category.
Percentages do not add up to 100% because of missing values.
Parity included previous live births and stillbirths.
Small for gestational age was defined as birthweight less than 2 standard deviations from the sex- and gestational age-specific mean.
Analyzed in a sub-cohort of 1,330,649 births, because of missing data before 1978.
Death before 28 days of age after live birth.
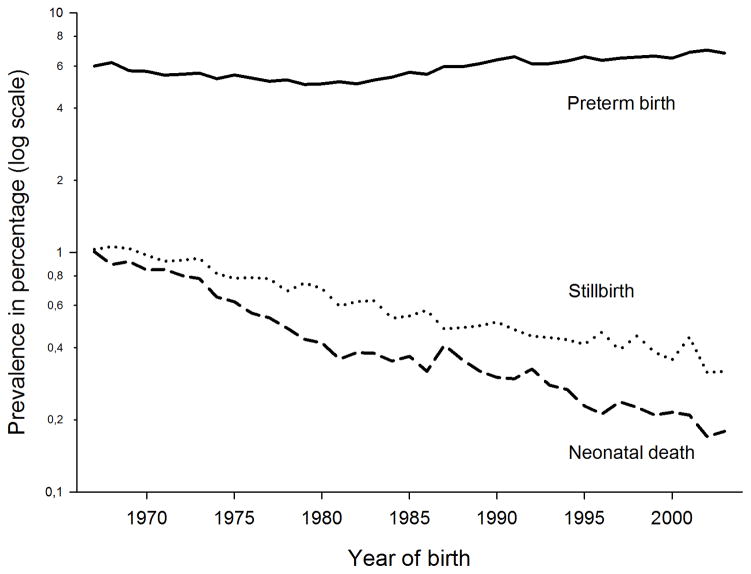
The recorded prevalence of maternal asthma increased from 0.7% in 1967–1978, to 1.6% in 1979–1990, and 3.2% in 1991–2003. The corresponding prevalences were 1.1%, 5.3%, and 4.0% for maternal atopic dermatitis and 0.1%, 0.5%, and 0.7% for maternal allergic rhinoconjunctivitis. Overall rate of preterm birth was 5.9%, of stillbirth 0.6%, and of neonatal death 0.5%. The prevalence of preterm birth was highest in winter (6.3%) and lowest in spring (5.6%). In summer and winter, the prevalence was 5.9%. The mortality rates declined throughout the study period while the preterm birth rate was relatively stable (Figure 1).
Figure 1.
Rates of preterm birth, stillbirth, and neonatal death among 1,974,226 births in the period 1967–2003
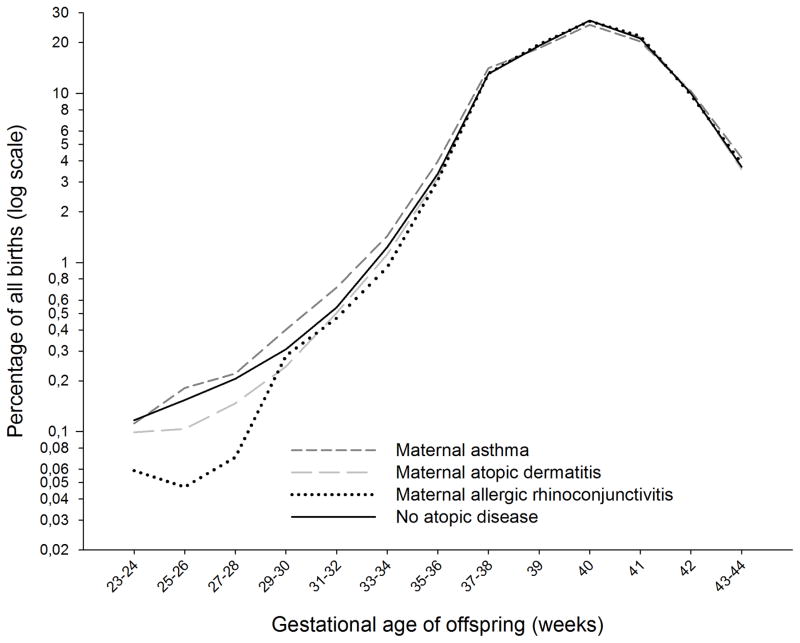
As expected, maternal asthma was associated with increased risk of preterm birth (RR 1.15, [95% confidence interval (CI) 1.10, 1.21]). However, maternal atopic dermatitis was associated with decreased risk (RR 0.90, [95% CI 0.86, 0.93]), as was maternal allergic rhinoconjunctivitis (RR 0.84, [95% CI 0.76–0.94]). All associations were more pronounced for early than for late preterm birth (Table 2). Only maternal asthma was associated with postterm birth (Table 2). The distribution of GA at birth with or without maternal atopic diseases is shown in Figure 2. When restricting the analyses to the 369,423 mother-infant dyads with complete GA data for both, the relative risks for mothers born at term (299,438 births) were similar to those of the whole cohort (Table S1).
Table 2.
Relative riska of preterm, term, and postterm birth for mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease in a cohort of 1,974,226 births
| Gestational age at birth | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 23–31 weeks (n=19,873) | 32–36 weeks (n=96,947) | 37–41 weeks (n=1,586,501) | 42–44 weeks (n=270,905) | |||||
|
| ||||||||
| Crude RRb [95% CI] | Adjusted RRc [95% CI] | Crude RRb [95% CI] | Adjusted RRc [95% CI] | Crude RRb [95% CI] | Adjusted RRc [95% CI] | Crude RRb [95% CI] | Adjusted RRc [95% CI] | |
|
| ||||||||
| Mothers with | ||||||||
| asthma | 1.25 [1.12, 1.38] | 1.33 [1.18, 1.50] | 1.18 [1.13, 1.24] | 1.13 [1.07, 1.18] | 0.98 [0.97, 0.98] | 0.98 [0.97, 0.99] | 1.06 [1.03, 1.09] | 1.05 [1.02, 1.08] |
| atopic dermatitis | 0.78 [0.71, 0.86] | 0.78 [0.70, 0.87] | 0.94 [0.90, 0.97] | 0.92 [0.88, 0.95] | 1.01 [1.00, 1.01] | 1.01 [1.01, 1.01] | 1.00 [0.98, 1.02] | 0.98 [0.96, 1.00] |
| allergic rhinoconjunctivitis | 0.64 [0.48, 0.86] | 0.75 [0.55, 1.03] | 0.87 [0.78, 0.97] | 0.86 [0.77, 0.96] | 1.01 [1.00, 1.02] | 1.01 [1.00, 1.02] | 1.00 [0.94, 1.05] | 1.01 [0.96, 1.07] |
| no atopic disease | Reference | Reference | Reference | Reference | ||||
Relative risks were obtained by comparing risks within each gestational age category.
Separate analyses were performed for each maternal atopic disease. The reference groups consisted of mothers without each atopic disease (not shown).
Adjusted for year of birth, other maternal diseases in the table, maternal age, single mother at birth, maternal and paternal education, parity, and seasonality.
CI, confidence interval; RR, relative risk
Figure 2.
Distribution of gestational age at birth in pregnancies of mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease
There was little evidence for an association of maternal asthma with either stillbirth or neonatal death (Tables 3 and 4). However, maternal atopic dermatitis was associated with decreased risk of stillbirth and neonatal death, and maternal allergic rhinoconjunctivitis was associated with decreased risk of stillbirth (Tables 3 and 4). The strong decline in perinatal mortality over time, together with the rise in recorded maternal atopic diseases, requires careful adjustment for year of birth. Once adjustment was made for year of birth, further adjustments by other potential confounding factors had little effect on the estimates.
Table 3.
Relative risk of stillbirth for mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease in a cohort of 1,974,226 births
| Stillbirth (n=12,487) | |||||
|---|---|---|---|---|---|
|
| |||||
| No.a | No. casesa | % | RRb [95% CI] | RRc [95% CI] | |
|
|
|||||
| Mothers with | |||||
| asthma | 35,771 | 179 | 0.5 % | 1.00 [0.86, 1.16] | 1.10 [0.95, 1.29] |
| atopic dermatitis | 66,535 | 257 | 0.4 % | 0.70 [0.61, 0.79] | 0.70 [0.62, 0.80] |
| allergic rhinoconjunctivitis | 8,505 | 16 | 0.2 % | 0.38 [0.23, 0.62] | 0.40 [0.25, 0.66] |
| no atopic disease | 1,876,211 | 12,079 | 0.6 % | Reference | |
Some mothers had a record of more than one disease.
Separate models were performed for each maternal atopic disease and were adjusted for year of birth. The reference groups consisted of mothers without each atopic disease.
Adjusted for year of birth, other maternal diseases in table, maternal age, single mother at birth, parity, and seasonality.
CI, confidence interval; RR, relative risk
Table 4.
Relative risk of neonatal death for mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease in a cohort of 1,974,226 births
| Neonatal death (n=9,039) | |||||
|---|---|---|---|---|---|
|
| |||||
| No.a | No. casesa | % | RRb [95% CI] | RRc [95% CI] | |
|
|
|||||
| Mothers with | |||||
| asthma | 35,771 | 124 | 0.3 % | 1.08 [0.90, 1.31] | 1.13 [0.92, 1.38] |
| atopic dermatitis | 66,535 | 186 | 0.3 % | 0.77 [0.66, 0.89] | 0.76 [0.65, 0.90] |
| allergic rhinoconjunctivitis | 8,505 | 20 | 0.2 % | 0.76 [0.49, 1.18] | 0.83 [0.52, 1.31] |
| no atopic disease | 1,876,211 | 8,752 | 0.5 % | Reference | |
Some mothers had a record of more than one disease.
Separate models were performed for each maternal atopic disease and were adjusted for year of birth. The reference groups consisted of mothers without each atopic disease.
Adjusted for year of birth, other maternal disease in table, maternal age, single mother at birth, parental education, parity, and seasonality.
CI, confidence interval; RR, relative risk
Sub-analyses within more restricted time periods generally showed stability in the associations of maternal atopic diseases with preterm birth and stillbirth over time (Tables S2 and S3). The exception was the association of maternal allergic rhinoconjunctivitis with stillbirth, which was much stronger in the latest period. In contrast, the associations of maternal atopic diseases with neonatal death showed greater variations over time (Tables S4).
In order to pursue the possibility that maternal diagnoses were underreported after poor pregnancy outcome, we explored the relations between other maternal health conditions and preterm birth, stillbirth and neonatal death. Maternal psoriasis (n=8,089) was associated with decreased risk of preterm birth (RR 0.89, [95% CI 0.81, 0.99]) and neonatal death (RR 0.78, [95% CI 0.51, 1.19]), but had no appreciable relation to stillbirth (RR 0.98, [95% CI 0.73, 1.33]). Infectious cystitis (n=26,520) was associated with decreased risk of stillbirth (RR 0.81, [95% CI 0.67, 0.97]) and neonatal death (RR 0.71, [95% CI 0.54, 0.92]), but not preterm birth (RR 0.98, [95% CI 0.93, 1.04]).
Discussion
In this large national cohort, maternal asthma was associated with increased risk of preterm birth and less definitely with increased risk of stillbirth and neonatal death. In contrast, other maternal atopic diseases were associated with decreased risk of preterm birth, stillbirth, and neonatal death.
There are several reports on associations between maternal asthma and adverse pregnancy outcomes,2, 3 while the literature on pregnancies of mothers with other atopic diseases is scarce. However, the few published results are consistent with our findings of protective effects. A Finnish study reported that allergic rhinoconjunctivitis was associated with lower risk of birthweight < 1000 g (OR:0.49, [95% CI 0.26, 0.89] and atopic dermatitis was more weakly associated (OR:0.64, [95% CI 0.36, 1.15]).11 Given that babies with extremely low birthweights are likely to be preterm, these results are consistent with our data. Similarly, Somoskövi et al12 found a significantly lower rate of preterm birth among mothers with allergic rhinoconjunctivitis (3.9%) than among mothers without (9.2%). Two studies have assessed relations between atopic dermatitis or allergic rhinoconjunctivitis and perinatal mortality. Seeger et al13 reported no cases of stillbirths in 225 pregnancies of women with atopic dermatitis compared with 0.6% in a control group. Metzger et al14 assessed incidence of preterm birth, stillbirth, and neonatal death in a small sample of atopic mothers, and found lower rates than those reported for the general population. These studies are far too limited to allow firm conclusions, but the consistency of protective effects across many study designs lends some credibility to our findings.
There are several possible explanations of the reduced risks of adverse pregnancy outcomes with maternal atopic dermatitis and allergic rhinoconjunctivitis. One is confounding. Affected mothers had higher levels of education, and higher socio-economic status is associated with increased prevalence of atopic dermatitis and allergy15 and also with decreased risk of poor pregnancy outcome.16 Mothers with high socio-economic status may also report more minor health issues than others. Mothers with atopic dermatitis or allergic rhinoconjunctivitis were more often nulliparous, and nulliparity has been associated with increased risk of preterm birth and perinatal death.17–19 Several studies have reported seasonal variations in prevalences of preterm birth and stillbirth,20, 21 and activity of atopic disorders may vary with seasons. However, the associations reported here were minimally changed after adjusting for socio-economic factors, parity, and seasonality. It is still possible that other sources of confounding have not been accounted for.
The observed associations may have been influenced by misclassification. Information on maternal health is acquired from the pregnancy health chart, and registration to the birth registry is completed during the mother’s stay at the maternity unit. With dramatic events, such as preterm delivery and perinatal death, maternal health conditions without obvious relations to the outcome may be underreported to the birth registry. This hypothesis was supported by the findings of decreased risk of adverse pregnancy outcomes with psoriasis and cystitis. The limited literature on psoriasis and pregnancy outcomes suggests that psoriasis has – if any – an adverse effect.22–24 Neither is there scientific support for a protective effect of infectious cystitis on perinatal mortality. However, as maternal psoriasis and cystitis in pregnancy were differently related to the outcomes, there was no consistent pattern of misclassification. If underreporting of atopic disorders after severe birth events is present, this bias would be differential and may exaggerate the negative associations of atopic dermatitis and allergic rhinoconjunctivitis with the outcomes and underestimate the positive associations of asthma.
We considered the possibility that disease treatments rather than the diseases themselves were associated with poor pregnancy outcomes. Topical steroids are the main treatment for atopic dermatitis, and antihistamines a mainstay in the treatment for allergic rhinoconjunctivitis. A Danish study found no association between use of topical steroids and preterm delivery.25 Another Scandinavian study found decreased risks of preterm delivery and low birthweight with antihistamine use, but only for mothers who had used these drugs for nausea and vomiting.26
We explored whether maternal atopic dermatitis or allergic rhinoconjunctivitis might be negatively associated with pregnancy disorders or birth characteristics that could have mediated a better pregnancy outcome. This was not supported by our findings. Pre-eclampsia, unspecified bleeding, and cesarean section (all associated with poorer outcome) were more prevalent among mothers with these diseases. Mean birthweight was slightly higher, and there were no important differences in Apgar scores. Thus, pregnancy and birth characteristics offered no obvious alternative explanation for the observed associations.
If atopic dermatitis and allergic rhinoconjunctivitis are in fact protective against preterm delivery and stillbirth, potential mechanisms may include immunological and genetic pathways. Both pregnancy and atopy are characterized by a shift from cellular (T-helper cell type 1-dominated) towards humoral immune responses (T-helper cell type 2-dominated).4, 5 Pregnancy promotes this immunological deviation in order to prevent rejection of the fetus while maintaining a defense against infections.5 There is evidence that immunological responses in pregnancy may influence success in pregnancy,5, 27 and the balance between cellular and humoral immunity seems to be a predictor for pregnancy length.27 It is thus possible that the immunological profile in atopic diseases may protect from adverse pregnancy outcomes. It is also possible that there are genes associated with both atopy and pregnancy outcomes. Genome-wide association studies have identified variants at genetic loci shared by atopic disorders,28 but possible links of these to pregnancy outcomes have, to our knowledge, not been explored.
In contrast, asthma has been associated with several adverse pregnancy outcomes.2, 3 A meta-analysis from 2011 reported that maternal asthma was associated with increased risk of preterm birth,2 and a recent meta-analysis found an increased risk of neonatal death, but not stillbirth.3 The risk of preterm birth seems to be lower if the pregnant women with asthma receive active management.2 This may indicate that manifestations of asthma (such as inflammation, reduced tissue oxygenation, and smooth muscle hyperresponsiveness) complicate pregnancies and overshadow possible favorable effects of atopy. Moreover, it has been proposed that less than half of the cases of asthma are attributable to atopy,29 and a potential protective effect related to atopy may be a less important factor in asthma than in other atopic disorders.
Strengths of this study include large sample size and minimal loss to follow-up. There are also important limitations. The birth registry diagnoses of maternal asthma, atopic dermatitis, and allergic rhinoconjunctivitis have not been validated. It was not possible to distinguish between self-reported and physician-diagnosed disease, or between past and current disease. Medication use during pregnancy, which could be a proxy parameter for active disease, was not recorded in the birth registry before 1999. A 1995 report on atopic diseases (self-reports of ever having had the disease) among Norwegian women found substantially higher prevalences than the present study (9% for asthma, 19% for atopic dermatitis, and 33% for allergic rhinoconjunctivitis).30 This implies that underreporting is likely. Such underreporting would probably be non-differential, which would weaken the observed associations. The low prevalences of maternal atopic disorder may also imply that severe disease is overrepresented, which may limit the generalizability of our findings to mild and moderate disease. Generalizability is also hampered by the long study period. During these decades, the diagnostic criteria, prevalence, and treatment of atopic diseases have evolved, while perinatal mortality rates have declined. For maternal atopic dermatitis and allergic rhinoconjunctivitis (although not for asthma), the risk estimates for preterm birth and stillbirth were generally slightly stronger towards more recent periods, suggesting that these associations could be valid in contemporary settings. In contrast, the risk estimates for neonatal death with atopic diseases varied considerably in different periods.
In conclusion, we found that maternal atopic dermatitis and allergic rhinoconjunctivitis were associated with decreased risk of several adverse pregnancy outcomes. These associations may be due to favorable immunological features of atopy, but the possibility of bias cannot be excluded. Further studies are warranted to confirm these findings. If verified, a search for specific protective perinatal factors related to atopic illness may lead to better understanding of perinatal health and new preventive strategies.
Supplementary Material
Table S1. Relative risk of preterm, term and postterm birth for mothers born at term (37–41 weeks) with asthma, atopic dermatitis, allergic rhinoconjuntivitis, and no atopic disease limited to 299,438 births where gestational age was known for both mother and child
Table S2. Relative risk of preterm, term, and postterm birth among mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods
Table S3. Relative risk of stillbirth with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods
Table S4. Relative risk of neonatal death with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods
Acknowledgments
The study was funded by the Western Norwegian Regional Health Authority and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Punekar YS, Sheikh A. Establishing the sequential progression of multiple allergic diagnoses in a UK birth cohort using the General Practice Research Database. Clinical and Experimental Allergy. 2009;39:1889–1895. doi: 10.1111/j.1365-2222.2009.03366.x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG: An International Journal of Obstetrics & Gynaecology. 2011;118:1314–1323. doi: 10.1111/j.1471-0528.2011.03055.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy VE, Wang G, Namazy JA, Powell H, Gibson PG, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120:812–822. doi: 10.1111/1471-0528.12224. [DOI] [PubMed] [Google Scholar]
- 4.Jutel M, Akdis CA. T-cell subset regulation in atopy. Current Allergy and Asthma Reports. 2011;11:139–145. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunology Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 6.Norwegian Institute of Public Health. [[Last accessed 3 May 2014]];Medical Birth Registry of Norway. 2012 Available at: http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25,7840:1:0:0:::0:0.
- 7.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:440–449. [PubMed] [Google Scholar]
- 8.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. American Journal of Epidemiology. 2008;167:474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 9.de Marco R, Pattaro C, Locatelli F, Svanes C, Group ES. Influence of early life exposures on incidence and remission of asthma throughout life. Journal of Allergy and Clinical Immunology. 2004;113:845–852. doi: 10.1016/j.jaci.2004.01.780. [DOI] [PubMed] [Google Scholar]
- 10.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. The association of preterm birth with severe asthma and atopic dermatitis: a national cohort study. Pediatric Allergy and Immunology. 2013;24:782–787. doi: 10.1111/pai.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savilahti E, Siltanen M, Pekkanen J, Kajosaari M. Mothers of very low birthweight infants have less atopy than mothers of full-term infants. Clinical and Experimental Allergy. 2004;34:1851–1854. doi: 10.1111/j.1365-2222.2004.02122.x. [DOI] [PubMed] [Google Scholar]
- 12.Somoskövi A, Bartfai Z, Tamasi L, Kocsis J, Puho E, Czeizel AE. Population-based case-control study of allergic rhinitis during pregnancy for birth outcomes. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2007;131:21–27. doi: 10.1016/j.ejogrb.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Seeger JD, Lanza LL, West WA, Fernandez C, Rivero E. Pregnancy and pregnancy outcome among women with inflammatory skin diseases. Dermatology. 2007;214:32–39. doi: 10.1159/000096910. [DOI] [PubMed] [Google Scholar]
- 14.Metzger WJ, Turner E, Patterson R. The safety of immunotherapy during pregnancy. Journal of Allergy and Clinical Immunology. 1978;61:268–272. doi: 10.1016/0091-6749(78)90202-6. [DOI] [PubMed] [Google Scholar]
- 15.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55:S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen LH, Helweg-Larsen K, Andersen AM. Socioeconomic differences in perinatal health and disease. Scandinavian Journal of Public Health. 2011;39:110–114. doi: 10.1177/1403494811405096. [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. British Medical Journal. 2013;346:f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond G, Langridge A, Leonard H, Hagan R, Jacoby P, DeKlerk N, et al. Changes in risk factors for preterm birth in Western Australia 1984–2006. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120:1051–1060. doi: 10.1111/1471-0528.12188. [DOI] [PubMed] [Google Scholar]
- 19.Vernier MC, Mackenzie CJ, Schulzer M, Vernier PR. Influence of the mother’s preceding pregnancies on fetal development and postnatal survival of the neonate, in normal pregnancy. An immunological phenomenon? American Journal of Human Biology. 2010;22:708–715. doi: 10.1002/ajhb.21071. [DOI] [PubMed] [Google Scholar]
- 20.Bodnar LM, Simhan HN. The prevalence of preterm birth and season of conception. Paediatric and Perinatal Epidemiology. 2008;22:538–545. doi: 10.1111/j.1365-3016.2008.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strand LB, Barnett AG, Tong S. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environmental Research. 2011;111:451–462. doi: 10.1016/j.envres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Barak E, Nachum Z, Rozenman D, Ziv M. Pregnancy outcomes in women with moderate-to-severe psoriasis. Journal of the European Academy of Dermatology and Venereology. 2011;25:1041–1047. doi: 10.1111/j.1468-3083.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 23.Lima XT, Janakiraman V, Hughes MD, Kimball AB. The impact of psoriasis on pregnancy outcomes. Journal of Investigative Dermatology. 2012;132:85–91. doi: 10.1038/jid.2011.271. [DOI] [PubMed] [Google Scholar]
- 24.Yang YW, Chen CS, Chen YH, Lin HC. Psoriasis and pregnancy outcomes: a nationwide population-based study. Journal of the American Academy of Dermatology. 2011;64:71–77. doi: 10.1016/j.jaad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Mygind H, Thulstrup AM, Pedersen L, Larsen H. Risk of intrauterine growth retardation, malformations and other birth outcomes in children after topical use of corticosteroid in pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2002;81:234–239. doi: 10.1034/j.1600-0412.2002.810308.x. [DOI] [PubMed] [Google Scholar]
- 26.Källén B. Use of antihistamine drugs in early pregnancy and delivery outcome. Journal of Maternal-Fetal and Neonatal Medicine. 2002;11:146–152. doi: 10.1080/jmf.11.3.146.152. [DOI] [PubMed] [Google Scholar]
- 27.El-Shazly S, Makhseed M, Azizieh F, Raghupathy R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. American Journal of Reproductive Immunology. 2004;52:45–52. doi: 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 28.Tamari M, Tanaka S, Hirota T. Genome-wide association studies of allergic diseases. Allergology International. 2013;62:21–28. doi: 10.2332/allergolint.13-RAI-0539. [DOI] [PubMed] [Google Scholar]
- 29.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith-Sivertsen T, Tchachtchine V, Lund E. Atopy in Norwegian and Russian adults: a population-based study from the common border area. Allergy. 2003;58:357–362. doi: 10.1034/j.1398-9995.2003.00034.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative risk of preterm, term and postterm birth for mothers born at term (37–41 weeks) with asthma, atopic dermatitis, allergic rhinoconjuntivitis, and no atopic disease limited to 299,438 births where gestational age was known for both mother and child
Table S2. Relative risk of preterm, term, and postterm birth among mothers with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods
Table S3. Relative risk of stillbirth with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods
Table S4. Relative risk of neonatal death with asthma, atopic dermatitis, allergic rhinoconjunctivitis, and no atopic disease among 1,974,226 births in different time periods