Abstract
Aim
Glucose-dependent insulinotropic peptide (GIP) is an incretin hormone that is released from intestinal K cells in response to nutrient ingestion. We aimed to investigate the therapeutic potential of the novel N- and C-terminally modified GIP analogue AC163794.
Methods
AC163794 was synthesized by solid-phase peptide synthesis. Design involved the substitution of the C-terminus tail region of the dipeptidyl peptidase IV (DPP-IV)-resistant GIP analogue [d-Ala2]GIP(1–42) with the unique nine amino acid tail region of exenatide. The functional activity and binding of AC163794 to the GIP receptor were evaluated in RIN-m5F β-cells. In vitro metabolic stability was tested in human plasma and kidney membrane preparations. Acute insulinotropic effects were investigated in isolated mouse islets and during an intravenous glucose tolerance test in normal and diabetic Zucker fatty diabetic (ZDF) rats. The biological actions of AC163794 were comprehensively assessed in normal, ob/ob and high-fat-fed streptozotocin (STZ)-induced diabetic mice. Acute glucoregulatory effects of AC163794 were tested in diet-induced obese mice treated subchronically with AC3174, the exendatide analogue [Leu14] exenatide. Human GIP or [d-Ala2]GIP(1–42) were used for comparison.
Results
AC163794 exhibited nanomolar functional GIP receptor potency in vitro similar to GIP and [d-Ala2]GIP(1–42). AC163794 was metabolically more stable in vitro and displayed longer duration of insulinotropic action in vivo versus GIP and [d-Ala2]GIP(1–42). In diabetic mice, AC163794 improved HbA1c through enhanced insulinotropic action, partial restoration of pancreatic insulin content and improved insulin sensitivity with no adverse effects on fat storage and metabolism. AC163794 provided additional baseline glucose-lowering when injected to mice treated with AC3174.
Conclusions
These studies support the potential use of a novel GIP analogue AC163794 for the treatment of type 2 diabetes.
Keywords: diabetes, DPP-IV resistant, GIP, rodents, Trp-cage
Introduction
Glucose-dependent insulinotropic peptide (GIP) is an incretin hormone that is released from intestinal K cells in response to nutrient ingestion 1,2. GIP has been shown to have a potent glucose-dependent stimulatory effect on insulin secretion 3–5 and direct stimulatory effects on β-cell neogenesis and preservation via pleiotropic signalling mechanisms 6–9.
The GIP receptor is not only expressed in β-cells but also in other tissues including the gut, heart, brain, adipose tissue, pituitary and inner layers of the adrenal cortex 10,11. Studies conducted in several different animal models suggest that GIP stimulates lipogenesis, enhances fatty acid uptake and can potentially increase adiposity 12.
Investigations on the contribution of diminished GIP action to the pathogenesis of type 2 diabetes mellitus (T2DM) provide contradictory data. In patients with T2DM, the effect of GIP to stimulate insulin secretion is impaired 13, and in some studies in patients with improved glucose control following bariatric bypass surgery, plasma GIP concentrations declined when compared with presurgery baseline 14. Of importance, it has been shown in the clinic that 4-week normalization of hyperglycaemia with insulin 15 or sulphonylurea in acute settings 16,17 improved the insulinotropic action of GIP in patients with T2DM. In preclinical studies, subchronic combination treatment with analogues of glucagon-like peptide 1 (GLP-1) and GIP provided additive, beneficial glucose-lowering and insulinotropic actions over monotherapy treatment in diabetic mice 18. In addition, reduction of hyperglycaemia by phlorizin reversed resistance to GIP in diabetic rats 19. These data suggest a potential therapeutic utility of GIP analogues especially in combination with other glucose-lowering agents.
The native human GIP(1–42) hormone has a very short half-life (2–5 min) in circulation. A major hurdle limiting the therapeutic potential of GIP is its rapid degradation in the bloodstream to the inactive form GIP(3–42) by the ubiquitous enzyme dipeptidyl peptidase IV (DPP-IV), a serine protease that cleaves N-terminal dipeptides from polypeptides with l-Pro or l-Ala at the penultimate position 20–22. Optimization efforts aimed at extending the circulation half-life of the native molecule have focused on modifications at the N-terminus region, primarily amino acid substitutions at the labile position two 23,24 or C-terminus truncations to GIP(1–30) 25,26. For instance, it has been reported that the analogue [d-Ala2]GIP(1–42) possesses greater resistance to enzymatic degradation with no loss of potency at the receptor and greater antidiabetic efficacy, suggesting that the GIP tail region can play a role in modulating receptor activity. In contrast, in our hands the C-terminally truncated GIP(1–30) analogue with d-Ala at amino acid position two has decreased in vitro potency at the GIP receptor (data not shown). Exenatide, a well-characterized GLP-1 agonist has a unique C-terminal extension of nine amino acid tail shown to be a part of a compact folding unit called a ‘Trp-cage’ 27–29. The Trp-cage offers unique function to exenatide by stabilizing it further against enzymatic degradation, and enhancing its in vivo potency.
In this study, we describe AC163794, a novel GIP analogue with biological activity enhanced via C-terminal extension of [d-Ala2]GIP(1–30) with the tail region (residues 31–39) of exenatide. We characterize the biological activity of AC163794 in vitro and in vivo. In some studies, native human GIP and/or [d-Ala2]GIP(1–42) were used for comparison purposes.
Materials and Methods
Animals
All procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee at Amylin Pharmaceuticals, LLC. Mice and rats used in these studies were 10- to 19-week-old female Hsd:NIHS mice (Harlan, Indianapolis, IN, USA), male 8- to 12-week-old male C57Bl/6 mice, 9-week-old male B6.V-Lepob/J (ob/ob) mice (Jackson Laboratories, Bar Harbor, ME, USA), 12–14-week-old male Sprague Dawley (HSD) rats (Harlan, Indianapolis, IN, USA) or 9-week-old male Zucker fatty diabetic (ZDF) rats (Charles River, Wilmington, MA, USA), which were maintained on Purina Diet 5008 (Newco Distributors, Rancho Cucamonga, CA, USA). Animals were acclimated for at least 6 days before use and housed 1–3 per cage at 21–24 °C with a 12 h light/dark cycle with ad libitum access to food and water with the exception of tests where fasting (no more than 16 h) was required. To induce experimental diabetes with insulin resistance and partial β-cell depletion, male C57Bl6/J mice, were fed a high fat (HF) diet (58% kcal/fat, D12331 Research Diets, New Brunswick, NJ, USA) starting from 4 weeks of age. At the age of 9 weeks, non-fasted mice were dosed intraperitoneally (IP) with 100 mg/kg streptozotocin (STZ) (Sigma-Aldrich, St. Louis MO, USA) reconstituted in 0.1 M citrate buffer once weekly for 4 weeks. Non-diabetic HF-fed or low fat (LF)-fed controls received citrate buffer at the same intervals as the STZ group.
Synthesis of AC163794
AC163794 was developed by C-terminal extension of [d-Ala2]GIP(1–30) with the nine amino acid tail region of exenatide in its amidated form. AC163794 was assembled on the Rink amide resin (EMD Chemicals, Gibbstown, NJ, USA) and [d-Ala2]GIP(1–42) was assembled on the Wang resin (EMD Chemicals) using standard solid-phase peptide synthetic protocols on a automated peptide synthesizer. The synthesis involved coupling of amino acids with HATU/DIEA [O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate/diisopropylethylamine] reagents in dimethylformamide (DMF) as solvent. The peptides were cleaved from the resin by using trifluoroacetic acid (TFA)/triisopropylsilane (TIS)/phenol/water as cleaving agents. The crude peptides were purified to >90% purity by high-performance liquid chromatography (HPLC) using a linear gradient of 0.1% TFA containing acetonitrile and water. The pure peptides thus obtained as a TFA salt were used for all biological evaluations. Synthetic human GIP was purchased from Bachem (Torrance, CA, USA). Peptide sequences are presented in Table 1.
Table 1.
AC163794 in vitro activity, metabolic stability and plasma glucose during an OGTT
| Name | Sequence | Assay | |||||
|---|---|---|---|---|---|---|---|
| rGIPR binding assay IC50 (nM) | rGIPR functional assay EC50 (nM) | Stability in human plasma (%) | Stability in human kidney membranes (%) | OGTT | |||
| ED50 (nmol/kg) | Maximal efficacy versus vehicle (%) | ||||||
| GIP(1–42) (GIP) | YAEGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ-OH | 0.16 | 19.9 | 56 ± 6 | 40 ± 4 | >400 | ND |
| [d-Ala2]GIP(1–42) | YaEGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ-OH | 0.63 | 19.0 | ND | 85 ± 2 | 17 | −21 |
| AC163794 = [d-Ala2]GIP(1–30)-exendin(31–39) | YaEGTFISDYSIAMDKIHQQDFVNWLLAQKPSSGAPPPS-NH2 | 3.8 | 26.2 | 87 ± 2 | 100 ± 3 | 4 | −22 |
Stability results (measured as AUC0–5h) are expressed as a percentage of peptide remaining versus the stable reference peptide [Leu14] exenatide. Assays were performed in a screening mode, compounds were ranked by result values in each assay, no statistical comparative analysis were performed (replicates within each assay n = 1–3). Bold indicates differences in specific sequence regions of listed peptides. ND, not determined.
In vitro Studies
rGIP Receptor Binding Assay
The RIN-m5F cell line, derived from a rat insulinoma, endogenously expresses the rat GIP receptor (ATCC® CRL-11605™, Manassas, VA, USA) 30. Crude membranes from RIN-m5F cell cultures were prepared by homogenization in ice cold 20 mM HEPES containing protease inhibitors (Roche, Indianapolis, IN, USA). AC163794, GIP or [d-Ala2]GIP(1–42) were incubated at increasing concentration (10 pM to 1 µM) with cell membranes in the presence of 30 pM 125I-GIP iodinated at tyrosine residues (2000 Ci/mmol, Product number NEX402, Perkin Elmer, Waltham, MA, USA) in 20 mM HEPES with 5 mM MgCl2, 1 mM CaCl2, 0.5% bovine serum albumin (BSA), 100 mg/ml bacitracin, 0.1 mg/ml phosphoramidon and 0.5 mg/ml bestatin for 1 h at room temperature. Incubations were terminated by rapid filtration through UniFilter-96 plates GF/B (Perkin Elmer), presoaked for at least 30 min in 0.5% polyethylenimine. Scintillant (Microscint 20, Perkin Elmer) was added to dried Unifilter plates, and CPM was determined using multiwell scintillation counter.
rGIP Functional Assay
RIN-m5F cells were incubated with increasing concentrations of AC163794, GIP or [d-Ala2]GIP(1–42) (1 fM to 1 µM) in assay buffer (HBSS, 0.1% BSA) in the presence of 250 µM IBMX (Calbiochem, San Diego, CA, USA) for 30 min. Cyclic-AMP (cAMP) accumulated in supernatants was measured using the cAMP Dynamic 2 assay (Cisbio US, Bedford, ME) according to the manufacturer’s instructions. cAMP was detected by a decrease in time-resolved fluorescence energy transfer (TR-FRET) using a GeniousPro plate reader (Tecan, Männedorf, Switzerland). Efficacy of peptides was determined relative to cell treatment with 10 µM forskolin (a constitutive activator of adenylate cyclase).
Metabolic Stability In vitro
Peptides (10–30 µM) were incubated at 37 °C in human plasma or human kidney membrane proteins (approximately 7 µg/ml protein in 25 mM HEPES buffer, pH 7.4). Samples were collected at the baseline and 1, 2, 3, 4 and 5 h thereafter. The amount of parent peptide was measured by on-line SPE-LC/MS/MS and area under the curve (AUC0–5h) was calculated. Data are expressed as a percentage of the peptide remaining versus the stable internal reference peptide [Leu14] exenatide.
Glucose-Stimulated Insulin Secretion From Isolated Islets
Mouse islets were isolated as previously described 31. Briefly, 0.35 mg/ml of Liberase RI (Roche Applied Science) solution was injected into the pancreatic duct, and the pancreas was digested for 18 min at 37 °C. Islets were then separated from exocrine tissue via centrifugation in Histopaque-1077 (Sigma-Aldrich) discontinuous density gradient. Prior to insulin secretion experiments, islets were cultured for 2 days in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% foetal calf serum, 10 mM HEPES, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C with 5% CO2. Glucose-stimulated insulin secretion (GSIS) was performed as described elsewhere 32. Briefly, after washing with fresh low-glucose (1.67 mM) medium, aliquots of 10 islets were placed for 1 h into stimulation media (16.7 mM glucose) prepared in 24-well plates with culture inserts (8.0 µm pore membrane, Millicell-PCF, Millipore Corp., Billerica, MA, USA). AC163794 and GIP were used at doses of 0.1, 1, 10 and 100 nM. At the end of insulin secretion experiments, inserts with islets were removed, media were collected, and frozen at −20 °C for insulin assay. Acid ethanol (1.5% HCl in 75% EtOH, pH 4.5) was applied for overnight extraction of insulin from remaining islets. Total islet insulin content per well was used to normalize insulin release results.
Administration of AC163794 in Mice and Rats
Acute Studies
An oral glucose tolerance test (OGTT) was performed in normal female Hsd:NIHS mice. Following a 4-h fast, mice received an IP injection of GIP (63–634 nmol/kg), [d-Ala2]GIP(1–42) (1–100 nmol/kg), AC163794 (1–30 nmol/kg) or vehicle. Five minutes later each mouse was gavaged with 120 µl of a 1.5 g/kg dextrose solution. A blood sample was obtained 30 min after the gavage for determination of plasma glucose concentration. Data were converted to percent difference from vehicle and fitted to a sigmoidal curve using non-linear regression to calculate ED50 and maximal efficacy (Graphpad Prism®, Graphpad Software, San Diego, CA, USA).
To evaluate the potential for combinatorial therapy, AC163794 was administered as a single IP bolus to HF-diet-induced obese (DIO) mice receiving GLP-1 receptor agonist AC3174 33 via continuous minipump (model 2002, Alzet, Durect Corp., Cupertino, CA, USA) subcutaneous (SC) infusion (0.5 nmol/kg/d for 4 weeks). AC163794 or GIP (80 nmol/kg) was injected IP into 2-h fasted mice at t = 0 immediately following baseline blood glucose sample. Blood glucose was measured at 30, 60, 120 and 180 min thereafter.
Insulinotropism of AC163794 was evaluated during intravenous glucose tolerance test (IVGTT) performed in rats. Fed, isoflurane anaesthetized male HSD rats were intubated and cannulated via femoral artery and vein and allowed to stabilize for 1 h. Following stabilization, a continuous IV infusion of vehicle (saline), GIP or AC163794 at doses of 10, 30 and 100 pmol/kg/min was started (t = −30). At t = 0, an IV bolus of 5.7 mmol/kg d-glucose was administered over 2 min. Samples for glucose measurement and insulin concentrations were taken at various time points before and after the glucose infusion. The slope of the pancreatic function was calculated as the relationship between (log) insulin and glucose (ng/ml)/(mg/dl).
To assess duration of action of GIP and GIP-based compounds, after the above described surgical procedure was completed and following stabilization, a SC bolus injection of GIP was administered 30 min, and AC163794 and [d-Ala2]GIP(1–42) 120 min prior to IV glucose challenge, respectively. Compounds were dosed at 3 µg per animal and saline was used as a vehicle control.
Comparison of glucoregulatory activity of equimolar doses (100 pmol/kg/min) of AC163794 and GIP was also performed in fed diabetic ZDF rats using IVGTT method as described for HSD rats.
Chronic Studies
Two different mouse models of diabetes were used to measure long-term effects of AC163794 on glucose metabolism and body weight. Mice were randomized into treatment groups based on glycated haemoglobin A1c (HbA1c) and dosed for 4 weeks using SC 2-week osmotic minipumps (model 2002; Alzet, Durect Corp.). After 2 weeks, mice were reimplanted with new minipumps with the same treatment administered for the next 2 weeks to retain a steady exposure of the compound. The preliminary in vitro assessment of AC163794 revealed that physical and chemical stability of the compound was not preserved completely over 4 weeks in a 4-week osmotic minipumps.
Diabetic and obese ob/ob mice were infused with AC163794 at 10, 30 and 100 nmol/kg/day (n = 9 per group). Control group received 50% dimethylsulphoxide (DMSO) in sterile water as a vehicle.
Diabetic HF-STZ mice were dosed with 100 nmol/kg/day AC163794 or vehicle (50% DMSO in sterile water with 0.1% BSA, n = 16 per group). HbA1c, blood glucose, body weight and terminal pancreatic insulin content were measured in both studies. At the end of the study in HF-STZ mice, an OGTT was performed after an overnight fast followed by body fat percent measurement using DEXA scan (dual-energy X-ray absorptiometry, GE Lunar PIXImus, Madison, WI, USA) in a subset of mice (n = 8). On the basis of pre-OGTT fasting glucose (FG, mM) and fasting insulin (FIns, µU/ml) values, homeostatic model assessment-insulin resistance (HOMA-R) [(FG × FIns)/22.5] and homeostatic model assessment-beta-cell function (HOMA-B) [FIns × 20/(FG − 3.5)] were calculated. Hepatic lipid content 34, plasma adipokines [interleukin-6 (Il-6), resistin and plasminogen activator inhibitor-1 (PAI-1)], cholesterol and triglycerides concentration and AC163794 levels were measured in the remaining animals (n = 8).
Biochemical Analysis
Plasma glucose in acute and chronic mouse studies was measured using a OneTouch® Ultra® blood glucose meter (LifeScan, Johnson & Johnson, Milpitas, CA, USA) and the AC2300 STAT glucose analyzer (YSI, Yellow Springs, OH, USA) in acute rat studies. Whole blood HbA1c, cholesterol and triglycerides were measured using the Olympus AU400e clinical analyzer (Olympus America, Irving, TX, USA). Insulin in mouse plasma, in islet culture samples and in pancreatic extracts was measured using insulin enzyme-linked immunosorbent assay (ELISA, Crystal Chem, Downers Grove, IL, USA). For rat studies, plasma insulin was measured using a rat insulin RIA (RI-13K, Millipore Corp.). Cytokine concentrations were assessed using multiplex immunoassay kit and adiponectin using kit according to manufacturer protocols (Linco/EMD, Millipore). Hepatic lipid content was measured as previously described 34. Plasma concentrations of AC163794 were measured using a validated immunoenzymetric assay developed at Amylin Pharmaceuticals, LLC (the coefficient of variation for intra- and inter-assay was <6.7 and 12.2%, respectively).
Statistical Analyses
Results are presented as mean ± standard error of mean, graphed and analysed using GraphPad Prism 5®. The potency of peptides in vitro functional and binding assays was determined by the analysis of a concentration-response curves using non-linear regression analysis. Statistical differences (p < 0.05) between multiple groups were identified with one-way analysis of variance (anova) or mixed model anova followed by post hoc Dunnett’s test for comparisons between treatment groups versus vehicle control, and Tukey’s test for comparison of multiple groups. Two group comparison was performed using unpaired Student’s t test. In vitro insulin data, FG, insulin and pancreatic insulin content from the study with HF-STZ mice were analysed using log-transformed data.
Results
In vitro Activity and Stability of AC163794
Results from the in vitro evaluation of AC163794 and parent molecules are summarized in Table 1. All three peptides displayed comparable activity in the GIP receptor functional assay measuring cAMP production in RIN-m5F cells. In contrast, AC163794 with a potency of 3.8 nM was 24-fold less potent than GIP and about 6-fold less potent than [d-Ala2]GIP(1–42) in its binding to the GIP receptor. AC163794 was stable in human plasma and kidney membrane proteins over 5 h. In contrast, GIP, was significantly degraded after an hour of incubation.
Ex vivo Effects of AC163794 on Isolated Mouse Islets
Following glucose stimulation of normal mouse islets, insulinotropic actions of AC163794 at 10 and 100 nM concentrations were similar to those observed for GIP, significantly stimulating insulin secretion by up to twofold when compared with vehicle control (figure 1).
Figure 1.

In vitro effects of AC163794 on glucose-stimulated insulin secretion in isolated mouse islets. Data are presented as fold increase over non-treated medium control (mean ± SEM). *p < 0.002 versus medium control (n = 3 experiments with three replicates).
Acute Effects of AC163794 in Mice and Rats
Mouse OGTT After a Single Injection of AC163794
When dosed 5 min prior to an oral glucose challenge, GIP, [d-Ala2]GIP(1–42) and AC163794 blunted the rise in plasma glucose relative to vehicle-treated mice. AC163794 and [d-Ala2]GIP(1–42) did so with similar efficacy and potency. Both were approximately 100-times more potent than GIP. Results are presented in Table 1.
Effect of a Single Injection of AC163794 in DIO Mice Being Treated with GLP-1 Receptor Agonist
After 4-week treatment with AC3174, a GLP-1 receptor agonist, DIO mice had a mean 2-h fasting plasma glucose of 170 ± 4 mg/dl, which was similar to that of mice maintained on a nutrient matched LF diet and 15% less than the mean 2-h fasting plasma glucose of 205 ± 8 mg/dl in the vehicle-treated mice (p < 0.01). Three hours after receiving an 80 nmol/kg IP bolus of GIP, plasma glucose in the AC3174 treated mice decreased modestly to 153 ± 9 mg/dl. The AC3174 treated mice that received 80 nmol/kg AC163794 IP had a much larger decrease in plasma glucose to 91 ± 2 mg/dl. Results, converted to percentage pretreatment, are presented in figure 2.
Figure 2.
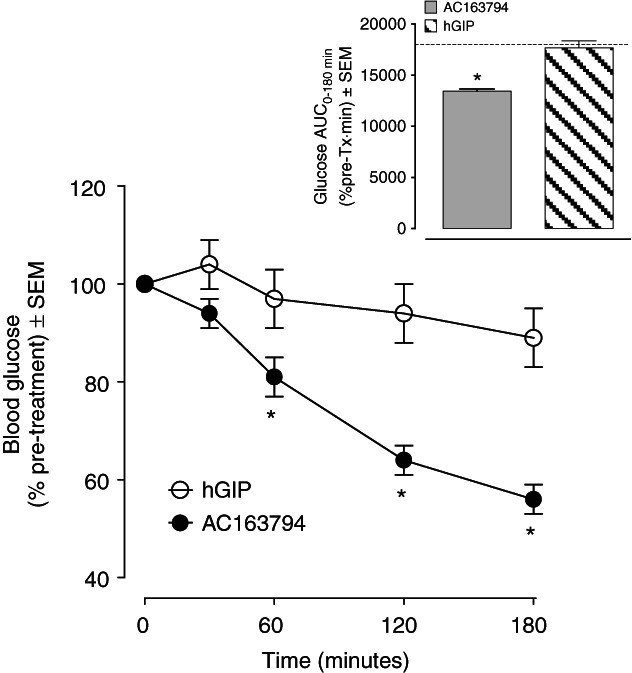
Effect of a single bolus injection of AC163794 or glucose-dependent insulinotropic peptide (GIP) on blood glucose in diet-induced obese (DIO) mice treated with the glucagon-like peptide 1 (GLP-1) receptor agonist AC3174 for 4 weeks via continuous subcutaneous (SC) infusion. Data are presented as mean ± SEM of calculated percent change from the glucose values measured immediately prior a single injection of GIP or GIP analogue. Inset represents calculated AUC for this change and dashed line represents theoretical AUC value for the initial glucose concentration remaining unchanged during the study.*p < 0.05 versus GIP, Student’s t test n = 8.
Potency and Duration of Action of AC163794 in Rat IVGTT
AC163794, administered as a continuous intravenous infusion at doses ranging from 10 to 100 pmol/kg/min during IVGTT in normal rats, produced dose-related decreases in plasma glucose concentrations (figure 3A) and increases in plasma insulin concentrations (figure 3C). In response to the glucose challenge, all doses of AC163794 resulted in significantly lower glucose excursions (figure 3A) and significantly higher insulin excursions (figure 3C, measured as AUC0–60) when compared with vehicle control. The AC163794 infusion elicited glucose (figure 3A) and insulin (figure 3C) excursions that were similar to GIP (figure 3B, D). The calculated slope of pancreatic function curve was dose-dependent for both peptides. AC163794 (ED50 = 12 pmol/kg/min) was more potent per unit infusion rate than GIP (ED50 = 28 pmol/kg/min, figure 3E). The insulin response to a single 3 µg dose of AC163794 administered 2 h prior to the IVGTT resulted in higher insulin excursions than after a single 3 µg dose of GIP or [d-Ala2]GIP(1–42) administered 30 min or 120 min, respectively, prior to the glucose challenge (figure 3F).
Figure 3.

Plasma glucose and insulin excursions in normal rats infused intravenously with glucose-dependent insulinotropic peptide (GIP) analogue peptides at 10, 30 and 100 pmol/kg/min during an intravenous glucose tolerance test (IVGTT). Effects of AC163794 on glucose (A) and insulin (C) excursions. Effects of GIP on glucose (B) and insulin (D) excursions. Insets in graphs A–D represent calculated AUC for specific peptide and specific excursion curves. (E) Calculated slope of pancreatic function curve from experiments presented in A–D. (F) Duration of insulinotropic action of AC163794 and [d-Ala2]GIP(1–42) administered as a single bolus 120 min prior to a glucose challenge (IVGTT). Data are presented as mean ± SEM. *p < 0.05 versus vehicle control (n = 6–9 control group and n = 4–6 peptide-treated groups).
The insulinotropic effect of AC163794 (100 pmol/kg/min) was also assessed in diabetic ZDF rats (figure 4A, B). The peptide induced a robust plasma insulin excursion with the AUC0–90 significantly higher than in animals administered vehicle control, while an equimolar dose of GIP had no significant effect (figure 4B).
Figure 4.

Plasma glucose (A) and insulin (B) excursions in diabetic Zucker fatty diabetic (ZDF) rats infused intravenously with 100 pmol/kg/min AC163794 and glucose-dependent insulinotropic peptide (GIP) during an intravenous glucose tolerance test (IVGTT). Insets represent calculated AUC for glucose (A) and insulin (B) excursion curves. Data are presented as mean ± SEM. *p < 0.05 versus vehicle control (n = 5–7 per group).
Glucoregulatory Effects of Subchronic Administration of AC163794 in Diabetic Mice
Continuous infusion of AC163794 for 4 weeks delayed the development of diabetes in ob/ob mice. At the study end, the change from baseline of HbA1c was 0.4 ± 0.2%, 0.3 ± 0.2% and 0.5 ± 0.2% for 10, 30 100 nmol/kg dose, respectively, however, it did not reach statistically significant difference from the control vehicle-treated group (1.0 ± 0.2%, p < 0.1) (figure 5A). Terminal pancreatic insulin content in animals treated with AC163794 was approximately twofold greater than in controls and was significantly different for 10 and 100 nmol/kg/day doses when compared with vehicle (p < 0.05, figure 5B). No changes were noted in body weight gain (figure 5C) during the study.
Figure 5.

Effects of 4-week continuous administration of AC163794 on HbA1c (A), terminal pancreatic insulin content (B) and body weight (C) in diabetic ob/ob mice. Data are presented as mean ± SEM. *p < 0.05 versus vehicle control [n = 9 (A and C), n = 5 (B)].
Continuous infusion of 100 nmol/kg/day AC163794 was initiated when diabetic HF-STZ mice were 16-week old with prior feeding the HF-diet for 12 weeks. Four-week treatment with compound significantly attenuated the rise in HbA1c values (change from baseline = 0.5 ± 0.2%) compared with vehicle-dosed controls (change from baseline 1.7 ± 0.3%) p < 0.01 (figure 6A). This correlated with the finding that pancreatic insulin content was significantly higher in animals treated with AC163794 than in diabetic controls (p < 0.05, figure 6B). No effect of AC163794 was observed on body weight (figure 6C) when compared with HF-STZ mice. Mean AC163794 concentration in plasma was 15.4 ± 2.9 ng/ml and terminal compound concentrations correlated with HbA1c values (r2 = 0.6). Non-diabetic control mice fed regular chow (LF) or HF diet were used as a reference for HF-STZ diabetic mouse model. As expected, they exhibited steady HbA1c values in the normal range of 5% (figure 6A), with HF-fed animals being heavier than LF-fed mice (figure 6C).
Figure 6.

Effects of 4-week continuous administration of AC163794 on HbA1c (A), terminal pancreatic insulin content (B) and body weight (C) in 16-week old high-fat-fed streptozotocin (HF-STZ) diabetic mice. Mice were fed a HF-diet for 12 weeks prior to the initiation of treatment. Data are presented as mean ± SEM. *p < 0.05 versus vehicle HF-STZ control [n = 16 HF-STZ groups (A and C), n = 6–8 HF-STZ group (B), HF and LF control groups (A and C)].
In a glucose tolerance test performed at the end of the study in HF-STZ mice, fasting plasma glucose in AC163794-treated group was similar to concentrations in non-diabetic mice and significantly lower than in diabetic controls (p < 0.05) (Table 2). Glucose excursions had a trend to be reduced when compared with diabetic controls and AUC0-2h to be elevated relative to non-diabetic controls (figure 7A). FIns concentrations were significantly higher in AC163794-treated group than in diabetic counterparts (p < 0.05, Table 2). Glucose-stimulated insulin concentrations and AUC0–2h were not significantly different between AC163794- and vehicle-treated diabetic groups and significantly reduced versus non-diabetic controls (p < 0.05, figure 7B). Calculated values for HOMA-R and HOMA-B along with measured terminal percentage of body fat, hepatic lipid content, plasma adipokines (Il-6, Resistin, PAI-1), cholesterol and triglycerides are presented in Table 2. AC163794 improved insulin sensitivity and β-cell function, and had no effect on body fat content and fat metabolism-related endpoints.
Table 2.
Effects of 4-week continuous AC163794 treatment on fasting glucose and insulin, lipid metabolism-related endpoints and inflammatory cytokines in HF-STZ diabetic mice
| Vehicle (HF-STZ) | AC163794 | Vehicle (HF) | Vehicle (LF) | |
|---|---|---|---|---|
| Fasting glucose (mg/dl) | 385 ± 38 | 206 ± 21* | 157 ± 8* | |
| Fasting insulin (ng/ml) | 0.27 ± 0.02 | 0.36 ± 0.02*† | 0.70 ± 0.08 | |
| HOMA-R | 7.7 ± 1.1 | 5.3 ± 0.7† | 10.1 ± 1.8 | |
| HOMA-B | 9.5 ± 1.1 | 28.5 ± 2.8*‡ | 81.3 ± 8.8* | |
| Body fat (%) | 18.4 ± 1.0 | 18.5 ± 0.8 | 28.7 ± 2.0* | |
| Hepatic lipid content | 38.1 ± 2.9 | 38.2 ± 1.7 | 38.4 ± 2.1 | |
| IL-6 (pg/ml) | 25.0 ± 2.6 | 25.6 ± 7.6 | 31 ± 6 | |
| Resistin (ng/ml) | 1.54 ± 0.06 | 1.6 ± 0.1 | 0.98 ± 0.05* | |
| PAI-1 (ng/ml) | 2.9 ± 0.3 | 3.1 ± 0.2 | 1.9 ± 0.2 | |
| Cholesterol (mg/dl) | 166 ± 7 | 172 ± 4‡ | 127 ± 8* | |
| Triglycerides (mg/dl) | 196 ± 35 | 130 ± 21 | 69 ± 5* |
Data are means ± SEM. N = 6–8 per group. HF-STZ, high-fat-fed streptozotocin; IL-6, interleukin 6; PAI-1, plasminogen activator inhibitor-1.
p < 0.05 versus HF-STZ control mice.
p < 0.05 versus HF control mice.
p < 0.05 versus LF control mice.
Figure 7.
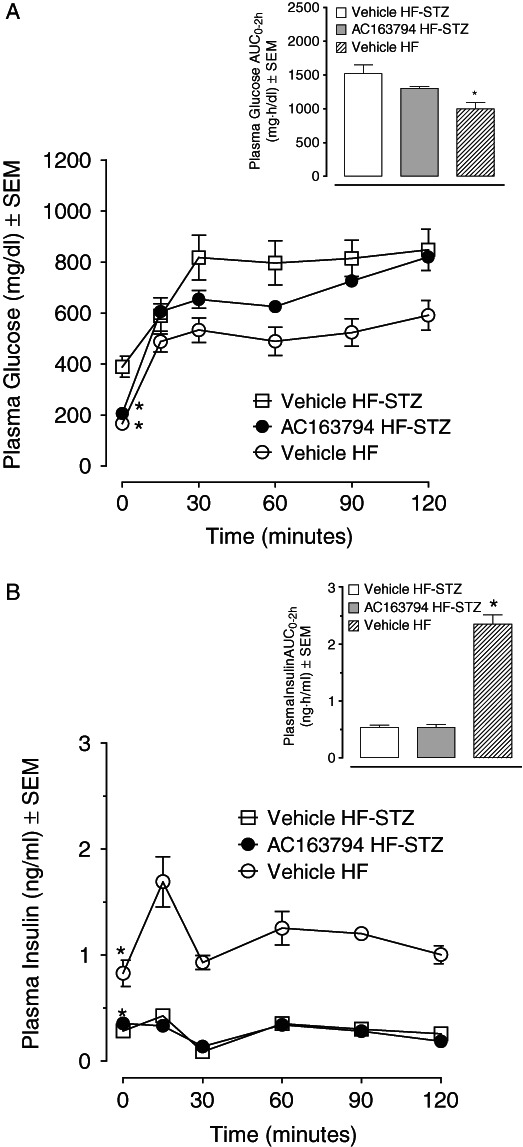
Plasma glucose (A) and insulin (B) excursions during an oral glucose tolerance test (OGTT) performed in high-fat-fed streptozotocin (HF-STZ) diabetic mice after 4-week continuous treatment with 100 nmol/kg/day AC163794. Insets represent calculated AUC for glucose (A) and insulin (B) excursion curves. *p < 0.05 versus HF-STZ vehicle-treated control (n = 6–8).
Discussion
Our GIP optimization strategy to design AC163794 focused on a combination of modifications that involved both the N- and C- termini of the GIP molecule. d-Ala substitution at the second position at the N-terminus conferred resistance to DPP-IV mediated enzymatic degradation. At the C-terminus, a more radical structural change was applied. Structurally, the GIP tail region, comprised of residues 31–42, is a flexible random coil 25,35, and recent reports have suggested that the C-terminus has a lipogenic function 36. The replacement of this tail region with a unique C-terminus tail of exenatide resulted in AC163794, a novel peptidic analogue of human GIP, with a significantly longer duration of insulinotropic action compared with native GIP or the DPP-IV-resistant [d-Ala2]GIP(1–42) peptide.
AC163794, GIP and [d-Ala2]GIP(1–42) displayed comparable nanomolar potencies for cAMP production when screened in RIN-m5F cells that express the GIP receptor 30. This finding indicates that our modifications to GIP did not adversely affect binding potency and functional activity at the GIP receptor relative to native GIP or another DPP-IV-resistant GIP analogue. In contrast to GIP, AC163794 was stable for at least 5 h when exposed to human plasma, and its stability in human kidney membrane milieu was significantly improved versus GIP or [d-Ala2]GIP(1–42). Thus, AC163794 is most likely resistant to cleavage by DPP-IV protease and a number of proteolytic enzymes that are found in these biological matrices.
As discussed below, our studies indicate that AC163794’s glucoregulatory effects were mediated by enhancement of glucose-induced insulin secretion in vitro and in vivo, and partial restoration of β-cell mass in ob/ob and HF-STZ diabetic mice. The subchronic treatment with AC163794 improved insulin sensitivity in HF-STZ mice. No effects of the compound on body weight and composition, plasma cholesterol, triglycerides and inflammatory cytokines were observed.
Isolated normal mouse islets and an IVGTT in normal rats were used to assess the acute insulinotropic action of AC163794. AC163794 exhibited dose-dependent insulinotropic activity in both tests and was similar to GIP. However, AC163794 was more potent when assessed using the calculated slope of a pancreatic function curve when compared with GIP. It was also superior to GIP and [d-Ala2]GIP(1–42) in the duration of its insulinotropic action. Administration of AC163794 2 h prior to the IVGTT resulted in a robust potentiation of glucose-induced insulin response whereas [d-Ala2]GIP(1–42) administered at the same time showed no notable activity similarly to GIP, which was administered only 30 min before the glucose challenge.
The acute glucoregulatory activity of AC163794 was investigated in normal mice during an OGTT. The maximal efficacy and ED50 of AC163794 were comparable with [d-Ala2]GIP(1–42), with both compounds being approximately 100-fold more potent than native GIP. Encouraged by these acute study results, we employed two diabetic mouse models for the subsequent chronic studies. AC163794 administered for 4 weeks in ob/ob and HF-STZ mice displayed significant HbA1c-lowering effects. The findings in the latter model were accompanied with lower terminal FG concentrations than in the diabetic control group. Increased terminal pancreatic insulin content, and improvement in HOMA-R and HOMA-B indices would suggest improved β-cell function and increase in insulin sensitivity. As pancreatic insulin content is commonly used as a surrogate measure of pancreatic β-cell mass in rodents, the present data suggest that AC163794 may enhance preservation of β-cells. These results are in the accordance with previously reported direct effects of GIP on β-cell maintenance 6–9,36; however, it is not clear if the observed beneficial effects of AC163794 are mediated by its effects on neogenesis, increased cell proliferation or inhibition of apoptosis, and further studies will be needed to assess the detailed mechanism.
The involvement of GIP in the pathogenesis of T2DM remains unclear despite the abundance of literature on its putative role. For instance, studies in patients with T2DM who underwent bariatric surgery and achieved normoglycaemia did not provide consistent results. In some studies, plasma GIP concentrations declined compared with prior-surgery baseline levels but other studies have reported increased levels of the hormone 14. Hence, both GIP receptor agonists and antagonists have been proposed for the development of GIP-based antidiabetic therapy 37.
One of the caveats of any preclinical evaluation of novel GIP analogues is the lack of a good translational model of GIP resistance. An initial study identifying Vancouver diabetic fatty Zucker (VDF) rats as GIP-resistant 38 was not confirmed by others 24. In our studies, we tested ZDF rats, the gold-standard rodent model of human T2DM. AC163794 potentiated insulinotropic activity in 9-week old diabetic ZDF rats in a manner similar to the insulin responses observed in normal rats during an IVGTT. The same test performed in older (15-week old) ZDF rats, showed a similar ability of AC163794 and GIP to stimulate insulin secretion (data not shown). These findings indicate that the insulinotropic activity of GIP or AC163794 is not impaired in the commonly used ZDF rat model. Additionally, our observations in two diabetic ob/ob and HF-STZ mouse models suggest that these mice were not GIP-resistant. These models were very responsive to GIP and AC163794 treatment, and a prior reduction of hyperglycaemia was not needed to achieve the expected GIP glucoregulatory activity. This is in contrast to clinical studies, where antidiabetic agents improving glycaemia in patients with T2DM were needed to restore GIP responsiveness 15–17. However, when AC163794 was administered acutely into DIO mice, which were chronically treated with the exenatide analogue, AC3174, an additional baseline glucose-lowering effect was observed. These data concur with other studies, where chronic combinatorial administration of GLP-1 and GIP analogues in diabetic mice result in markedly improved glucose-lowering and insulinotropic actions compared with either peptide given individually 18. Thus, our observations support the notion that a GIP-based analogue either alone or as an adjunct with other incretin-derived agents, has a potential utility for the treatment of T2DM.
There is considerable evidence that GIP plays a role in fat homeostasis, therefore, one potential side effect of GIP-based therapies may be to increase adiposity 12. In our studies, AC163794 had no effect on body weight in two diabetic mouse strains after 4 weeks of continuous dosing. Body fat content was not affected when measured in HF-STZ mice, nor in non-diabetic DIO mice and rats (data not shown). Additionally, AC163794 did not change certain lipid metabolism-related endpoints, including triglycerides and cholesterol, did not promote fat deposition in the liver and did not increase inflammatory cytokines in plasma. These findings indicate that AC163794 has no adverse impact on fat storage and metabolism in these rodent models similarly to other GIP analogues 36,39. A comprehensive toxicology assessment was not performed for this molecule, however, we did not see any evidence of adverse effects when AC163794 was tested acutely up to 6 mg/kg (˜1400 nmol/kg) in mice.
In summary, we have shown that an N-terminal placement of a DPP-IV-resistant amino acid residue in GIP, coupled with substitution of its C-terminus tail region with the unique C-terminal domain of exenatide, provides a novel GIP analogue, AC163794 that displays enhanced antidiabetic activity and significantly prolonged duration of action versus the native hormone. The antidiabetic activity of AC163794 in preclinical rodent models is manifested through its β-cell effects and improved insulin sensitivity. Our findings also suggest that further investigation is warranted to determine whether AC163794 could provide additional glycaemic control if used as adjunctive therapy with other antidiabetic agents such as GLP-1 receptor agonists.
Acknowledgments
The authors are thankful to Dr Alain Baron for his intellectual contribution to GIP Development Program at Amylin Pharmaceuticals. We appreciate the excellent technical assistance of Augustine J. Cho, Jenne Pierce, Kim DeConzo, Christiane Villescaz, Lisa Adams, Tina Whisenant and Melissa G. W. Lu in conducting the study. We are grateful to John Herich, Jennifer Athanacio and Carmelle V. Remillard for their assistance in preparation of the manuscript.
The majority of results from this study were previously presented at the annual American Diabetes Association Scientific Sessions in 2007 and 2008, and at the Keystone Symposia on Molecular and Cellular Biology meetings in 2007 and 2009.
Conflict of Interest
At the time when the work for this manuscript was performed all authors were employed by Amylin Pharmaceuticals, LLC. All authors held stock in Amylin Pharmaceuticals, LLC.
All the authors were involved in study design, data analysis, interpretation of results and preparation of the manuscript, including decisions on content and editing. K. T. wrote the manuscript and prepared tables and figures. All authors approved the final manuscript.
References
- 1.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 2.Jorde R, Burhol PG, Waldum HL, Schulz TB, Lygren I, Florholmen J. Diurnal variation of plasma gastric inhibitory polypeptide in man. Scand J Gastroenterol. 1980;15:617–619. doi: 10.3109/00365528009182224. [DOI] [PubMed] [Google Scholar]
- 3.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metabol. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 4.Pederson RA, Schubert HE, Brown JC. Gastric inhibitory polypeptide. Its physiologic release and insulinotropic action in the dog. Diabetes. 1975;24:1050–1056. doi: 10.2337/diab.24.12.1050. [DOI] [PubMed] [Google Scholar]
- 5.Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest. 1978;62:152–161. doi: 10.1172/JCI109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehses JA, Casilla VR, Doty T, et al. Glucose-dependent insulinotropic polypeptide promotes β-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology. 2003;144:4433–4445. doi: 10.1210/en.2002-0068. [DOI] [PubMed] [Google Scholar]
- 7.Trümper A, Trümper K, Hörsch D. Mechanisms of mitogenic and anti-apoptotic signaling by glucose-dependent insulinotropic polypeptide in β(INS-1)-cells. J Endocrinol. 2002;174:233–246. doi: 10.1677/joe.0.1740233. [DOI] [PubMed] [Google Scholar]
- 8.Trümper A, Trümper K, Trusheim H, Arnold R, Göke B, Hörsch D. Glucose-dependent insulinotropic polypeptide is a growth factor for β (INS-1) cells by pleiotropic signaling. Mol Endocrinol. 2001;15:1559–1570. doi: 10.1210/mend.15.9.0688. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Nian C, Widenmaier S, McIntosh CH. Glucose-dependent insulinotropic polypeptide-mediated up-regulation of beta-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol Cell Biol. 2008;28:1644–1656. doi: 10.1128/MCB.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 11.Yip RG, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 12.Flatt PR. Dorothy Hodgkin Lecture 2008. Gastric inhibitory polypeptide (GIP) revisited: a new therapeutic target for obesity-diabetes? Diabet Med. 2008;25:759–764. doi: 10.1111/j.1464-5491.2008.02455.x. [DOI] [PubMed] [Google Scholar]
- 13.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis. 2008;18:574–579. doi: 10.1016/j.numecd.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Hojberg PV, Vilsboll T, Rabol R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 16.Aaboe K, Knop FK, Vilsboll T, et al. KATP channel closure ameliorates the impaired insulinotropic effect of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:603–608. doi: 10.1210/jc.2008-1731. [DOI] [PubMed] [Google Scholar]
- 17.Meneilly GS, Bryer-Ash M, Elahi D. The effect of glyburide on beta-cell sensitivity to glucose-dependent insulinotropic polypeptide. Diabetes Care. 1993;16:110–114. doi: 10.2337/diacare.16.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Gault VA, Kerr BD, Harriott P, Flatt PR. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin Sci (Lond) 2011;121:107–117. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 19.Piteau S, Olver A, Kim SJ, et al. Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat. Biochem Biophys Res Commun. 2007;362:1007–1012. doi: 10.1016/j.bbrc.2007.08.115. [DOI] [PubMed] [Google Scholar]
- 20.Pospisilik JA, Hinke SA, Pederson RA, et al. Metabolism of glucagon by dipeptidyl peptidase IV (CD26) Regul Pept. 2001;96:133–141. doi: 10.1016/s0167-0115(00)00170-1. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 22.Ahren B, Foley JE. The islet enhancer vildagliptin: mechanisms of improved glucose metabolism. Int J Clin Pract Suppl. 2008;159:8–14. doi: 10.1111/j.1742-1241.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- 23.Gault VA, Flatt PR, O’Harte FP. Glucose-dependent insulinotropic polypeptide analogues and their therapeutic potential for the treatment of obesity-diabetes. Biochem Biophys Res Commun. 2003;308:207–213. doi: 10.1016/s0006-291x(03)01361-5. [DOI] [PubMed] [Google Scholar]
- 24.Hinke SA, Gelling RW, Pederson RA, et al. Dipeptidyl peptidase IV-resistant [d-Ala(2)]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002;51:652–661. doi: 10.2337/diabetes.51.3.652. [DOI] [PubMed] [Google Scholar]
- 25.Alana I, Hewage CM, Malthouse JP, Parker JC, Gault VA, O’Harte FP. NMR structure of the glucose-dependent insulinotropic polypeptide fragment, GIP(1–30)amide. Biochem Biophys Res Commun. 2004;325:281–286. doi: 10.1016/j.bbrc.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Gault VA, Porter DW, Irwin N, Flatt PR. Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1–30) and GIP(1–42) in high-fat fed mice. J Endocrinol. 2011;208:265–271. doi: 10.1530/JOE-10-0419. [DOI] [PubMed] [Google Scholar]
- 27.Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH. Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry. 2001;40:13188–13200. doi: 10.1021/bi010902s. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Xu W, Tang L, Gong M, Zhang J. A novel GLP-1 analog exhibits potent utility in the treatment of type 2 diabetes with an extended half-life and efficient glucose clearance in vivo. Peptides. 2011;32:1408–1414. doi: 10.1016/j.peptides.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Doyle ME, Theodorakis MJ, Holloway HW, Bernier M, Greig NH, Egan JM. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul Pept. 2003;114:153–158. doi: 10.1016/s0167-0115(03)00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallwitz B, Witt M, Folsch UR, Creutzfeldt W, Schmidt WE. Binding specificity and signal transduction of receptors for glucagon-like peptide-1(7–36)amide and gastric inhibitory polypeptide on RINm5F insulinoma cells. J Mol Endocrinol. 1993;10:259–268. doi: 10.1677/jme.0.0100259. [DOI] [PubMed] [Google Scholar]
- 31.Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Tatarkiewicz K, Garcia M, Omer A, Van Schilfgaarde R, Weir GC, De Vos P. C-peptide responses after meal challenge in mice transplanted with microencapsulated rat islets. Diabetologia. 2001;44:646–653. doi: 10.1007/s001250051672. [DOI] [PubMed] [Google Scholar]
- 33.Hargrove DM, Kendall ES, Reynolds JM, et al. Biological activity of AC3174, a peptide analog of exendin-4. Regul Pept. 2007;141:113–119. doi: 10.1016/j.regpep.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Anti-obesity effects of the β-cell hormone amylin in diet induced obese rats: effects on food intake, body weight, composition, energy expenditure and gene expression. Endocrinology. 2006;147:5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 35.Venneti KC, Malthouse JP, O’Harte FP, Hewage CM. Conformational, receptor interaction and alanine scan studies of glucose-dependent insulinotropic polypeptide. Biochim Biophys Acta. 1814;2011:882–888. doi: 10.1016/j.bbapap.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Widenmaier SB, Kim SJ, Yang GK, et al. A GIP receptor agonist exhibits beta-cell anti-apoptotic actions in rat models of diabetes resulting in improved beta-cell function and glycemic control. PLoS One. 2010;5:e9590. doi: 10.1371/journal.pone.0009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin N, Flatt PR. Therapeutic potential for GIP receptor agonists and antagonists. Best Pract Res Clin Endocrinol Metab. 2009;23:499–512. doi: 10.1016/j.beem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Lynn FC, Pamir N, Ng EH, McIntosh CH, Kieffer TJ, Pederson RA. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes. 2001;50:1004–1011. doi: 10.2337/diabetes.50.5.1004. [DOI] [PubMed] [Google Scholar]
- 39.Martin CM, Irwin N, Flatt PR, Gault VA. A novel acylated form of (d-Ala(2))GIP with improved antidiabetic potential, lacking effect on body fat stores. Biochim Biophys Acta. 1830;2013:3407–3413. doi: 10.1016/j.bbagen.2013.03.011. [DOI] [PubMed] [Google Scholar]


