Abstract
Objective
To determine the cause of refractory hypercalcemia in a patient with metastatic renal cell carcinoma.
Methods
We describe the clinical, pathologic, and immunostain findings in a patient with metastatic renal cell carcinoma and hypercalcemia of malignancy refractory to intravenous bisphosphonates.
Results
A 57-year-old man with a remote history of clear cell renal cell carcinoma was referred to our clinic for evaluation of resistant hypercalcemia 12 years after nephrectomy. The patient had simultaneous elevation of serum 1,25-dihydroxyvitamin D and parathyroid hormone–related peptide. Computed tomographic scan of the chest and abdomen demonstrated numerous ring-enhancing lesions in the liver, and histologic examination of a biopsy specimen revealed liver tissue infiltrated by a malignant neoplasm composed of cells with clear and eosinophilic cytoplasm, arranged in tubules and nests. Findings were morphologically consistent with renal cell carcinoma of clear cell type, and positive immunostaining with the epithelial markers EMA and CAM 5.2 were supportive of the morphologic impression of renal cell carcinoma. The tumor showed expression of 25-hydroxyvitamin D 1α-hydroxylase by immunostaining. After failing to respond to intravenous bisphosphonates, the hypercalcemia improved with prednisone treatment.
Conclusions
In some patients with renal cell carcinoma, hypercalcemia of malignancy is associated with simultaneous elevation in serum 1,25-dihydroxyvitamin D and parathyroid hormone–related peptide. As our case exemplifies, it is imperative to identify such patients because hypercalcemia due to elevated 1,25-dihydroxyvitamin D levels may respond better to glucocorticoid treatment than to the conventional management with bisphosphonates.
Keywords: hypercalcemia, calcium, 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 1-alpha-hydroxylase, parathyroid hormone, parathyroid hormone-related protein, neoplasms, renal cell carcinoma
INTRODUCTION
Malignancy-associated hypercalcemia is the most common cause of hypercalcemia in the hospitalized patient and is a predictor of a poor prognosis (1). Although it can be secondary to bone resorption by bone metastases, the most common cause of hypercalcemia of malignancy is the paraneoplastic syndrome referred to as humoral hypercalcemia of malignancy (HHM). In HHM, secretion of parathyroid hormone–related peptide (PTHrP) by the underlying tumor promotes bone resorption and reduces urinary calcium excretion (2). A second, less common, paraneoplastic syndrome is 1,25-dihydroxyvitamin D (1,25[OH]2D)–mediated hypercalcemia, in which the active form of vitamin D is secreted by the tumor (3). Far less common than HHM, 1,25(OH)2D–mediated hypercalcemia has been mostly reported in patients with lymphomas (4).
PTHrP is a polypeptide with an N-terminal segment that is closely related to parathyroid hormone (PTH), allowing it to act at the PTH receptor in a similar fashion as PTH (5). Therefore, patients with cancer who have HHM demonstrate many similarities to patients with primary hyperparathyroidism, including hypercalcemia, enhanced renal calcium reabsorption, and increased osteoclastic bone resorption (6). However, a notable difference in vitamin D metabolism is seen between primary hyperparathyroidism and HHM (7). While serum 1,25(OH)2D is generally elevated in patients with primary hyperparathyroidism, it tends to be suppressed in patients with HHM and elevated PTHrP levels (8,9). In contrast, PTH and PTHrP have no role in 1,25(OH)2D–mediated hypercalcemia; instead, the active vitamin D enhances osteoclastic activity and increases intestinal calcium absorption, with consequent hypercalcemia and suppression of serum PTH (4,10).
We describe a patient with metastatic renal cell carcinoma (RCC) and associated hypercalcemia of malignancy in whom comprehensive evaluation for hypercalcemia revealed simultaneous elevation of serum 1,25(OH)2D and PTHrP.
CASE REPORT
Patient Description
A 57-year-old man with a remote history of clear cell RCC was referred to our clinic for evaluation of resistant hypercalcemia 12 years after nephrectomy. Medical history was notable for prostate cancer diagnosed at age 49 years and treated with prostatectomy. Lymph nodes were negative for metastases, and prostate-specific antigen had been undetectable since prostatectomy.
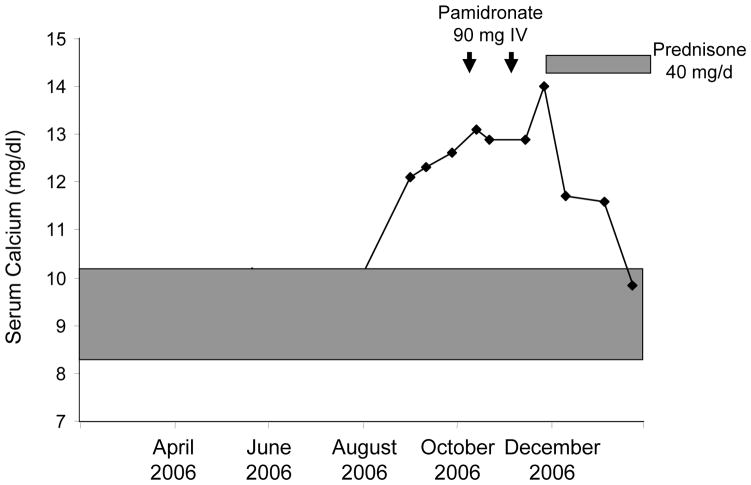
Clinical symptoms at the time of presentation included fatigue, lethargy, constipation, nausea, polyuria, and polydipsia. Physical examination findings were notable for dry mucous membranes. Findings from neurologic, cardiovascular, and musculoskeletal examinations were unremarkable. Serum calcium was essentially within the reference range (8.3–9.9 mg/dL when checked on 2 occasions, 9 and 4 months before presentation. Three months before referral, the serum calcium concentration started increasing, ranging between 12.1 and 13.1 mg/dL (Fig. 1). At that time, serum PTH was undetectable (<2.5 pg/mL) and PTHrP was above the upper limit of the reference range (2.7 pmol/L [normal <2.0 pmol/L]). He also had hypercalciuria with mild renal insufficiency (Table 1). Serum prostate-specific antigen was undetectable.
Fig. 1.
Course of serum calcium levels in a patient with metastatic renal cell carcinoma and simultaneous elevation in serum parathyroid hormone–related peptide and 1,25-dihydroxyvitamin D before and during bisphosphonate and glucocorticoid therapy. Shaded area represents reference range for serum calcium. Arrows represent timing of 90-mg pamidronate intravenous infusions. Bisphosphonate therapy was ineffective in treating the hypercalcemia, but resolution occurred with glucocorticoids.
Table 1.
Serum and Urinary Laboratory Values Before and During Glucocorticoid Treatment
| Parameter (reference range) | Before prednisone | During prednisone therapy |
|---|---|---|
| Serum total calcium (8.4–10.2 mg/dL) | 12.9 | 9.8 |
| Serum ionized calcium (4.7–5.1 mg/dL) | 7.3 | 5.8 |
| Serum phosphorus (2.4–4.5 mg/dL) | 2.3 | 2.8 |
| Serum parathyroid hormone (12–65 pg/mL) | <2.5 | <2.5 |
| Serum parathyroid hormone–related peptide (<2.0 pmol/L) | 3.4 | 2.5 |
| Serum 25-hydroxyvitamin D (25–80 ng/mL) | 38 | ND |
| Serum 1,25-dihydroxyvitamin D (22–67 pg/mL) | 79 | 25 |
| Serum creatinine (0.6–1.2 mg/dL) | 1.5 | 1.7 |
| 24-hour urinary calcium (100–250 mg/24 h) | 470 | ND |
Abbreviation: ND, not done.
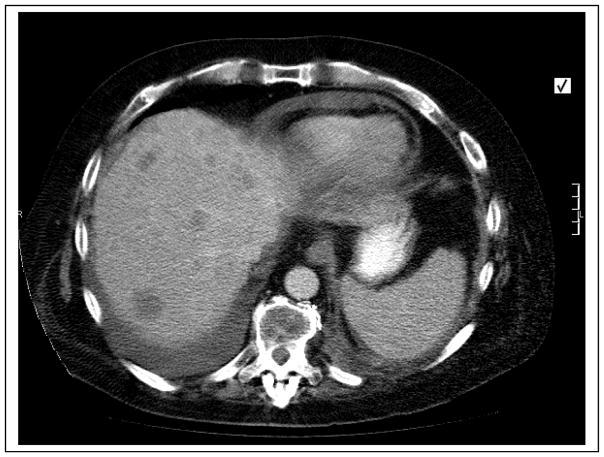
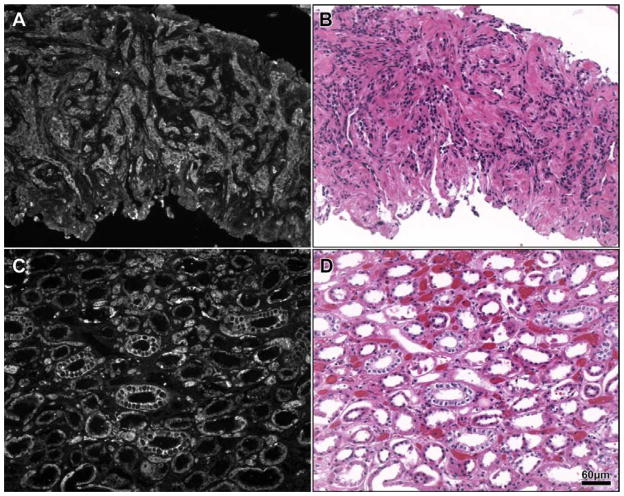
Computed tomographic scan of the chest and abdomen demonstrated numerous ring-enhancing lesions in the liver (Fig. 2). Histologic examination of the specimen obtained by computed tomography–guided liver biopsy revealed liver tissue infiltrated by a malignant neoplasm composed of cells with clear and eosinophilic cytoplasm, arranged in tubules and nests (Fig. 3). Findings were morphologically consistent with RCC of clear cell type, and positive immunostaining with the epithelial markers EMA and CAM 5.2 were supportive of the morphologic impression of RCC. On immunohistologic examination, the lesion stained positive for 25-hydroxyvitamin D 1α-hydroxylase (Fig. 3). The patient was initially treated for hypercalcemia with 2 intravenous infusions of 90 mg pamidronate 4 weeks apart without improvement in serum calcium. Because of the poor response to intravenous pamidronate, the serum 1,25(OH)2D concentration was checked, and it was elevated at 79 pg/mL (reference range, 22–67 pg/mL).
Fig. 2.
Axial computed tomographic image of the abdomen demonstrating numerous ring-enhancing lesions in the liver.
Fig. 3.
Immunostaining showing expression of 25-hydroxyvitamin D 1α-hydroxylase by the metastatic tumor. Panel A, Darkfield microscopy showing expression of 25-hydroxyvitamin D 1α-hydroxylase in tumor cells (center of section), but not in the adjacent normal liver parenchyma (bottom right) (original magnification ×100). Panel B, Corresponding hematoxylin and eosin stain showing liver tissue infiltrated by a malignant neoplasm composed of cells with clear and eosinophilic cytoplasm (original magnification ×100). Panel C, Expression of 25-hydroxyvitamin D 1α-hydroxylase by renal tubular epithelial cells (positive control) (original magnification ×100). Panel D, Hematoxylin and eosin stain of section corresponding to Panel C (original magnification ×100).
Because of the elevated serum 1,25(OH)2D and further worsening of his hypercalcemia, a trial of prednisone, 40 mg orally per day, was started 3 weeks after the last pamidronate infusion. The patient’s condition responded well with prompt improvement of symptoms and decrease in serum calcium (9.8 mg/dL) and 1,25(OH)2D (25 pg/mL) concentrations (Table 1). On last follow-up, his serum calcium concentration was still within the reference range while on prednisone. Unfortunately, the patient’s tumor was poorly responsive to chemotherapy, and he developed a malignant pericardial effusion and eventually died.
Tissue Harvest and Preparation
Patient liver samples obtained for diagnostic histopathology and immunohistochemistry were taken via computed tomography–guided fine-needle aspiration biopsy. Cadaveric kidney served as a positive control for 25-hydroxyvitamin D 1α-hydroxylase. Tissues were fixed, and subsequent paraffin processing, embedding, sectioning, and histologic stains were performed by standard procedures.
Immunohistochemistry
Immunostaining of paraffin sections was performed according to published methods and to commercial vendor recommendations. Polyclonal sheep antisera against 25-hydroxyvitamin D 1α-hydroxylase were obtained from Binding Site, Inc (San Diego, California). Briefly, paraffin sections were dewaxed, hydrated through graded ethanols to water, microwaved for 2.5 minutes at 100°C in 1× Antigen Retrieval Citra buffer (BioGenex Laboratories, Inc, San Ramon, California), blocked with normal rabbit serum, and incubated with diluted primary antibody overnight at 4°C. Bound primary was detected with biotinylated rabbit antisheep secondary antibody and streptavidin fluorescein from Vector Laboratories (Burlingame, California). Coverslips were applied with Vectashield (Vector Laboratories, Burlingame, CA).
Microscopy
Review and photography of histologic preparations were performed on a Zeiss Axioplan 2iE photomicroscope (Carl Zeiss Microimaging, Thornwood, New York) equipped with brightfield and epifluorescence illumination. Photomicrography was done using this microscope, a Zeiss Axiocam CCD monochromatic camera, and color filter wheel (CRi, Woburn, Massachusetts)–controlled OpenLab image acquisition software (Improvision, Waltham, Massachusetts).
DISCUSSION
We describe a unique patient with metastatic RCC and hypercalcemia of malignancy who exhibited simultaneous elevation of 1,25(OH)2D and PTHrP, with the tumor expressing 25-hydroxyvitamin D 1α-hydroxylase. After failing to respond to intravenous bisphosphonates, hypercalcemia improved with prednisone treatment. This case illustrates the importance of reviewing all possible factors that can be responsible for hypercalcemia in the presence of a solid tumor because it may redirect management towards a more effective treatment.
Hypercalcemia secondary to RCC is generally attributable to elevated PTHrP levels (11,12), and other investigators have detected PTHrP in RCC tissues by immunostaining (13). PTHrP and PTH act through the same G-protein–coupled receptor, and patients with HHM share some features with patients who have primary hyperparathyroidism (14,15). These features include hypercalcemia, elevated urinary cyclic adenosine monophosphate excretion, and accelerated osteoclastic bone resorption (9). However, there is a key difference between the 2 conditions in vitamin D metabolism: while serum 1,25(OH)2D concentrations are typically elevated in patients with hyperparathyroidism, they are generally suppressed in patients with HHM (7–9). The mechanism for this underlying difference remains unclear, as both PTH and PTHrP have comparable affinity to the PTH receptor and produce similar increments in circulating 1,25(OH)2D in rodents. It is possible that the marked elevation in serum calcium in HHM directly inhibits the renal 1α-hydroxylase, overcoming the stimulatory effects of PTHrP (16). Another potential explanation is the elaboration of a factor that inhibits renal 1,25(OH)2D production by the underlying tumor (17). A third explanation is that the PTHrP and PTH molecules have discordant effects on 1,25(OH)2D production in humans, due to differences in signaling pathways involved in the regulation of 1α-hydroxylase that are not entirely understood (6).
Our patient is unusual in that he presented with simultaneous elevation of serum PTHrP and 1,25(OH)2D. We demonstrated by immunostaining that the RCC cells that had metastasized to the liver expressed 25-hydroxyvitamin D 1α-hydroxylase, the enzyme needed for 1,25(OH)2D synthesis (Fig. 3). Similar synthesis of 1,25(OH)2D by tumor cells has been shown to occur in dysgerminomas, which express high levels of 25-hydroxyvitamin D 1α-hydroxylase (18).
Simultaneous elevation in serum PTHrP and 1,25(OH)2D has been already been reported in the literature, typically in association with hematologic malignancies (10). Few case reports have described this occurrence in patients with solid tumors, such ovarian malignancy (19) and a pancreatic neuroendocrine tumor (20). To our knowledge, there are no reported cases of patients with RCC and simultaneous elevation of serum PTHrP and 1,25(OH)2D. Identification of such cases probably has clinically significant therapeutic implications. After adequate hydration, bisphosphonates are the most valuable and successful agents available for the treatment of HHM due to PTHrP excess. Unlike HHM, in 1,25(OH)2D–mediated hypercalcemia, glucocorticoids are a more successful modality of treatment through suppression of extrarenal 25-hydroxyvitamin D 1α-hydroxylase (3). In 2 previously described patients with simultaneous elevation in PTHrP and 1,25(OH)2D, hypercalcemia was resistant to bisphosphonate treatment (19,20). Our patient exhibited a similar resistance to bisphosphonates, but his hypercalcemia responded well to glucocorticoid treatment. The improvement in serum calcium and normalization of 1,25(OH)2D despite persistent elevation in serum PTHrP with glucocorticoids suggests that 1,25(OH)2D was at least in part responsible for his hypercalcemia.
CONCLUSION
Hypercalcemia of malignancy may be associated with simultaneous elevation in serum 1,25(OH)2D and PTHrP in some patients with RCC. As our case exemplifies, it is imperative to identify such patients because hypercalcemia due to elevated 1,25(OH)2D levels may respond better to glucocorticoid treatment than to the conventional management with bisphosphonates.
Abbreviations
- 1, 25[OH]2D
1,25-dihydroxyvitamin D
- HHM
humoral hypercalcemia of malignancy
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone–related peptide
- RCC
renal cell carcinoma
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
References
- 1.Mundy GR, Guise TA. Hypercalcemia of malignancy. Am J Med. 1997;103:134–145. doi: 10.1016/s0002-9343(97)80047-2. [DOI] [PubMed] [Google Scholar]
- 2.Broadus AE, Mangin M, Ikeda K, et al. Humoral hypercalcemia of cancer. Identification of a novel parathyroid hormone-like peptide. N Engl J Med. 1988;319:556–563. doi: 10.1056/NEJM198809013190906. [DOI] [PubMed] [Google Scholar]
- 3.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 4.Breslau NA, McGuire JL, Zerwekh JE, Frenkel EP, Pak CY. Hypercalcemia associated with increased serum calcitriol levels in three patients with lymphoma. Ann Intern Med. 1984;100:1–6. doi: 10.7326/0003-4819-100-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Suva LJ, Winslow GA, Wettenhall RE, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz MJ, Tedesco MB, Sereika SM, et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res. 2005;20:1792–1803. doi: 10.1359/JBMR.050602. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AF, Horst R, Deftos LJ, Cadman EC, Lang R, Broadus AE. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. N Engl J Med. 1980;303:1377–1383. doi: 10.1056/NEJM198012113032401. [DOI] [PubMed] [Google Scholar]
- 8.Schilling T, Pecherstorfer M, Blind E, Leidig G, Ziegler R, Raue F. Parathyroid hormone-related protein (PTHrP) does not regulate 1,25-dihydroxyvitamin D serum levels in hypercalcemia of malignancy. J Clin Endocrinol Metab. 1993;76:801–803. doi: 10.1210/jcem.76.3.8445039. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama K, Fukumoto S, Takeda S, et al. Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J Clin Endocrinol Metab. 1996;81:607–611. doi: 10.1210/jcem.81.2.8636276. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal N, Insogna KL, Godsall JW, Smaldone L, Waldron JA, Stewart AF. Elevations in circulating 1,25-dihydroxyvitamin D in three patients with lymphoma-associated hypercalcemia. J Clin Endocrinol Metab. 1985;60:29–33. doi: 10.1210/jcem-60-1-29. [DOI] [PubMed] [Google Scholar]
- 11.Papworth K, Grankvist K, Ljungberg B, Rasmuson T. Parathyroid hormone-related protein and serum calcium in patients with renal cell carcinoma. Tumour Biol. 2005;26:201–206. doi: 10.1159/000086953. [DOI] [PubMed] [Google Scholar]
- 12.Klatte T, Said JW, Belldegrun AS, Pantuck AJ. Differential diagnosis of hypercalcemia in renal malignancy. Urology. 2007;70:179, e7–e8. doi: 10.1016/j.urology.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh A, Kitazawa S, Mizuno Y, et al. Common expression of parathyroid hormone-related protein and no correlation of calcium level in renal cell carcinomas. Cancer. 1993;71:2803–2806. doi: 10.1002/1097-0142(19930501)71:9<2803::aid-cncr2820710919>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Sourbier C, Massfelder T. Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Lett. 2006;240:170–182. doi: 10.1016/j.canlet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi N, Caulfield MP, Fisher JE, et al. Similarity of synthetic peptide from human tumor to parathyroid hormone in vivo and in vitro [erratum in Science. 1988;239:128] Science. 1987;238:1566–1568. doi: 10.1126/science.3685994. [DOI] [PubMed] [Google Scholar]
- 16.Budayr AA, Zysset E, Jenzer A, et al. Effects of treatment of malignancy-associated hypercalcemia on serum parathyroid hormone-related protein. J Bone Miner Res. 1994;9:521–526. doi: 10.1002/jbmr.5650090412. [DOI] [PubMed] [Google Scholar]
- 17.Fukumoto S, Matsumoto T, Yamoto H, et al. Suppression of serum 1,25-dihydroxyvitamin D in humoral hypercalcemia of malignancy is caused by elaboration of a factor that inhibits renal 1,25-dihydroxyvitamin D3 production. Endocrinology. 1989;124:2057–2062. doi: 10.1210/endo-124-5-2057. [DOI] [PubMed] [Google Scholar]
- 18.Evans KN, Taylor H, Zehnder D, et al. Increased expression of 25-hydroxyvitamin D-1alpha-hydroxylase in dysgerminomas: a novel form of humoral hypercalcemia of malignancy. Am J Pathol. 2004;165:807–813. doi: 10.1016/s0002-9440(10)63343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoekman K, Tjandra YI, Papapoulos SE. The role of 1,25-dihydroxyvitamin D in the maintenance of hypercalcemia in a patient with an ovarian carcinoma producing parathyroid hormone-related protein. Cancer. 1991;68:642–647. doi: 10.1002/1097-0142(19910801)68:3<642::aid-cncr2820680334>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Van den Eynden GG, Neyret A, Fumey G, et al. PTHrP, calcitonin and calcitriol in a case of severe, protracted and refractory hypercalcemia due to a pancreatic neuroendocrine tumor. Bone. 2007;40:1166–1171. doi: 10.1016/j.bone.2006.11.009. [DOI] [PubMed] [Google Scholar]