Abstract
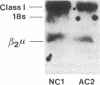
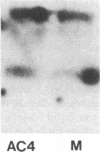
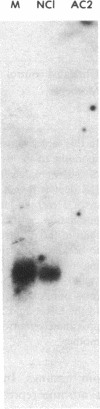
The bare lymphocyte syndrome is a disorder in which class I histocompatibility antigens fail to be expressed normally on the surface of lymphocytes. Utilizing complementary DNA probes for both beta 2-microglobulin and class I genes, the molecular basis for this syndrome was investigated in a family with two siblings exhibiting the bare lymphocyte syndrome. Southern blot analysis demonstrated no gross internal defect in either class I or beta 2-microglobulin genes. Northern blot analysis of class I and beta 2-microglobulin messenger RNAs also revealed no qualitative difference between affected and unaffected family members. In contrast, quantitation of both class I and beta 2-microglobulin transcripts demonstrated each to be decreased in patients when compared to controls. Moreover, the decrease in both transcripts was coordinate. These results suggest that the bare lymphocyte syndrome may represent a pretranslational regulatory defect of both class I and beta 2-microglobulin gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Bourgeade M. F., Creasey A. A., Merigan T. C. Interferon increases HLA synthesis in melanoma cells: interferon-resistant and -sensitive cell lines. Proc Natl Acad Sci U S A. 1982 May;79(10):3265–3269. doi: 10.1073/pnas.79.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buc-Caron M. H., Condamine H., Jacob F. The presence of F9 antigen on the surface of mouse embryonic cells until day 8 of embryogenesis. J Embryol Exp Morphol. 1978 Oct;47:149–160. [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Durandy A., Virelizier J. L., Griscelli C. Enhancement by interferon of membrane HLA antigens in patients with combined immunodeficiency with defective HLA expression. Clin Exp Immunol. 1983 Apr;52(1):173–178. [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. The cell surface antigens of mouse embryonal carcinoma cells. Biochim Biophys Acta. 1978 Sep 18;516(1):27–60. doi: 10.1016/0304-419x(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Gladstone P., Pious D. Identification of a trans-acting function regulation HLA-DR expression in a DR-negative B cell variant. Somatic Cell Genet. 1980 Mar;6(2):285–298. doi: 10.1007/BF01538802. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lisowska-Grospierre B., Charron D. J., de Préval C., Durandy A., Griscelli C., Mach B. A defect in the regulation of major histocompatibility complex class II gene expression in human HLA-DR negative lymphocytes from patients with combined immunodeficiency syndrome. J Clin Invest. 1985 Jul;76(1):381–385. doi: 10.1172/JCI111974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Morello D., Gachelin G., Dubois P., Tanigaki N., Pressman D., Jacob F. Absence of reaction of a xenogenic anti-H-2 serum with mouse embryonal carcinoma cells. Transplantation. 1978 Aug;26(2):119–125. doi: 10.1097/00007890-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Brodsky F. M., Peterlin B. M., Young L. M. "Bare lymphocytes" without immunodeficiency. Hum Immunol. 1983 Apr;6(4):219–227. doi: 10.1016/0198-8859(83)90095-2. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Schuurman R. K., van Rood J. J., Vossen J. M., Schellekens P. T., Feltkamp-Vroom T. M., Doyer E., Gmelig-Meyling F., Visser H. K. Failure of lymphocyte-membrane HLA-A and -B expression in two siblings with combined immunodeficiency. Clin Immunol Immunopathol. 1979 Dec;14(4):418–434. doi: 10.1016/0090-1229(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L., Betuel H., Souillet G., Jeune M. Combined immunodeficiency disease associated with absence of cell-surface HLA-A and -B antigens. J Pediatr. 1978 Jul;93(1):47–51. doi: 10.1016/s0022-3476(78)80598-8. [DOI] [PubMed] [Google Scholar]
- Touraine J. L., Bétuel H. Immunodeficiency diseases and expression of HLA antigens. Hum Immunol. 1981 Mar;2(2):147–153. doi: 10.1016/0198-8859(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Touraine J. L. The bare-lymphocyte syndrome: report on the registry. Lancet. 1981 Feb 7;1(8215):319–321. doi: 10.1016/s0140-6736(81)91922-x. [DOI] [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Lengyel P., Reddy E. S., Morgan W. R., Weissman S. M. Transcripts of human HLA gene fragments lacking the 5'-terminal region in transfected mouse cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):649–653. doi: 10.1073/pnas.81.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Reddy E. S., Weissman S., Lengyel P. Mouse interferons enhance the accumulation of a human HLA RNA and protein in transfected mouse and hamster cells. J Biol Chem. 1982 Nov 25;257(22):13169–13172. [PubMed] [Google Scholar]
- Zegers B. J., Heijnen C. J., Roord J. J., Kuis W., Schuurman R. K., Stoop J. W., Ballieux R. E. Defective expression of mononuclear cell membrane HLA antigens associated with combined immunodeficiency: impaired cellular interactions. Birth Defects Orig Artic Ser. 1983;19(3):93–96. [PubMed] [Google Scholar]
- Zegers B. J., Heijnen C. J., Roord J. J., Kuis W., Schuurman R. K., Stoop J. W., Ballieux R. E. Defective expression of mononuclear cell membrane HLA antigens associated with combined immunodeficiency: impaired cellular interactions. Birth Defects Orig Artic Ser. 1983;19(3):93–96. [PubMed] [Google Scholar]