Abstract
A weak T-cell response plays a key role in the persistence of hepatitis B virus (HBV) infection. We aimed to confirm that T-cell defects in patients with chronic HBV infection are associated with HBV DNA infection of bone marrow (BM) hematopoietic stem cells (HSCs). Using reverse transcription polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH), we observed the transcription of HBsAg coding genes and confirmed the integration of HBV DNA in CD34+ BM HSCs from chronic HBV infection patients. T cells were generated by coculturing the HSCs with delta-like ligand 1-expressing OP9 (OP9-DL1) cells. The phenotypes of the T cells were then evaluated by flow cytometric (FACS) analysis on days 14 and 25. The results demonstrated that fewer CD3+TCRaβ+ CD3+CD4+ and CD4+CD8+ T cells were generated from the HSCs of the patients than from the healthy controls, (P < 0.01) but the frequency of CD3+D8+ T cells was not significantly different between the two group (P > 0.05). In contrast, CD4+CD25+ T cells were more in the patient group than in healthy controls (P < 0.01) on both days 14 and 25. There were fewer CD3+CD4+/CD3+CD8+ cells in the patient group than in the healthy control group on day 25 (P < 0.05). Less proliferation and lower levels of IL-2 and IFN- γ were also observed in the patient group compared with the control group (P < 0.05).These data suggest that HBV DNA infected and integrated into the BM HSCs from patients with chronic HBV infection and that these BM HSCs generated defective T cells.
Keywords: HBV DNA, HSCs, integration, replication, T-cell defects
INTRODUCTION
Patients with chronic HBV infection show immune tolerance, which has been attributed to the poor T-cell responses in the peripheral blood 1–4. However, few studies have investigated whether differentiation of T cells from HSCs is associated with a weak T-cell response in chronic HBV infection. Ilan et al. reported that bone marrow transplantation led to the ablation of persistent hepatitis B, as immune reconstitution of the recipient's bone marrow resulted in clearance of both circulating HBsAg and e HBV DNA 5. Few reports demonstrated the development of immunity against HBV after stem cell transplantation 6. Furthermore, some studies have reported seroconversion in chronic hepatitis B infection after stem cell transplantation 7,8. These studies implied that stem cell transplantation led to immunologic reconstitution in patients with chronic HBV infection. CD34+ cells are present mainly in the bone marrow (BM) and can generate all hematopoietic cell lineages. T lymphocytes are also derived from CD34+BM cells 9,10. Thus, healthy T lymphocytes should be continually generated from CD34+BM HSCs in chronic HBV infection patients. However, there is little immunologic reconstitution with T-cell regeneration from the CD34+ BM HSCs in patients with chronic HBV infection. Therefore, we hypothesized that CD34+BM HSCs could be infected with HBV and that the viral DNA would integrate into the genome of the HSCs, causing deficient T-cell differentiation and development. If true, this hypothesis would explain the reduced immune reconstitution capability of CD34+ BM HSCs in patients with chronic HBV infection. To investigate this hypothesis, we sought to confirm the replication and integration of HBV DNA in CD34+ BM HSCs and to measure the phenotype, proliferative activity and secretory function of T cells generated from the HSCs in chronically HBV-infected patients.
Conventionally, CD34+ hematopoietic precursor cells differentiate to T cells in the thymus 11. However, Zuniga-Pflucker and his fellow researchers discovered that when murine hematopoietic precursor cells (HPC) were cocultured with delta-like ligand 1 (OP9-DL1) cells in the presence of IL-7 and Flt3L, these cells differentiated into double-positive (DP) cells and finally into mature CD3+ T cells that were able to produce IFN-γ 12. T cells could also be generated from various sources of human hematopoietic precursors including human bone marrow CD34+ hematopoietic cells in OP9-DL1 cultures 13–15. Van Coppernolle et al. suggested that the generation of T cells by the OP9-DL1 system may be dependent on positive selection mechanisms similar to those operative in the thymus 16. This OP9-DL1 system also has the capacity to generate regulatory T cells (Tregs) 17. Our goal was to study T cells generated from CD34+BM HSCs from chronic HBV infection patient, and the OP9-DL1 system constituted a simple and convenient approach for generating T lymphocytes in vitro.
Chronic HBV infection can lead to cirrhosis and hepatocellular carcinoma. Although antiviral therapy has improved dramatically in the last few years, it still cannot be produce a cure. Consequently, the persistent poor immune responses are an important factor. Thus, our investigation will provide a theoretical basis for immune tolerance in patients with chronic HBV infection.
PATIENT AND METHODS
Patients
For this study, eight patients diagnosed with persistent HBV infection more than one year at the Infectious Department of Harbin University First Affiliated Hospital were consecutively recruited between May 2011 and June 2012, and seven healthy individuals as control group were also enrolled. The inclusion criteria included a diagnosis of HBV DNA ≥105copies/mL, HBsAg and anti-HBc positive, anti-HBs negative and no history of antiviral treatment. Patients were excluded if they had other mixed causes of chronic liver disease such as hepatitis C, alcohol abuse or autoimmune liver disease, or if they had a diagnosis of liver cirrhosis and hypersplenia The study was performed in accordance with the principles of the Declaration of Helsinki and its appendices and was approved by the hospital's Internal Review Board and Ethics Committee. Written informed consent was obtained from all patients prior to participation.
Methods
Isolation of human CD34+ hematopoietic cells
Bone marrow cell suspensions (10 mL) were obtained, respectively, by bone marrow paracentesis from the patients and healthy donors, for isolation of CD34+ hematopoietic cells. Mononuclear cells were then obtained by density gradient centrifugation with Lymphoprep (Boster Bio, China). CD34+ hematopoietic cells were isolated by anti-CD34 (BD Biosciences, Franklin, NJ, USA) antibodies and the MACS system (Stem Cell Technology, Vancouver, Canada) from the mononuclear cells according to the manufacturer's protocols.
REVERSE TRANSCRIPTION POLYMERASE CHAIN REACTION (RT-PCR) AND DETECTION OF HBSAG
CD34+ BM HSCs of patients with chronic HBV infection and healthy donors were purified for the measurement of hepatitis B (HB) S gene expression. The levels of messenger RNA (mRNA) were assessed by RT-PCR as follows: first total RNA was extracted, purified and DNase I-treated using the RNeasy mini kit (QIAGEN, Dusseldorf, Germany) according to the manufacturer's instructions. Then, reverse transcription was performed according to the One Step RT-PCR kit (QIAGEN) protocol. For the HB S gene mRNA detection, the following HB S gene-specific primers were used: forward(fw), 5′-TATCGCTGGATGTGTCTG C-3′; reverse, 5′-AGACTTGG CCCCCAATACC-3′ which amplified a 403-bp region as used by Huang and Li X-M et al. 18. To ensure that the amplified HB S gene bands originated from cDNA from the patients’ HSC, the total RNA from the negative controls (healthy HSC) was amplified at same time. For GAPDH, the following primers were used: fw, 5-TGCACCACCAACTGCTTAGC-3 and GAPDH; rev, 5-GGCATG GACTGTGGTCATGAG-3 19. In these experiments, GAPDH was used as an internal control gene to normalize the amount of RNA loaded for each sample. The genes were amplified using 5 μL of cDNA as template. A total of 10 μL of each RT-PCR product was made visible by staining with ethidium bromide, after electrophoresis on 1% agarose gel.
The HBsAg secreted in the coculture supernatants of the two groups of subjects was measured using the Roche Elecsys HBsAg kit (Roche, Basel, Switzerland) according to the manufacturer's protocols on day 0, day 14 and day 25.
Fluorescence in situ hybridization (FISH)
FISH was performed according to Wang 20. In our study, material from three sources was examined; CD34+ cells from the patients, CD34+ cells from healthy donors and HepG2.2.15 cells as the positive control (obtained from Medical University Chong Qing, China). HepG2.2.15 cells are human hepatocarcinoma cells stably transfected with HBV. The suspension of BM CD34+ cells from the three cell types was treated, respectively, with colchicine for 3 h and then incubated in 10 mL of 75 mm KCl for 30 min at 37 °C. The cells were then treated with 2 mL of freshly prepared fixative, three times for 30 min each. The nuclei suspensions (50 μL) from each of the three samples were then placed onto microscope slides. Thus, nuclei and metaphase chromosomal samples were obtained for FISH analysis. The samples were treated with 0.01 m PBS for 10 min at room temperature and then with 200 μg/mL pepsin for 10 min, followed by 2 min each in 70%, 80%, 95% and 100% ethanol. The samples were denatured at 75 °C for 4 min in 73% formamide in 2 × SSC, and 40 μL of HBV DNA probe (Tbd science Biotechnology, Tianjin, China) was then added. The samples with the probe were incubated for 8–16 h in a dark moist chamber at 37 °C. Subsequently, the slides were washed for 5 min each in 2 × SSC, 0.2 × SSC and 0.1 m TBS at 37 °C. FITC compound (Tbd science Biotechnology) was dropped onto slides to fluorescently label the HBV DNA probe for 2 h. The nuclei and chromosomes were counterstained with 5 μL DAPI for 5 min. The slides were analysed by an Olympus BX61 fluorescent microscope, and images were acquired with 3.93 image analysis software (Olympus, Tokyo, Japan)
CD34+ BM HSCs and OP9-DL1 cocultures
The coculture experiments followed the method of Zúñiga-Pflücker et al. 21. OP9-DL1 cells were obtained from Dr. J. C. Zuniga-Pflucker (University of Toronto, Toronto, Canada). In this study, two groups were investigated; a CHB patient group and a healthy control group. A total of 1 × 103 OP9-DL1 cells were seeded onto 12-well tissue culture plates (Boster Bio, Wuhan, China) 24 h before the experiment. Sorted CD34+ BM HSCs from the two groups were then seeded, respectively, at 2 × 105 cells/well into 12-well plates containing a subconfluent monolayer of OP9-DL1 cells. All coculture experiments were performed in culture medium consisting of IMDM (GIBCO, Carlsbad, CA, USA) supplemented with 20% FCS (GIBCO, Grand Island, NY, USA), 100 IU/mL streptomycin, penicillin and L-glutamine in the presence of 5 ng/mL IL-7 (BD Biosciences), 5 ng/mL Flt3L (BD Biosciences) and 2.5 ng/mL stem cell factor (SCF) (BD Biosciences). Every 4–5 days, the cells were harvested by forceful pipetting and transferred onto a fresh confluent monolayer of OP9-DL1 cells. Fresh culture medium was then added while the aforesaid cytokine was added again. The cocultures were maintained for 25 days for the generation of T cells.
T-cell expansion and detection of T-cell subpopulations
The T cells were, respectively, harvested from the OP9-DL1 cocultures on day 14 and day 25, and the T-cell phenotype was studied using CD3+TCRaβ+, CD3+CD4+, CD3+ CD8+, CD4+CD8+ and CD4+CD25+ as markers. A FACSAria flow cytometer (BD Biosciences) was used for flow cytometry analysis (FACS) of T-cell subsets. The following monoclonal antibodies (mAbs) were used: allophycocyanin(APC)-conjugated CD3, phycoerythrin(PE)-conjugated TCRaβ, fluorescein isothiocyanate (FITC)-conjugated CD4, PE-conjugated CD8 and PE-conjugated CD25 (all from BD Biosciences). The cell suspensions were labelled and stained with the above mAbs in the dark, according to the manufacturer's protocols. Viable cells were gated based on the lymphocyte gate. The data were analysed by FACS to determine the percentage of positive cells in quadrant corners on day 14 and day 25.
T-lymphocyte proliferation and cytokine production assays
Carboxyfluorescein diacetate succinimidyl ester (CFSE) is a fluorescent dye used to label cells, the proliferative activity of which can then be detected by flow cytometry. Using this method, cells may be traced through successive generations without the use of radioactivity 22. CFSE dilution was measured by flow cytometry for assessing T-lymphocyte proliferation. Labelling of T cells with CFSE has been described previously 23. T cells from the patient group and healthy controls were harvested from OP9-DL1 cocultures on day 25 were incubated with CFSE (Invitrogen, Carlsbad, CA, USA) in 10 mm/L PBS at 37 °C for 15 min. The cell labelling was terminated by addition of one-tenth the volume of FCS. Cells were then washed twice in 37 °C PBS for 10 min and suspended at 2 × 106 cells/mL, and half of the CFSE-labelled T cells were then cultured and stimulated with 5 μg/mL phytohemagglutinin (PHA) for 72 h. The rest of the cells were cultured without PHA. Following the 72-h incubation, the percentage of decreased fluorescence intensity of the PHA-stimulated and non-PHA-stimulated cells was analysed by FACS. The cell division index (CDI) is the ratio of percentage of decreased fluorescence intensity of the PHA-stimulated to non-PHA-stimulated cells, which was used to calculate cell proliferation dynamics. The culture supernatants of lymphocytes stimulated with PHA for 72 h were collected and frozen for analysis of IL2 and IFN-γ levels. The lymphocyte culture supernatant was analysed for cytokine content by IL-2 and IFN-γ specific ELISA kits as recommended by the manufacturer (Anke Bio, Beijing, China) and described previously 24.
RESULTS
RT-PCR and HBsAg quantitation
RT-PCR for HB S gene-specific RNA was shown to be reliable for detecting HBV gene(s) expression. The 403-bp RT-PCR product corresponding to the amplified HB S gene fragment was detected in CD34+ HSCs from all patients by agarose gel electrophoresis, but no expression of these gene fragments was observed in the HSCs from the healthy controls (Fig.1). These results demonstrated that HB S encoding mRNAs are expressed in the CD34+ BM HSCs from patients.
Figure 1.
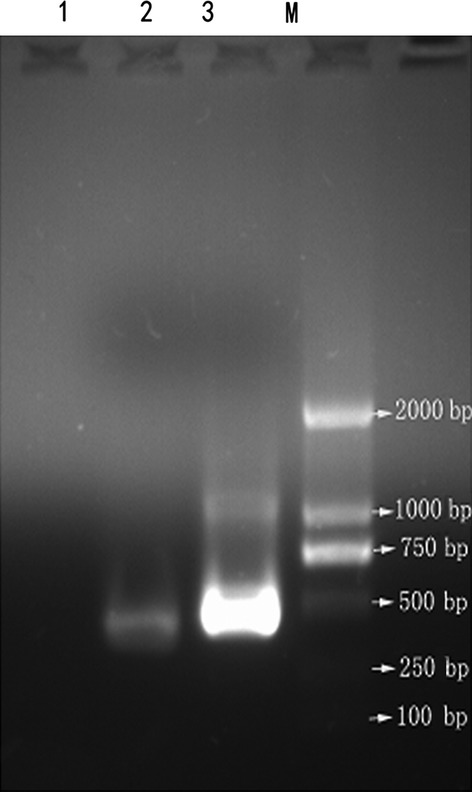
Reverse transcription polymerase chain reaction (RT-PCR) products: Lane M, DNA marker (DL 2000); lane 1, healthy control; lane 2, hepatitis B (HB) S gene (403 bp); lane 3, internal control gene(GAPDH).
The results showed that no HBsAg was detected on day 0. However, HBsAg quantitation indicated levels of 65.43 ± 8.21 ng/mL on day 14 and 89.35 ± 13.04 ng/mL on day 25 in the patient group. No HBsAg was detected on days 0, 14 and 25 in the healthy control group.
Fluorescence in situ hybridization
To perform FISH, HB S gene fragments were used as a specific probe. Positive and green fluorescent signals were observed in the nucleus of CD34+ HSCs from all the patients and chromosomes of five of the patients (Fig.2a, d, d*), as well as in the nucleus and chromosomes of HepG2.2.15 cells (Fig.2b, e, e*). No Positive signals were detected in the healthy controls (Fig.2c, f).
Figure 2.
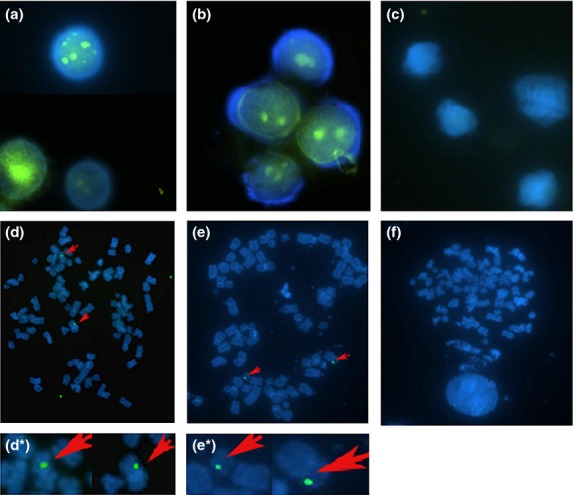
FISH analysis of HBV DNA integration into CD34+ BM HSCs. 100 × magnification. (a) Green fluorescence hybridization signals in nuclei of CD34+ BM HSCs in the patient group demonstrating HBV DNA integration into BM HSC DNA. (b) Nuclei of HepG2. 2.15 cells showing green fluorescence signal (positive control). (c) Absence of green fluorescence hybridization signal in the nuclei of CD34+ BM HSCs in healthy controls (negative control). (d) Chromosomes of CD34+ BM HSCs of the patient group. Some chromosomes show punctate green fluorescence hybridization signals (red arrows), indicating that HBV DNA gene fragments were integrated into some chromosomes of BM HSCs. (d*): Clipped and amplified chromosomes showing green fluorescence signal from figure d. (e) Chromosomes of HepG22.15 cells with some chromosomes showing green fluorescence hybridization signals (red arrows). (e*): Clipped and amplified chromosomes showing green fluorescence signal from figure e. (f): Absence of green fluorescence signal in the chromosomes of BM CD34+ cells from healthy controls.
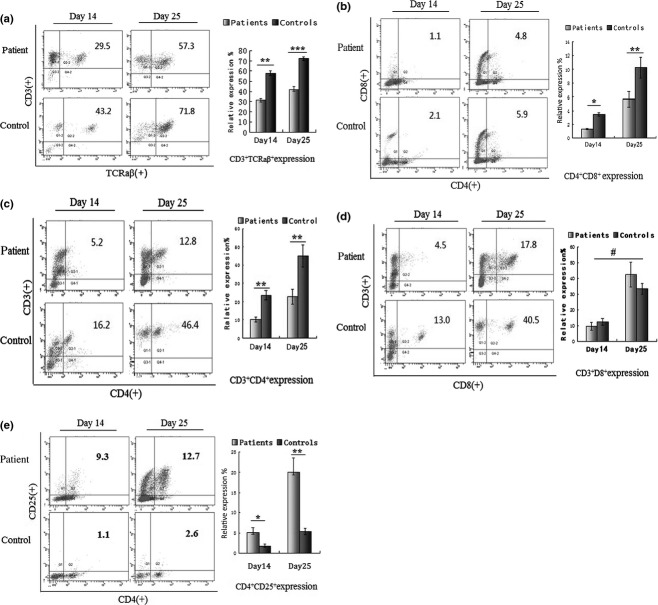
Phenotypic analysis of T lymphocytes
T lymphocytes were generated in OP9-DL1 cultures. Phenotypically mature CD3+CD4+ and CD3+CD8+ single positive T cells as well as immature double-positive CD4+CD8+ T cell, CD3+TCRaβ+ and CD4+CD25+ regulatory T cells could be discerned on day 14, and the expression of these T cells gradually increased till the T cells reached maturity on day 25. The results of this experiment are shown as the mean values ± standard deviation. The frequencies of CD3+TCRaβ+, CD4+CD8+ and CD3+CD4+T cells, respectively, were significantly lower in the patient group [(31.63 ± 1.65%), (1.28 ± 0.04%) and (10.27 ± 1.37%)] than in the healthy controls [(41.83 ± 2.85%), (3.44 ± 0.27%) and (23.51 ± 2.60%)] on day 14 (P < 0.01; Fig.3a, b, c). However, the frequencies of the CD3+CD8+ subsets were similar in the patient group (9.59 ± 2.40%) and in the healthy controls (12.30 ± 2.05%) (P > 0.05; Fig.3d). In contrast, the frequencies of CD4+CD25+ T cells were higher in the patient (5.15 ± 1.22%) than in the healthy control group (1.82 ± 0.39%) (P < 0.05; Fig.3e). On day 25, the frequencies of CD3+CD4+ T cells in the patient (22.67 ± 4.03%) also were lower than in the healthy control group (44.98 ± 5.96%) (P < 0.01; Fig.3c) while the frequency of CD3+TCRaβ+ T cells in the patient (57.98 ± 1.95%) was even lower than in the healthy control group (72.35 ± 1.70%) (P < 0.001; Fig.3a). The CD4+CD8+ expression in the patient (5.66 ± 1.14%) was also lower than in healthy controls group (10.26 ± 1.52%) (P < 0.05; Fig.3b). However, the CD3+CD8+ expression was no different between the patient (42.31 ± 7.77%) and the healthy control group (33.34 ± 3.83%) (P > 0.05; Fig.3d). The frequencies of CD4+CD25+ T cells in the patient group (20.00 ± 3.49%) increased more obviously than in the controls (5.40 ± 0.74%) (P < 0.01; Fig.3e). Although the CD3+CD4+/CD3+CD8+ ratio was similar in the patient group (1.79 ± 0.52) and in the healthy control group (2.21 ± 0.36) on day 14, this ratio was lower in the patient group (0.58 ± 0.09) than in the healthy control group (1.42 ± 0.26) on day 25 (P < 0.05) as shown in Fig.3f.
Figure 3.
Expression analysis of T-cell subpopulations generated from CD34+ BM HSCs cocultured with OP9-DL1 cells. (a–e): Flow cytometric and statistical analysis of CD3+TCRaβ+ (a) CD4+CD8+ (b), CD3+CD4+ (c),CD3+CD8+ (d) and CD4+CD25+ (e)T cells on days 14 and 25 in the two groups. The percentage of T cell phenotype expression is indicated on the upper-right side of the cytometric graph. (f) T-cell ratio analysis of CD3+CD4+/CD3+CD8+ on days 14 and 25. Data are shown as mean values ± SD representative of eight independent experiments. P-values were determined using Student's t test. (*P < 0.05, **P < 0.01,***P < 0.001, #P > 0.05).
CDI and cytokines produced by T lymphocytes
The fluorescence intensity of the CFSE-labelled HSCs was remarkably decreased in the healthy control group (Fig.4b) than in the patient group (Fig.4a) by FACS. The CDI values were lower in the patient group (1.28 ± 0.06) than e in the healthy control group (2.18 ± 0.24) as shown in Fig.4c (P < 0.05). When the cytokine production by the T cells of the two groups was compared following PHA stimulation, the following values were observed for the patient group: IL-2 (157.83 ± 54.46 pg) and IFN-γ (356.11 ± 57.9 pg), while for the healthy controls, these were IL-2 (392.92 ±122.17 pg) and IFN-γ (769.02 ± 99.59 pg). The production of IL-2 and IFN-γ was lower in the patient group compared to the control group, as shown in this Fig.4d.
Figure 4.
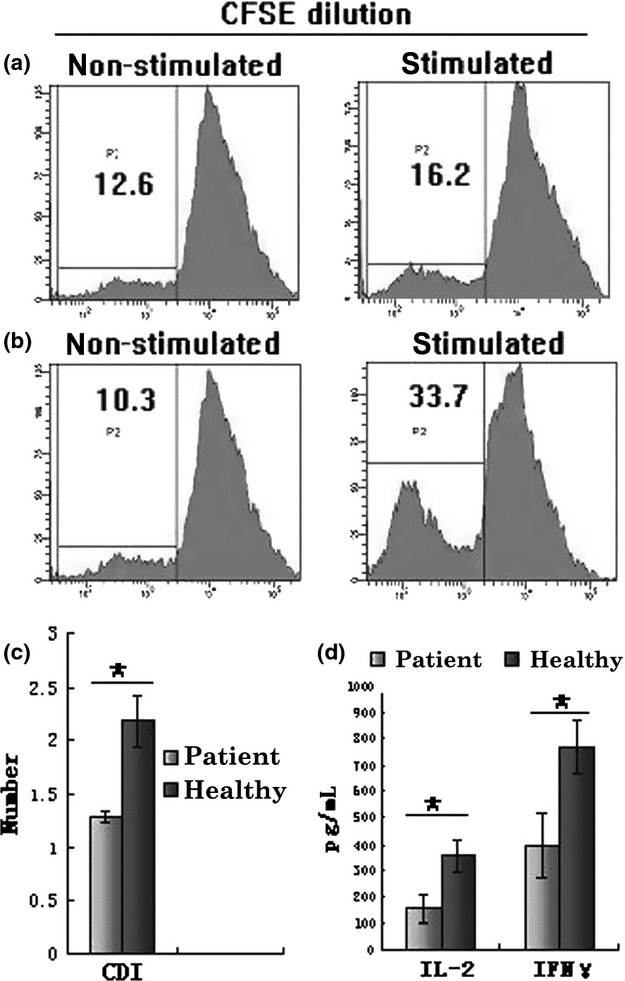
Analysis of T-lymphocyte proliferation and cytokine production. (a, b) Flow cytometric analysis demonstrating the percentage of decreased fluorescence intensity of PHA-stimulated and non-PHA-stimulated CFSE-labelled T cells from the two groups. The percentage is indicated on the upper left side of the cytometric graph. (a) Healthy control group; (b) patient group. (c) Comparative analysis data of CDI in the two groups. (d) Comparative analysis data of IFN-γ and IL-2 production by T cells harvested from HSC/OP9-DL1 of the two groups following stimulation with PHA for 72 h. The data are represented as mean values ± SD of individual experiments. P-values were determined using Student's t test (*P < 0.05).
DISCUSSION
In this study, we describe for the first time the immune deficiency mechanism of chronic HBV infection in terms of HSCs. The HBV genome contains four open reading frames (ORF), of which the S-ORF is divided into the pre-S1, pre-S2 and S genes. Expression of the HB S gene (403 bp) mRNA is an important marker of HBV replication and transcription. HBsAg encoded by the S gene is the major viral antigen. The results of the HB S gene (403 bp) mRNA detection and HBsAg expression in patient HSCs by RT-PCR and HBsAg quantitative determination suggested that HBV DNA is able to replicate in the BM CD34+ HSCs of patients and that HBsAg is synthesized and secreted into the supernatant. The results of no HBsAg in coculture supernatants on day 0 implied that the detected HBsAg in coculture supernatants on days 14 and 25 was not due to contamination. FISH analysis suggested that HB S gene segments are irregularly integrated into HSCs DNA. The integrated HB S gene segments can also express HBsAg followed by release into the supernatant.
T-cell development is usually divided into three stages; double negative (DN), double positive (DP) and single positive (SP) 25. The DP cell is an immature T cell expressing CD4+CD8+, but the SP cell is a mature T cell expressing CD3+CD4+ CD8− which presents peptides in association with MHCII or CD3+CD8+CD4− which utilize MHC. The T-cell receptor mainly expresses TCRaβ+ which recognizes antigen and delivers activation signals. The expression of TCRaβ+, CD4+ and CD8+ are crucial for the T-cell response. CD4+CD25+ Tregs have been demonstrated to maintain immunotolerance and suppress the antigen-specific or antigen-nonspecific T-cell responses. Therefore, in our study, CD3+TCRaβ+, CD3+CD4+, CD3+CD8+, CD4+CD8+ and CD4+CD25+ T-cell phenotypes were measured. The OP9-DL1 system not only simulated the microenvironment of the thymus but also obviated individual differences in the thymic microenvironment in vivo. This method can avoid exogenous confounding factors impacting T-cell development. The results revealed lower frequency of CD3+TCRaβ+, CD3+CD4+ and CD4+CD8+ both in the early and late stage of development in the patient group, but CD3+CD8+ expression was not significantly different between the two groups both on days 14 and 25. However, the ratio of CD3+CD4+ to CD3+CD8+T cells was lower in the patient group than in the healthy control group at day 25. This observation supports the viewpoint that abnormal expression of T-cell phenotype could be present in both the mature and immature stage of development and that differentiation and development of these T-cell subsets become more and more disproportional over time. Many previous investigators indicated that chronic HBV infection patients have also been suggested to have reduced numbers of CD3+ and CD4+ cells, CD4+/CD8+ ratios and higher numbers of circulating CD4+ CD25+ Tregs in peripheral blood 26–28. Thus, there is no need to study T-cell subsets in peripheral blood. However, in this study, the results implied that the decreased CD4+ and TCRaβ+ expression, reduced CD4+/CD8+ T-cell ratios and up-regulated CD4+ CD25+ Tregs were already present during the T-lymphocyte development. Some studies have found that HBV-specific CD8 T cells are present at extremely low frequencies in peripheral blood and liver of patients with chronic HBV infection 29. Conversely, Schurich et al. suggested that CD8 T cells in patients with chronic HBV infection have an increased propensity to express the coinhibitory receptor CTLA-4 30. Furthermore, there is a standpoint that the functionality of HBV-specific CD8 T cells is more important than the number of T cells to control HBV replication 31. Analysing the data in ours and previous studies, we assumed that the number of CD8+ T cells was not significantly different in the stage of T-cell development until entering the blood circulation. Cytokines modulate the host immune response to infectious microorganisms such as interferon gamma (IFN-γ) and interleukin (IL-2). These play a key role in cell-mediated immune responses against bacteria, fungi and viruses 32. Therefore, IL-2 and IFN-γ production was also measured. The lower IFN-γ and IL-2 production and reduced proliferation in the patient group indicated that both the T-cell phenotype and function were defective in the patient group. Down-regulation of CD3+CD4+ and CD3+TCRaβ+ and up-regulation of CD4+CD25+ inhibited antigen recognition, presentation and activation of T cells. Therefore, the defective T-cell phenotype expression is an important factor of immune tolerance in patients with chronic HBV infection. Why does the phonotype and function of the T cells in the patient group become abnormal? There are two possible reasons. Firstly, some HBV DNA gene segments as exogenous sequences are irregularly integrated into the HSCs DNA, which could lead to gene rearrangements in HSCs. Therefore, the HSCs with chromosomal variation could cause differentiation into abnormal lymphatic progenitor cells, and this process could interact with the gene rearrangement of TCRaβ T cells. Some studies have also reported that HBV DNA integration in hepatocytes is a causal risk factor for primary liver cancer 33. Another report demonstrated that both HB S and pre-C/C coding genes are integrated into embryos 34. Moreover, continual expression of HBsAg because of replication and integration of HBV DNA in HSCs could affect positive and negative selection during T-cell development, which could lead to abnormal expression of T-cell phenotype or apoptosis of some T-cell subpopulations.
Accordingly, we presumed that T-cell defects associated with CHB infection could be improved by hematopoietic stem cell transplantation, similar to the aforementioned reports. Mesenchymal hematopoietic stem cell transplantation has gained a great deal of attention in the last few years because of its ability to improve liver function in patients with liver failure, caused by hepatitis B 35. However, this treatment is rarely used for immune reconstitution against hepatitis B virus. In the immune tolerance phase of HBV, the immune system does not recognize virus, so there is no attack and no damage by the immune system. According to the 2010 American Association for Study of Liver Disease (AALSD) guidelines, patients in this phase do not need antiviral treatment. However, these individuals are still a source of infection and are at risk of disease progression. However, hematopoietic stem cell transplantation (HSCT) would likely remove HBV and cause HBsAg seroconversion in these patients. In recent years, autologous hematopoietic stem cell transplantation (AHSCT) has gained attention, because this therapy does not cause graft versus host disease (GVHD) and does not require a donor. However, according to our results, immune reconstruction is unlikely to be acquired after AHSCT in chronic HBV infection patients, because the HSCs would generate defective T cell following infection and integration of HBV DNA. Therefore, allogeneic hematopoietic stem cell transplantation could be more suitable than AHSCT. However, it is not clear whether the elimination of HBV DNA in the BM HSCs can restore normal differentiation of HSCs in patients.
In conclusion, we have shown that the HSCs of chronically HBV-infected patients with integrated HBV DNA generated defective T cells, because of the effect on differentiation and development of T lymphocytes. This suggestion has provided a theoretical basis for the use of hematopoietic stem cell transplantation as a therapy for chronically HBV-infected patients.
Acknowledgments
We thank Dr. J. C. Zuniga-Pflucker (University of Toronto, Toronto, Canada) for making available the OP9-DL1 cell line and Yu Zhang(Department of Immunology Peking university Health Science Center) for providing guidance on the transportation and cultivation of the OP9-DL1 cells. In addition, we thank the participating families for taking part in the study and the field staff for their tremendous work.
Glossary
- AHSCT
autologous hematopoietic stem cell transplantation
- APC
allophycocyanin
- BM
bone marrow
- CDI
cell division index
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DN
double negative
- DP
differentiated into double positive
- FACS
flow cytometry analysis
- FISH
fluorescence in situ hybridization
- FITC
fluorescein isothiocyanate
- GVHD
graft versus host disease
- HBV
persistence of hepatitis B virus
- HPC
murine hematopoietic precursor cells
- HSCs
hematopoietic stem cells
- HSCT
hematopoietic stem cell transplantation
- mAbs
monoclonal antibody
- OP9-DL1
delta-like ligand 1-expressing OP9
- ORF
four open reading frames
- PE
phycoerythrin
- PHA
phytohemagglutination
- RT-PCR
reverse transcription polymerase chain reaction
- SCF
stem cell factor
- SP
single positive
- Tregs
regulatory T cells
FINANCIAL SUPPORT
This work was supported by Natural Science Foundation of China [0571638], Postdoctoral Science Special Foundation of China [200902416] and Ph.D. programs Foundation of ministry of education of China [200923071100].
CONFLICT OF INTEREST
All authors: No conflict of interest exits in the submission and commercial of this manuscript, and manuscript is approved by all authors for publication.
References
- 1.Ferrari C, Missale G, Boni C, Urbani S. Immunopathogenesis of hepatitis B. J Hepatol. 2003;39(Suppl 1):S36–S42. doi: 10.1016/s0168-8278(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 2.Peng G, Luo B, Li J, et al. Hepatitis B e-antigen persistency is associated with the properties of HBV-specific CD8 T cells in CHB patients. J Clin Immunol. 2011;31(2):195–204. doi: 10.1007/s10875-010-9483-5. [DOI] [PubMed] [Google Scholar]
- 3.Maini MK, Schurich A. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol. 2010;52(4):616–619. doi: 10.1016/j.jhep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Dimitropoulou D, Karakantza M, Tsamandas AC, Mouzaki A, Theodorou G, Gogos CA. T-lymphocyte subsets in peripheral blood and liver tissue of patients with chronic hepatitis B and C. In Vivo. 2011;25(5):833–840. [PubMed] [Google Scholar]
- 5.Ilan Y, Nagler A, Adler R, Tur-Kaspa R, Slavin S, Shouval D. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology. 1993;104(6):1818–1821. doi: 10.1016/0016-5085(93)90664-x. [DOI] [PubMed] [Google Scholar]
- 6.Chiang LT, Yao M, Ko BS, Chen CH. Development of immunity against hepatitis B virus after donor lymphocyte infusion in a peripheral blood stem cell transplantation recipient with chronic hepatitis B. Infection. 2011;39(4):363–365. doi: 10.1007/s15010-011-0120-x. [DOI] [PubMed] [Google Scholar]
- 7.Teh BW, Slavin MA, Szer J, Sasadeusz JJ. Hepatitis B serological changes following allogeneic bone marrow transplantation. Transpl Infect Dis. 2012;11(10):1399–3062. doi: 10.1111/j.1399-3062.2012.00762.x. [DOI] [PubMed] [Google Scholar]
- 8.Serap A, Funda O, Bengu K, et al. Sustained seroconversion of chronic hepatitis B infection after stem cell transplantation. Pediatr Transplant. 2011;15(5):E92–E95. doi: 10.1111/j.1399-3046.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 9.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 10.Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99(9):3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- 11.Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2(10):760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt TM. Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 13.Awong G, Herer E, La Motte-Mohs RN. Zúñiga-Pflücker JC. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol. 2011;12:22. doi: 10.1186/1471-2172-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34 progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33(3):227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.La Motte-Mohs RN, Herer E, Zúñiga-Pflücker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105(4):1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 16.Van Coppernolle S, Verstichel G, Timmermans F, et al. Functionally Mature CD4 and CD8 TCR alphabeta Cells Are Generated in OP 9-DL1 Cultures from Human CD34+ Hematopoietic Cells1. J Immunol. 2009;183(8):4859–4870. doi: 10.4049/jimmunol.0900714. [DOI] [PubMed] [Google Scholar]
- 17.Hutton JF, Gargett T, Sadlon TJ, et al. Development of CD4+ CD25+ FoxP3+ regulatory T cells from cord blood hematopoietic progenitor cells. J Leukoc Biol. 2009;85(3):445–451. doi: 10.1189/jlb.1008620. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Lu D, Ji G, et al. Hepatitis Bvirus (HBV) vaccine-induced escape mutants of HBV S gene among children from Qidong area, China. Virus Res. 2004;99(1):63–68. doi: 10.1016/j.virusres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Vandesompele J, De PK, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Cong S, Ma H, Zhang X. Mutation analyses of integrated HBV genome in hepatitis B patients. J Genet Genomics. 2008;2:35. doi: 10.1016/S1673-8527(08)60013-2. [DOI] [PubMed] [Google Scholar]
- 21.Holmes R, Zúñiga-Pflücker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc. 2009;2(4):1–12. doi: 10.1101/pdb.prot5156. [DOI] [PubMed] [Google Scholar]
- 22.Banks HT, Thompson WC, Peligero C, Giest S, Argilaguet J, Meyerhans A. A division-dependent compartmental model for computing cell numbers in CFSE-based lymphocyte proliferation assays. Math Biosci Eng. 2012;9(4):699–736. doi: 10.3934/mbe.2012.9.699. [DOI] [PubMed] [Google Scholar]
- 23.Wallace PK, Tario JD, Jr, Fisher JL, Wallace SS, Ernstoff MS, Muirhead KA. Tracking antigen-driven responses by flow cytometry: monitoring proliferation by dye dilution. Cytometry A. 2008;73(11):1019–1034. doi: 10.1002/cyto.a.20619. [DOI] [PubMed] [Google Scholar]
- 24.McHugh S, Deighton J, Rifkin I, Ewan P. Kinetics and functional implications of Th1 and Th2 cytokine production following activation of peripheral blood mononuclear cells in primary culture. Eur J Immunol. 1996;26(6):1260–1265. doi: 10.1002/eji.1830260612. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8(1):9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TrehanPati N, Geffers R, Sukriti S, et al. Gene expression signatures of peripheral CD4+ T cells clearly discriminate between patients with acute and chronic hepatitis B infection. Hepatology. 2009;49(3):781–90. doi: 10.1002/hep.22696. [DOI] [PubMed] [Google Scholar]
- 27.You J, Sriplung H, Geater A, et al. Effect of viral load on T-lymphocyte failure in patients with chronic hepatitis B. World J Gastroenterol. 2008;14(7):1112–1119. doi: 10.3748/wjg.14.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123(1):57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster GJ, Reignat S, Brown D, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B:implications for immunotherapy. J Virol. 2004;78(11):5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurich A, Khanna P, Lopes AR, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53(5):1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 31.Maini MK, Boni C, Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191(8):1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schelonka RL, Maheshwari A, Carlo WA, Taylor S, et al. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53(2):249–255. doi: 10.1016/j.cyto.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52(4):594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Ali BA, Huang TH, Salem HH, Xie QD. Expression of hepatitis B virus genes in early embryonic cells originated from hamster ova and human spermatozoa transfected with the complete viral genome. Asian J Androl. 2006;8(3):273–279. doi: 10.1111/j.1745-7262.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54(3):820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]