Abstract
Patient: Female, 21
Final Diagnosis: Systemic lupus erythematosus pancreatitis
Symptoms: Abdominal pain
Medication: —
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Challenging differential diagnosis
Background:
Acute pancreatitis is uncommon in systemic lupus erythematosus (SLE). When recognized early and properly treated with IV steroids and hydration, the course may be benign, as exemplified in the following report.
Case Report:
A 21-year-old woman with history of SLE and stage IV lupus nephritis, was admitted to the Sergio Bernales Hospital ICU (Lima, Peru), complaining of worsening epigastric pain radiating to the back, and nausea and vomiting for 1 week. She denied prior cholelithiasis, alcohol use, or recent medication changes.
On examination, she was tachycardic and normotensive, with a slightly distended abdomen and epigastric tenderness on deep palpation, without signs of peritoneal irritation.
Laboratory results demonstrated leukocytosis without left shift, creatinine of 2.26 mg/dL, amylase of 750 U/L, and lipase of 1038 U/L. Liver chemistries, calcium, lactic acid, triglycerides, and IgG4 were normal and alcohol level was undetectable. Ultrasound did not show cholelithiasis, biliary sludge, or common bile duct dilation. CT of the abdomen showed pancreas head (parenchyma) stranding with uniform enhancement consistent with interstitial pancreatitis.
Despite receiving IV fluids, opiates, anti-emetics, and nothing by mouth, her clinical condition deteriorated, prompting the use of IV methylprednisolone. After completing 1 week of IV steroids, she was transferred to the medical floor clinically improved. The patient was discharged with an oral steroid taper and complete resolution of symptoms.
Conclusions:
After ruling out common causes, such as hepatobiliary pathology or toxin-related insults like alcohol, hypercalcemia, hypertriglyceridemia or medications, steroids may be used in SLE pancreatitis because they might improve the overall prognosis.
MeSH Keywords: Lupus Erythematosus, Systemic; Pancreas; Pancreatitis
Background
SLE is a multi-systemic autoimmune connective tissue disorder with a broad range of clinical manifestations. Although uncommon, the presented case highlights the importance of considering acute pancreatitis secondary to SLE, as early recognition will guide appropriate treatment and result in favorable outcomes [1–4].
Case Report
A 21-year-old woman with history of SLE and stage IV lupus nephritis, treated with daily oral prednisone and IV cyclophosphamide pulsed doses every 6 months, was admitted to the Sergio Bernales National Hospital intensive care unit (Lima, Peru), complaining of worsening epigastric pain radiating to the back, nausea, and bilious vomiting for 1 week. She denied prior cholelithiasis, alcohol use, abdominal trauma, recent medication changes, or the use of over-the-counter medications or herbal remedies. Her last dose of cyclophosphamide was 6 months prior to presentation, and she reported adherence with all of her prescribed medications.
On examination, the patient was tachycardic with heart rate of 110 beats/minute and was normotensive. Her abdomen was slightly distended; the epigastrium was tender to deep palpation without signs of peritoneal irritation.
Laboratory results demonstrated 20 870 white blood cells/mL 92% segmented and 1% bands (normal value: 3500–11 000 cell/mL, Segmented: 54–62% and Bands 3–5%), creatinine of 2.26 mg/dL (normal value: 0.40–1.10 mg/dL), amylase 750 U/L (normal value: 30–110 U/L), lipase 1038 U/L (normal value: 75–395 U/L), IgG4 53 mg/dL (normal value: 8–140 mg/dL), anti-dsDNA antibody 125 IU/mL (normal value <30.0 IU/mL), and decreased complement C3 and C4 (34 mg/dL and 2 mg/dL, respectively, normal values C3: 88–206 mg/dL and C4: 13–75 mg/dL). Liver chemistries, calcium, lactic acid, and triglycerides were normal, with an undetectable alcohol level. Ultrasound did not show cholelithiasis, common bile duct dilation, or biliary sludge. CT of the abdomen was performed in the emergency department to look for necrotizing pancreatitis, given an APACHE II score of seventeen and one week-long symptom duration. CT revealed pancreas head stranding with uniform enhancement, consistent with inflammation from interstitial pancreatitis (Figure 1).
Figure 1.
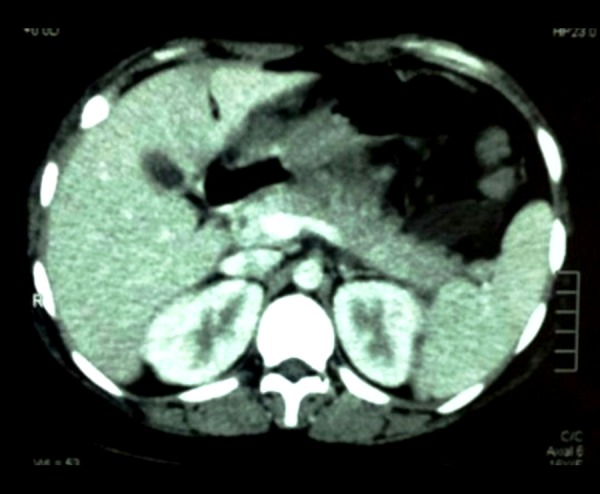
CT of abdomen showing pancreas head stranding, with uniform enhancement, consistent with inflammation and interstitial pancreatitis.
Despite receiving IV NS at 70 cc/h, opiates, anti-emetics, and nothing by mouth, her clinical condition deteriorated, with APACHE II score of 19 the following day, prompting the use of IV methylprednisolone. Clinical symptoms rapidly improved and pancreatic enzymes began to decrease.
The patient received 1 week of IV methylprednisolone 100 mg every 8 hours and then was transferred to the general medical floor to complete an oral taper of prednisone. She was discharged home 1 week later and was seen as an outpatient within 2 weeks, without complaints. She maintained outpatient clinical care with gastroenterology for 10 months following discharge without further symptoms attributed to pancreatitis and persistently normal pancreatic enzymes.
Discussion
Common precipitants of acute pancreatitis include hepatobiliary tract disease-related mechanical obstruction and metabolic insults including alcohol, drugs, hypercalcemia, and hypertriglyceridemia. Around 20% of cases of acute pancreatitis are deemed idiopathic and, as in the case presented here, other etiologies such as lupus pancreatitis should be considered in the appropriate clinical setting. Pathogenic features of SLE pancreatitis may include vasculitis, micro-thrombus formation, anti-pancreatic antibodies, and inflammation due to T-cell infiltration and complement activation [5–8]. Infectious processes, such as cytomegalovirus, have also been associated with lupus pancreatitis [9,10].
Pancreatitis in the setting of SLE was first reported by Reifenstein, [11] and has an estimated annual incidence of 0.4–1.1 per 1000 patients. Patients are typically female, with clinically active SLE developing within the first 2 years of disease onset, as in the case presented here [12].
The diagnosis of acute pancreatitis is based on clinical symptoms and pancreatic enzyme elevation and may be supported by characteristic imaging findings. However, SLE patients can have subclinical presentations of pancreatitis, with elevation of pancreatic enzymes in the absence of clinical symptoms [5,13]. Another distinguishing feature of lupus pancreatitis is that it can present in association with cytopenias. Anemia, leukopenia, and thrombocytopenia occur in 81%, 59%, and 48% of cases, respectively, while leukocytosis is infrequent (15%) [4]. Our patient presented with leukocytosis, likely explained by her chronic ingestion of oral prednisone.
Among the general population, glucocorticoids have been implicated as a potential cause of acute pancreatitis; however, in patients with SLE this association is less clear. In fact, as seen in our patient, there appears to be a paradoxical benefit of steroids in SLE pancreatitis, evidenced by studies demonstrating decreased mortality with glucocorticoids [3,4]. Prior immunosuppressive therapy does not affect the mortality from lupus pancreatitis (25% for patients prescribed steroids vs. 31% in patients not using steroids). Similarly, for those SLE patients started on steroids following the diagnosis of pancreatitis, their mortality rate was 20% compared to 61% among those not prescribed steroids [4].
The active presence of lupus symptoms among pancreatitis patients considerably increases mortality risk. One series reported no mortality events among patients without SLE symptoms at the onset of pancreatitis, compared to 40% mortality among those with SLE manifestations [4,5]. The astute clinician must have a high index of suspicion for pancreatitis in patients with SLE presenting with abdominal pain and vomiting. However, more common causes of pancreatitis should be ruled out first. Possibilities to consider include drug-induced pancreatitis, classified based on potential for causing pancreatitis from I through IV, with class I and II drugs having the greatest likelihood. Our patient was prescribed both prednisone and cyclophosphamide, which have been described as class II and IV, respectively [14].
Once common causes of pancreatitis have been excluded, steroids or other immune modulating therapies may be initiated if the clinician suspects lupus pancreatitis. In severe cases, plasmapheresis and intravenous gamma-globulin infusion may be also helpful [5,12].
Conclusions
SLE pancreatitis portends an increased mortality rate. Although uncommon, it should be considered in the differential diagnosis of acute abdominal pain in SLE patients.
After first excluding the more common causes of pancreatitis, such as hepatobiliary pathology, toxin-related insults like alcohol, hypercalcemia, hypertriglyceridemia, or medications, and with the appropriate clinical setting, steroids may improve the overall prognosis in lupus pancreatitis.
References:
- 1.Hoffman BI, Katz WA. The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature. Semin Arthritis Rheum. 1980;9(4):237–47. doi: 10.1016/0049-0172(80)90016-5. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds JC, Inman RD, Kimberly RP, et al. Acute pancreatitis in systemic lupus erythematosus: report of twenty cases and a review of the literature. Medicine (Baltimore) 1982;61(1):25–32. doi: 10.1097/00005792-198201000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Pascual-Ramos V, Duarte-Rojo A, Villa AR, et al. Systemic lupus erythematosus as a cause and prognostic factor of acute pancreatitis. J Rheumatol. 2004;31(4):707–12. [PubMed] [Google Scholar]
- 4.Breuer GS, Baer A, Dahan D, et al. Lupus-associated pancreatitis. Autoimmun Rev. 2006;5(5):314–18. doi: 10.1016/j.autrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Essaadouni L, Samar E, Krati K. Pancreatitis as initial manifestation of systemic lupus erythematosus. Lupus. 2010;19(7):884–87. doi: 10.1177/0961203309356456. [DOI] [PubMed] [Google Scholar]
- 6.Duncan HV, Achara G. A rare initial manifestation of systemic lupus erythematosus–acute pancreatitis: case report and review of the literature. J Am Board Fam Pract. 2003;16(4):334–38. doi: 10.3122/jabfm.16.4.334. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Wang NS, Zhao BH, et al. Acute pancreatitis as an initial symptom of systemic lupus erythematosus: a case report and review of the literature. World J Gastroenterol. 2005;11(30):4766–68. doi: 10.3748/wjg.v11.i30.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebbar M, Gosse D, Hatron PY, et al. Acute pancreatitis, systemic lupus erythematosus and antiphospholipid syndrome. Rev Med Interne. 1994;15(2):146–47. doi: 10.1016/s0248-8663(05)81190-7. [DOI] [PubMed] [Google Scholar]
- 9.Soyibo AK, Alfred R. A Case of Lupus-Associated Pancreatitis in Jamaica. West Indian Med J. 2010;59(3):338–41. [PubMed] [Google Scholar]
- 10.Ikura Y, Matsuo T, Ogami M, et al. Cytomegalovirus associated pancreatitis in a patient with SLE. J Rheumatol. 2000;27(11):2715–17. [PubMed] [Google Scholar]
- 11.Reifenstein E, Reifenstein EJ, Reifenstein G. A variable complex of undetermined etiology with fatal termination. Arch Intern Med. 1939;65:553–74. [Google Scholar]
- 12.Tian XP, Zhang X. Gastrointestinal involvement insystemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010;16(24):2971–77. doi: 10.3748/wjg.v16.i24.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Wang NS, Zhao BH, et al. Acute pancreatitis an initial symptom of systemic lupus erythematosus: a case report and review of the literature. World J Gastroenterol. 2005;11(30):4766–68. doi: 10.3748/wjg.v11.i30.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5(6):648–61. doi: 10.1016/j.cgh.2006.11.023. quiz 664. [DOI] [PubMed] [Google Scholar]


