Summary
'Idiopathic' herniation of the brain is a rare entity previously reported in 13 cases. It may be incidentally encountered in neuroimaging studies acquired for various clinical indications. We herein describe two cases of idiopathic brain herniation that were incidentally diagnosed. A 12-year-old boy presented with a six-month history of daytime sleepiness and sudden spells of sleep. Herniation of the left inferior temporal gyrus was revealed in MRI acquired with the suspicion of epilepsy. His overnight polysomnogram and multiple sleep latency tests were compatible with the diagnosis of narcolepsy. The other case, a two-year-old girl, was transferred from an outside hospital due to partial seizures with the fever. Herniation of the precuneal gyrus was encountered in MRI acquired after controlling her seizures with the initiation of phenytoin. The brain herniations of both patients were considered to be inconsistent with their medical conditions, so that they were symptom-free with only medical treatment for following three and six months, respectively. This is a rare presentation of idiopathic brain herniation as an incidental finding that accompanied narcolepsy and epilepsy. Awareness of this entity would avoid excessive surgical and medical treatments.
Keywords: brain herniation, idiopathic, precuneus, inferior temporal gyrus, MR imaging
Introduction
The cause of acquired brain herniation is either acute or chronic increased intracranial pressure due to space-occupying pathologies 1,2. Type II Chiari malformation and related posterior fossa shunting may rarely lead to uncal herniation secondary to a negative pressure gradient 3,4.
Contrary to acquired brain herniations, 'idiopathic herniation' is an exceedingly rare entity that is incidentally diagnosed in neuroradiology studies acquired for various clinical indications 5.
To our knowledge, only 13 idiopathic gyral herniation cases have been reported in the English language literature 5-7.
We herein describe for the first time two paediatric cases of idiopathic inferior temporal and precuneal gyrus herniation that had highly characteristic appearances.
Case Report
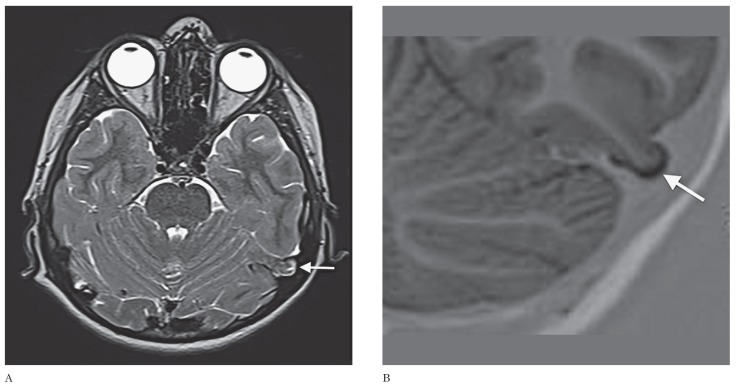
A 12-year-old boy had complaints of daytime sleepiness and sudden spells of sleep at school for the last six months. He was the youngest of three children of consanguineous parents and did not have hypnagogic hallucinations, sleep paralysis or weight change. The physical examination and routine blood tests were all normal. In order to exclude epilepsy caused by a potential epileptogenic focus, a work-up of electroencephalogram (EEG) and brain MRI were performed. EEG was normal while MRI revealed no abnormality other than an incidentally encountered mass-like appearance of a pedunculated herniation of the left posterior inferior temporal gyrus. The herniation was 9×12×15 mm in size, isointense to the adjacent cerebral parenchyma and surrounded by a cerebrospinal fluid-filled sac (Figure 1). No bony defect was observed. MR venography was planned as a further imaging modality. There was no evidence of venous sinus anomaly or thrombosis. His overnight polysomnogram and multiple sleep latency tests (mean sleep latency: 6 min, sleep onset rapid eye movement: 3) were compatible with the diagnosis of narcolepsy and he was treated with methylphenidate 27 mg per day. The patient's sleep pattern improved in the month following medical therapy.
Figure 1.
T2-weighted axial (A) and zoomed coronal T1-weighted inversion recovery (B) MR images depicting left inferior temporal gyrus herniation. Note that the herniation has a similar signal intensity to that of the adjacent cortex (arrows).
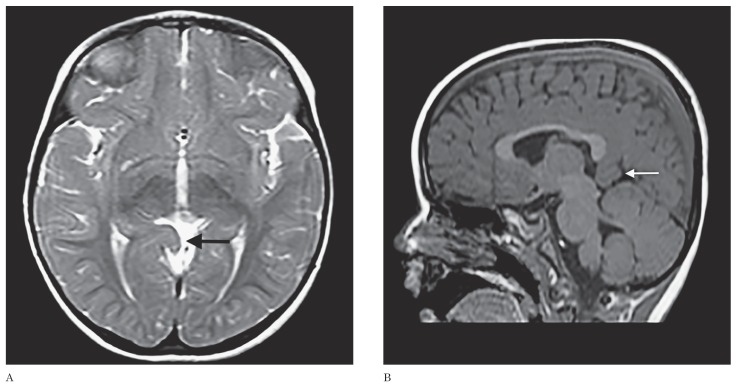
A two-year-old girl transferred from an outside hospital due to uncontrolled partial seizures accompanied with the fever was admitted to our paediatric neurology inpatient clinic. She was unconscious with prolonged partial seizures and a fever of 38.5°C. The phenytoin was initiated to control the seizures. Routine laboratory investigations of blood, urine, CSF examination, plasma amino acid concentrations, and viral PCR panel were all negative for any disease. Subsequently, EEG and the cranial MRI protocolled for the epilepsy were carried out to investigate the epileptogenic focus. MRI manifested an 18×22×25 mm right precuneal gyrus herniation through the quadrigeminal cistern with no detectable signal abnormality (Figure 2). There was no other pathology on brain MRI to serve as an epileptogenic focus. The patient's EEG showed sharp and slow wave activity in the temporal and parieto-occipital regions of both hemispheres and was considered to be incompatible with the precuneal gyrus herniation. Based on these findings the patient was diagnosed with complex febrile seizures, and treated with levetiracetam. The patient's seizures were controlled with the medical treatment.
Figure 2.
T2-weighted axial (A) and T1-weighted sagittal (B) MR images revealing a precuneal gyrus herniation into the quadrigeminal cistern (arrows).
Due to the lack of related symptoms with the herniated gyri, the patients have been followed up with the medical treatment for epilepsy and narcolepsy and had no complaints in the following three and six months, respectively.
Neither of our cases had undergone previous cranial imaging for comparison, so we presumed their condition to be congenital. There was no evidence of increased or decreased intracranial pressure. Neither patient had a definitive history of head trauma, operation or infection that may have caused the herniation, so that the herniations were considered to be 'idiopathic'.
Discussion
Idiopathic brain herniations are extremely rare and, unlike acquired brain herniations that usually occur as a consequence of a mass effect produced by infection, ischaemia, trauma or neoplasia, are not associated with a causative factor. A recent prospective study focused on idiopathic cuneal gyrus herniation found a prevalence rate of 0.073 % 5. The patients with cuneal gyrus herniation were managed conservatively while a herniated parahippocampal gyrus encountered in a patient diagnosed with trigeminal neuralgia was biopsied due to its mass-like appearance 7. The diagnosis of our first patient of idiopathic narcolepsy was based on the diagnostic criteria of the International Classification of Sleep Disorders (the presence of excessive daytime sleepiness for at least three months, sleep latency test findings as mean sleep latency ≤8 min, ≥2 sleep onset rapid eye movement). As idiopathic herniation of the brain has not been previously associated with narcolepsy, and the location of a herniated precuneal gyrus was not consistent with the EEG findings of second patient, the herniations were considered to be incidental findings in our current cases.
The meningeal coverings of the brain first appear in the fourth week of the developing embryo as they are derived from the mesoderm. The dura mater, a thick sheet of dense fibrous tissue, surrounds and supports the brain and arachnoid mater located inside. Although controversial, we consider that idiopathic herniation would occur due to localized defective constitution of the dura and dural extensions (e.g., tentorium cerebelli).
Idiopathic brain herniation may occupy the spaces confined with the meninges and need to be differentiated from intracranial mass lesions. Their identical signal characteristics to adjacent cerebral parenchyma, lack of signal abnormalities, and contrast enhancement on MR images may help discriminate herniations from masses and avoid unnecessary surgical procedures.
Temporal lobe encephaloceles involving the laterotemporal cranial vault should be considered the foremost element of the differential diagnosis of lateral temporal lobe herniation. Unlike herniations, they occur through a dural−osseous defect of the skull, and affected patients may present with recurrent ear infections, progressive hearing loss, and medically refractory temporal lobe epilepsy 8,9. Detection of bony defects is possible with MRI in the coronal and sagittal planes, but may be facilitated by high-resolution computed tomography.
Because of the close localization of the herniated inferior temporal gyrus to the transverse sinus, this condition may mimic either dural venous sinus thrombosis or arachnoid granulation. Detection of the typical signal characteristics of the herniated gyral segment (isointense to other gyri) and its connection to adjacent brain parenchyma in conjunction with normal MR venography examination would rule out the pathologies and variations of the dural venous sinuses.
In conclusion, despite its rarity, recognition and differentiation of idiopathic brain herniations from other pathologies such as mass lesion, encephalocele or dural venous sinus thrombosis would avoid excessive surgical and medical treatments.
References
- 1.Gopakumar H, Sivji R, Rajiv PK. Vitamin K deficiency bleeding presenting as impending brain herniation. J Pediatr Neurosci. 2010;5(1):55–58. doi: 10.4103/1817-1745.66681. doi: 10.4103/1817-1745.66681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duransoy YK, Mete M, Barutçuoğlu M, et al. Intracranial hydatid cyst is a rare cause of midbrain herniation: A case report and literature review. J Pediatr Neurosci. 2013;8(3):224–227. doi: 10.4103/1817-1745.123683. doi: 10.4103/1817-1745.123683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udayakumaran S, Ben Sira L, Constantini S. Temporal lobe herniation of developmental origin: a novel radiological association with open spina bifida and Chiari II malformation. Childs Nerv Syst. 2010;26(3):277–278. doi: 10.1007/s00381-009-1068-3. doi: 10.1007/s00381-009-1068-3. [DOI] [PubMed] [Google Scholar]
- 4.Udayakumaran S, Ben Sira L, Constantini S. Chronic uncal herniation secondary to posterior fossa shunting: case report and literature review. Childs Nerv Syst. 2010;26(2):267–271. doi: 10.1007/s00381-009-1027-z. doi: 10.1007/s00381-009-1027-z. [DOI] [PubMed] [Google Scholar]
- 5.Maldjian C, Adam R. Prevalence of idiopathic cuneate gyrus herniation based on emergency room CT examinations. Emerg Radiol. 2014 doi: 10.1007/s10140-014-1212-6. doi: 10.1007/s10140-014-1212-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Duarte MP, Maldjian TC, Tenner M, et al. Magnetic resonance imaging of idiopathic herniation of the cuneus gyrus. J Neuroimaging. 2007;17(4):353–354. doi: 10.1111/j.1552-6569.2007.00123.x. doi: 10.1111/j.1552-6569.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz M, Kassam A, Levy E, et al. Misinterpretation of parahippocampal herniation for a posterior fossa tumor: imaging and intraoperative findings. J Neuroimaging. 2002;12(1):78–79. doi: 10.1111/j.1552-6569.2002.tb00097.x. doi: 10.1111/j.1552-6569.2002.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Sdano MT, Pensak ML. Temporal bone encephaloceles. Curr Opin Otolaryngol Head Neck Surg. 2005;13(5):287–289. doi: 10.1097/01.moo.0000179247.51476.f5. doi: 10.1097/01.moo.0000179247.51476.f5. [DOI] [PubMed] [Google Scholar]
- 9.Yang E, Yeo SB, Tan TY. Temporal lobe encephalocoele presenting with seizures and hearing loss. Singapore Med J. 2004;45(1):40–42 . [PubMed] [Google Scholar]