Abstract
Background
Reliable methods of labeling human enteric nervous system (ENS) stem cells for use in novel cell replacement therapies for enteric neuropathies are lacking. Here, we explore the possibility of using lentiviral vectors expressing fluorescent reporter genes to transduce, label, and trace mouse and human ENS stem cells following transplantation into mouse gut.
Methods
Enteric nervous system precursors, including ENS stem cells, were isolated from enzymatically dissociated mouse and human gut tissues. Lentivirus containing eGFP or mCherry fluorescent reporter genes was added to gut cell cultures at a multiplicity of infection of 2–5. After fluorescence activated cell sorting for eGFP and subsequent analysis with markers of proliferation and cell phenotype, transduced mouse and human cells were transplanted into the gut of C57BL/6 and immune deficient Rag2-/gamma chain-/C5 mice, respectively and analyzed up to 60 days later.
Key Results
Mouse and human transduced cells survived in vitro, maintained intense eGFP expression, proliferated as shown by BrdU incorporation, and formed characteristic neurospheres. When transplanted into mouse gut in vivo and analyzed up to 2 months later, transduced mouse and human cells survived, strongly expressed eGFP and integrated into endogenous ENS networks.
Conclusions & Inferences
Lentiviral vectors expressing fluorescent reporter genes enable efficient, stable, long-term labeling of ENS stem cells when transplanted into in vivo mouse gut. This lentiviral approach not only addresses the need for a reliable fluorescent marker of human ENS stem cells for preclinical studies, but also raises the possibility of using lentiviruses for other applications, such as gene therapy.
Keywords: cell replacement therapy, enteric nervous system stem cells, enteric neuropathies, Hirschsprung disease, human gut, lentiviral labeling
Key Messages.
For ENS stem cells to be used in novel cell replacement therapies for enteric neuropathies such as Hirschsprung disease then ‘proof of principle’ animal experiments need to be extended using human cells. For such studies, robust methods of labeling transplanted human ENS stem cells need to be established.
Our aim was to assess the ability of lentiviral vectors expressing fluorescent reporter genes to label ENS stem cells and trace them following transplantation into mouse gut in vivo.
Mouse and human gut-derived cells, including ENS stem cells, were transduced with a lentiviral vector containing eGFP (green) or mCherry (red) reporter genes and analyzed in vitro and in vivo.
In vitro, mouse and human lentivirus-transduced cells survived, maintained intense eGFP expression, proliferated as shown by BrdU incorporation, and formed neurospheres. When transplanted into mouse gut in vivo, transduced cells survived up to 2 months, strongly expressed eGFP and integrated into endogenous ENS networks.
INTRODUCTION
Stem cell transplantation therapies for congenital defects and degenerative diseases are rapidly becoming a reality. A number of groups, including ours, are focusing on establishing stem cell therapies for enteric neuropathies such as Hirschsprung disease (HSCR), which is characterized by an absence of the enteric nervous system (ENS) in the distal bowel.1–3 The ultimate aim is to transplant ENS stem cells into defective gut to replace missing enteric neurons and restore gut function.4–6 Supporting this idea, studies have shown that neural crest-derived cells, the precursors of the ENS,7 can be harvested from embryonic and postnatal gut, expanded in culture to form ‘neurospheres’ that contain ENS stem cells, and transplanted into aganglionic gut, where they differentiate into enteric neurons, and in some studies, affect gut activity8–11 (and reviewed in Refs. 4,5,12,13). Much of this work has been carried out in mouse models of disease, both as source and recipient of ENS stem cell-containing neurospheres. Genetic manipulation leading to expression of fluorescent proteins14 has been the labeling method of choice for visualizing cells prior to and following transplantation in vivo.11 Although such mouse genetic approaches are powerful and effective, their use is restricted to transgenic animals and is not applicable for human cells. If ENS stem cells are to be used as a therapy in HSCR patients these ‘proof of principle’ animal experiments need to be extended using ENS stem cells obtained from humans. For such studies to occur, robust methods of labeling human ENS stem cells need to be established. Here, we demonstrate that lentiviral vectors can be used to introduce fluorescent reporter genes into mouse and human ENS stem cells in order to label and trace them prior to and following transplantation into in vivo mouse gut.
MATERIALS AND METHODS
We used a self-inactivating (SIN) second generation HIV-1 based lentiviral vector containing the spleen focus forming virus LTR promoter, and the mutated Woodchuck Posttranscriptional Regulatory Element, which, when placed downstream of the complementary DNA to be expressed (eGFP or mCherry reporter genes) causes a posttranscriptional increase in transgene expression independent of transgene, promoter or vector sequences15 (Fig.1A).
Figure 1.
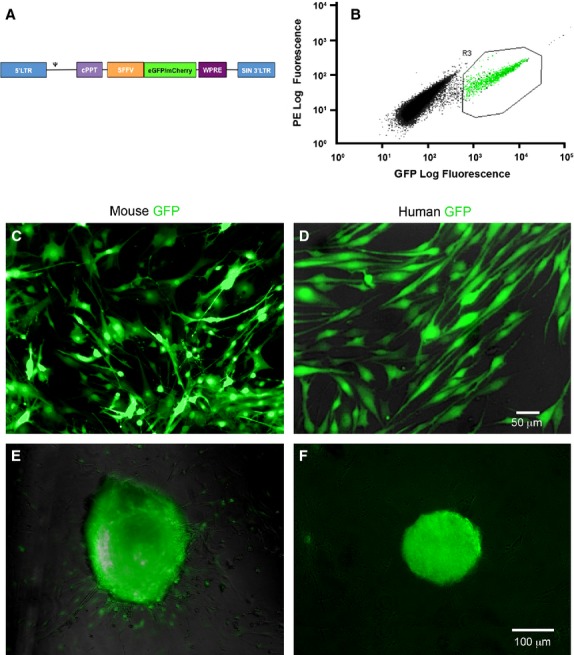
Lentiviral vector and transduced mouse and human gut-derived cells. (A) Map showing the lentiviral plasmid construct that expresses either eGFP or mCherry under the spleen focus forming virus (SFFV) promoter and the mutated Woodchuck Posttranscriptional Regulatory Element (WPRE) which enhances transgene expression and titer. (B) Representative gating for FACS analysis of lentivirally transduced (eGFP) mouse or human gut-derived cells showing separation of transduced vs non-transduced cells. (C and D) Transduced cells highly express the eGFP reporter gene for prolonged time in culture (C, mouse ENS cells after 65 days and D, human ENS cells after 35 days). (E and F) Transduced cells form characteristic neurospheres similar in appearance to previously reported non-transduced cells.10 Scale bars = 50 μm (C and D) and 100 μm (E and F).
For lentiviral vector production, a previously published protocol was followed16,17 whereby 80% confluent 293T cells were transfected for 4 h with 40 μg of plasmids encoding the viral backbone (pHR'SIN), 10 μg of VSV-G envelope glycoprotein (pMDG.2) and 30 μg of packaging proteins (gag-pol, rev, tat, in pCMVΔR8.74) using 1 μm polyethyleneimine as a transfection agent. Cells were washed and refreshed with Dulbecco's Modified Eagle Medium - DMEM containing glutamine, 10% Fetal Calf Serum - FCS and antibiotic mix and viral particles harvested after 36 h, filtered and stored frozen unconcentrated as 200 μL aliquots. They were titered by flow cytometry.
To isolate mouse ENS precursor cells, including ENS stem cells, gut tissues were harvested from C57BL/6 mice aged postnatal day 4–6, and the outer muscle layers, containing the ENS plexus layers, were peeled and enzymatically dissociated using a method previously described.8,10 Dissociated cells were plated onto fibronectin-coated dishes and cultured in DMEM F12 supplemented with N2 and B27 (Life Technologies, Paisley, UK) and Primocin antibiotics (InvivoGen, Source BioScience LifeSciences, Nottingham UK) and 20 ng/mL of both EGF and FGF (both Peprotech, London, UK). Human gut tissues were either full thickness biopsies from surgical resections or mucosal biopsies from routine endoscopy procedures carried out on pediatric patients. All samples were obtained with ethical approval and informed consent at Great Ormond Street Hospital, London, UK. Human tissue samples were dissociated and cultured using a similar protocol to that used for mouse gut.
Dissociated cells were transduced with lentivirus carrying either eGFP or mCherry reporter genes. Lentivirus was added to cultures at multiplicities of infection in the range of 2–5 (100 μL for 105 cells per well) and left for 36–48 h ensuring that the cells attached to the dish, were transduced, and the lentivirus had inactivated. Following trypsinization, FACS analysis was performed in order to both quantify the percentage of transduced cells, and to select eGFP+ cells which were spun down, plated on fibronectin-coated dishes, and cultured for 7–10 days for further analysis, including transplantation.
For BrdU labeling of cells prior to transplantation, 10 μM BrdU (Sigma-Aldrich, Dorset, UK) was applied for 48 h followed by fixation for immunolabeling using a previously described protocol.8 Antibodies used were rabbit anti-SMA (1:100; Abcam, Cambridge, UK), mouse anti-TuJ1 (1:500; Covance, Leeds, UK), and rat anti-BrdU (1:500, AbD Serotec, Kidlington, UK). Corresponding secondary antibodies were Alexa Fluor goat anti-rabbit 568, goat anti-mouse 568, and anti-rat 568 (all 1:500; Life Technologies, Paisley, UK).
Lentivirus-transduced mouse cells were transplanted into wild-type C57BL/6 mice, and human transduced cells were transplanted into immune deficient Rag2-/gamma chain-/C5 mice. For transplantations, the distal hindgut of 8–10 weeks old animals was exposed by laparotomy and cells were microinjected into the gut wall using a beveled syringe needle. Recipient mice were maintained for up to a further 60 days before sacrifice. Whole-mount gut analysis was performed using immunohistochemistry (anti-TuJ1) and confocal microscopy imaging (Zeiss LSM 710; Zeiss, Cambridge, UK).
RESULTS
After lentiviral transduction with a fluorescent reporter gene, 78 ± 3.9% (n = 3) of mouse and 69 ± 6.4% (n = 5) of human cells were labeled with eGFP and selected by gating with FACS (Fig.1B). When transduced cells were visualized by eGFP expression and manually counted under a microscope, 88.4 ± 13% of mouse and 92.1 ± 3.4% of human cells were eGFP+ (Fig.1C and D). After FACS, eGFP+ cells were cultured for up to 65 days for mouse cells and 35 days for human cells. During this time transduced cells survived in culture and maintained eGFP expression and intensity. They also formed characteristic neurospheres within 1 week of culture for mouse cells (Fig.1E), and within 10 days to 2 weeks for human cells (Fig.1F).
Characterization of the cultures showed that cells proliferated as shown by BrdU (15.3 ± 1.3% of non-transduced cells were BrdU+ compared to 13.3 ± 1.0% of transduced mouse cells, p > 0.05, indicating that lentiviral transduction does not adversely affect proliferation; Fig.2A). Both neural crest-derived cells such as neurons (15.01 ± 2.5%), and non-neural crest cells such as smooth muscle cells (64 ± 6.3%) were transduced by lentivirus in human gut cell cultures as shown by eGFP expression and co-immunolabeling with the neuronal marker TuJ1 (Fig.2B) and the smooth muscle marker SMA (Fig.2C). 84.4 ± 8.8% of TuJ1+ neurons were also GFP+. Lentiviral transduction did not significantly affect cell fate as 18.9 ± 3.4% of cells were TuJ1+ in untransduced cultures vs 15.01 ± 2.5% in transduced cultures (p > 0.05). Similar findings were obtained with SMA immunostaining where 60.4 ± 3.6% of cells were SMA+ in untransduced cultures vs 64 ± 6.3% in transduced cultures. In mouse cell cultures the proportions of cells immunopositive for TuJ1, the glial marker S100, and for SMA in untransduced vs transduced cultures were 30.12 ± 1.68% vs 32.38 ± 1.6%; 18 ± 5.29% vs 16.63 ± 3.1%; and 69.55 ± 4.8% vs 66.38 ± 1.89%, respectively (p > 0.05 in all cases). Of the transduced mouse cells, 90.6 ± 10.8% of the TuJ1+ cells were GFP+. Again, lentivirus did not preferentially transduce a particular cell type (neural crest-derived or non-neural crest-derived). This was confirmed by FACS sorting human gut cells into p75+ve (neural crest-derived) and p75−ve (non-neural crest-derived) populations that were equally highly transduced with eGFP (p75+ve/eGFP 95.01 ± 3.83%; p75−ve/eGFP: 97.7 ± 0.88% p = 0.22).
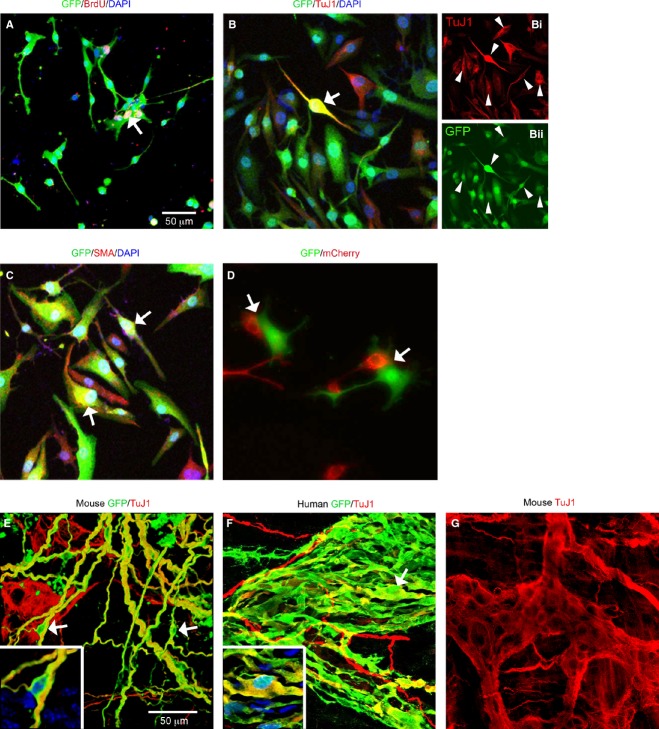
Figure 2.
Characteristics of lentivirus-transduced mouse and human gut-derived cells in vitro and following transplantation in vivo. (A) Transduced (eGFP) mouse cells proliferate as shown by BrdU incorporation (red). Arrow shows double-labeled lentivirus-transduced eGFP expressing cell also positive for BrdU. (B and C) Human cell phenotypes that were transduced by lentivirus include neural crest-derived neurons (B, arrow; Bi, arrowheads, TuJ1 in red; Bii, eGFP in green) and non-neural crest-derived cells such as smooth muscle (C, SMA, red). DAPI (blue) labels all nuclei. (D) Mixed cultures of mouse cells transduced with either eGFP (green) or mCherry (red) lentiviruses. After 2 weeks in culture, even though some cells were juxtaposed (arrows), yellow cells (expressing both expressing eGFP and mCherry) were not observed, indicating that lentivirus cross infection does not occur. (E) Transduced mouse ENS stem cell-containing neurospheres transplanted into recipient wild-type mouse gut. (F) Transduced human ENS stem cell-containing neurospheres transplanted into recipient immune deficient mouse gut. In both cases, transduced cells were observed up to 2 months (E, mouse) and up to one month (F, human) after transplantation. High eGFP expression was maintained and transduced cells integrated into the endogenous ENS network (TuJ1 immunolabeling, red). Transduced cells expressing TuJ1 are indicated by arrows and shown at high magnification in insets (also showing DAPI in blue) in both panels. (G) Untransplanted TuJ1 immunolabeled mouse gut for comparison showing enteric ganglion. Scale bars = 50 μm; A applies to A–D and E applies to E–G.
In order to verify that lentiviruses cannot remobilize and do not spread to neighboring cells, we transduced mouse gut-derived cells with either the eGFP-containing lentiviral construct (green), or the mCherry-containing construct (red). Both groups of cells were cultured for 48 h to allow virus particles to self-inactivate, cultured for 1 further week, then mixed together and co-cultured for 2 weeks. After this time, cells expressing exclusively eGFP or mCherry were observed (Fig.2D) and no double-labeled (yellow) cells expressing both eGFP and mCherry were evident.
In order to test the suitability of lentivirally labeled cells for stem cell transplantation, lentivirally transduced cells derived from both mouse and human gut were transplanted (n = 10 for each) into recipient mouse gut and analyzed up to 2 months later. Transduced eGFP expressing cells were observed in transplanted gut by fluorescence microscopy even without immunolabeling for eGFP, suggesting that the eGFP intensity had not diminished in vivo. The transduced mouse and human cells included smooth muscle cells and enteric neurons, as evidenced by eGFP expression and immunoreactivity for SMA and for TuJ1 (Fig.2E and F), and TuJ1+ cells showed integration into the endogenous ENS networks (Fig.2E and F).
DISCUSSION
Here, we report the use of lentiviruses to fluorescently label mouse and human gut-derived cells for gut transplantation studies. Some previous studies have employed retroviral or adenoviral vectors to label and track enteric or CNS-derived neural stem cells transplanted into gut.8,18,19 However, lentiviruses have advantages over these previous approaches by infecting both dividing and non-dividing cells, providing long-term, stable transgene expression, low immunogenicity, and adequate payload capacity for the majority of cDNAs and most marker genes, making them very useful for transducing a range of cell types.20–22 Specifically, we found the transduction efficiency of lentivirus to be high (∼90%) compared with that reported for adenovirus transduction of mouse neural stem cells (up to 25% in 72 h19). In our study, lentivirus maintained efficient, stable, long-term labeling of gut-derived cells, including ENS stem cells. Transduced cells survived, proliferated and expressed fluorescent reporter genes for an extended period of time in vitro and in vivo. They also formed neurospheres that were similar in appearance to neurospheres derived from non-transduced cells as previously reported.10 Our findings therefore highlight the use of lentiviruses for cell tracing and lineage analysis following transplantation of transduced human cells into mouse gut.
This lentiviral method for labeling human cells can also be utilized prior to, or following, selection of cells with a NCC-specific antibody such as p75NTR to obtain enriched populations of neural crest-derived cells.23 This combined methodology, generating eGFP+/p75+ cells, may enhance the ability of selected cells to rescue the ENS compared to a non-enriched, mixed cell population derived from dissociated gut. Further exciting applications of lentivirally transduced ENS stem cells include the possibility of delivering genes to target genetically defective human ENS stem cells (e.g., those from patients with mutations in RET or EDNRB3,24) as a somatic gene therapy tool for restoration of cell function.18,19,25
Despite the fact that further research is required in order to understand and refine the use of lentiviruses in cell or gene therapy, the labeling method described here is likely to become an essential part of the toolkit for preclinical transplantation studies of human ENS stem cells.
Acknowledgments
The authors thank Dr Ayad Eddaoudi (UCL Institute of Child Health FACS Facility) for technical support.
FUNDING
The experiments carried out in this study were funded by a grant from Great Ormond Street Hospital Children's Charity, London, UK (to NT and AJB).
DISCLOSURE
No conflicts of interest declared.
AUTHOR CONTRIBUTION
DN, SC, JC, JMD, and CMcC, performed experiments and analyzed data; SH provided essential lentiviral constructs; DN, JC, SH, AJB and NT undertook the study design; AJB, DN and NT wrote the draft manuscript. All authors provided critical review of the manuscript. All authors approved the submitted version of the manuscript.
References
- 1.Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013;10:43–57. doi: 10.1038/nrgastro.2012.234. [DOI] [PubMed] [Google Scholar]
- 2.McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2:113–29. doi: 10.1002/wdev.57. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein AM, Hofstra RMW, Burns AJ. Building a brain in the gut: development of the enteric nervous system. Clin Genet. 2013;83:307–16. doi: 10.1111/cge.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns AJ, Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol. 2014;11:317–28. doi: 10.1038/nrgastro.2013.226. [DOI] [PubMed] [Google Scholar]
- 5.Hotta R, Natarajan D, Burns AJ, Thapar N. Stem cells for GI motility disorders. Curr Opin Pharmacol. 2011;11:617–23. doi: 10.1016/j.coph.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Hotta R, Natarajan D, Thapar N. Potential of cell therapy to treat pediatric motility disorders. Semin Pediatr Surg. 2009;18:263–73. doi: 10.1053/j.sempedsurg.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- 9.Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–96. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–25. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Hotta R, Stamp LA, Foong JP, McConnell SN, Bergner AJ, Anderson RB, Enomoto H, Newgreen DF, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123:1182–91. doi: 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–79. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 13.Thapar N. New frontiers in the treatment of Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2009;48(Suppl. 2):S92–4. doi: 10.1097/MPG.0b013e3181a15d62. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–13. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 16.Howe SJ, Chandrashekran A. Vector systems for prenatal gene therapy: principles of retrovirus vector design and production. Methods Mol Biol. 2012;891:85–107. doi: 10.1007/978-1-61779-873-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Cooray S, Howe SJ, Thrasher AJ. Retrovirus and lentivirus vector design and methods of cell conditioning. Methods Enzymol. 2012;507:29–57. doi: 10.1016/B978-0-12-386509-0.00003-X. [DOI] [PubMed] [Google Scholar]
- 18.Sun NF, Zhong WY, Lu SA, Tian YL, Chen JB, Hu SY, Tian AL. Coexpression of recombinant adenovirus carrying GDNF and EDNRB genes in neural stem cells in vitro. Cell Biol Int. 2013;37:458–63. doi: 10.1002/cbin.10060. [DOI] [PubMed] [Google Scholar]
- 19.Shu X, Meng Q, Jin H, Chen J, Xiao Y, Ji J, Qin T, Wang G, et al. Treatment of aganglionic megacolon mice via neural stem cell transplantation. Mol Neurobiol. 2013;48:429–37. doi: 10.1007/s12035-013-8430-x. [DOI] [PubMed] [Google Scholar]
- 20.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 21.Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18:477–90. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443:603–18. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 23.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 24.Alves MM, Sribudiani Y, Brouwer RW, Amiel J, Antinolo G, Borrego S, Ceccherini I, Chakravarti A, et al. Contribution of rare and common variants determine complex diseases-Hirschsprung disease as a model. Dev Biol. 2013;382:320–9. doi: 10.1016/j.ydbio.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Kumar P, Woon-Khiong C. Optimization of lentiviral vectors generation for biomedical and clinical research purposes: contemporary trends in technology development and applications. Curr Gene Ther. 2011;11:144–53. doi: 10.2174/156652311794940782. [DOI] [PubMed] [Google Scholar]