Abstract
In the field, plants are challenged by more than one biotic stressor at the same time. In this study, the molecular interactions between potato (Solanum tuberosum L.), Colorado potato beetle (Leptinotarsa decemlineata Say; CPB) and Potato virus YNTN (PVYNTN) were investigated through analyses of gene expression in the potato leaves and the gut of the CPB larvae, and of the release of potato volatile compounds. CPB larval growth was enhanced when reared on secondary PVYNTN-infected plants, which was linked to decreased accumulation of transcripts associated with the antinutritional properties of potato. In PVYNTN-infected plants, ethylene signalling pathway induction and induction of auxin response transcription factors were attenuated, while no differences were observed in jasmonic acid (JA) signalling pathway. Similarly to rearing on virus-infected plants, CPB larvae gained more weight when reared on plants silenced in JA receptor gene (coi1). Although herbivore-induced defence mechanism is regulated predominantly by JA, response in coi1-silenced plants only partially corresponded to the one observed in PVYNTN-infected plants, confirming the role of other plant hormones in modulating this response. The release of β-barbatene and benzyl alcohol was different in healthy and PVYNTN-infected plants before CPB larvae infestation, implicating the importance of PVYNTN infection in plant communication with its environment. This was reflected in gene expression profiles of neighbouring plants showing different degree of defence response. This study thus contributes to our understanding of plant responses in agro-ecosystems.
Keywords: gene expression, insect midgut transcriptional response, Leptinotarsa decemlineata, plant defence, Solanum tuberosum (potato), volatile organic compounds
Introduction
Plant pathogens and pests are the cause of substantial loss of crop yields throughout the world. Understanding the principles of plant–pathogen and plant–herbivore interactions is necessary to establish effective systems for plant protection. Substantial knowledge has been gathered during past decades on the mechanisms of attack and defence in one-to-one interaction studies under laboratory conditions (Pieterse et al. 2012). In the field, however, plants have to face combinations of different pathogens and pests.
Colorado potato beetle (Leptinotarsa decemlineata Say; CPB) and Potato virus Y (PVY) are the most important pest and viral pathogen, respectively, in potato (Solanum tuberosum L.), and these can result in major economic damage to potato production worldwide (Alyokhin et al. 2008; Scholthof et al. 2011). Thus, the aim of this study was to determine the responses of potato plants when simultaneously exposed to both of these biotic stressors.
Plants respond to pathogen or herbivore attack by activation of different plant defence pathways. Biotrophic pathogens, including viruses, and a majority of phloem feeding insects induce salicylic acid (SA) pathway-related defences, whereas necrotrophic pathogens, some phloem feeding insects and chewing herbivores, such as CPB, mainly activate jasmonic acid (JA) and ethylene (ET) pathway defences (Pieterse et al. 2012). Infestation with CPB larvae induces JA biosynthesis (Kruzmane et al. 2002) and upregulates the expression of JA-pathway-responsive antinutritional proteins, such as proteinase inhibitors, arginase, threonine deaminase and polyphenol oxidase, which decrease the amino acid bioavailability in the CPB midgut (Rivard et al. 2004; Lawrence et al. 2008; Chung & Felton 2011). The potato response to the most aggressive strain of PVY, PVYNTN, has been characterized in several studies (Kogovšek & Ravnikar 2013), although the dynamics and interactions between the phytohormone pathways during infection remain largely unknown.
In the context of multitrophic interactions, pre-existing plant viral infections can alter the attraction of a plant to herbivores (Castle et al. 1998) or improve the nutritional assimilation of the plant by the herbivores (Belliure et al. 2005; Wang et al. 2012). Such interactions are commonly explained by differences in plant defence responses to viruses and herbivores (Thaler et al. 2010; Zhang et al. 2012), such as the antagonistic crosstalk between the SA and JA signalling pathways (Van der Does et al. 2013).
In the JA pathway, the COI1 protein has been identified as a receptor for the bioactive form of the jasmonates: jasmonic acid–isoleucine (JA-Ile). Upon JA-Ile treatment, COI1 targets a repressor protein, JAZ, to a SCFCOI1 complex for degradation, which ultimately leads to the activation of herbivore defence response genes (Thines et al. 2007). In Arabidopsis, the COI1-dependent defence response is transcriptionally regulated by the MYC and ERF/AP2 transcription factors, which act antagonistically (Verhage et al. 2011; Schweizer et al. 2013). Silencing of the COI1 gene results in desensitization of the JA signalling pathway. When attacked by herbivores, COI1-silenced plants (coi1 plants) produce lower levels of defence compounds and release lower amounts of volatile organic compounds (VOCs; Li et al. 2004; Halim et al. 2009; Schweizer et al. 2013).
These VOCs also have important roles in communication between plants and other organisms. Insect infestation induces the production and release of VOCs, such as fatty acid derivatives and terpenoids, which are mainly regulated by the JA pathway. The VOCs are involved in direct plant defence, as their release can deter herbivores and indirect defence as they attract insects that pred-ate upon or parasitize herbivorous insects (Mithöfer & Boland 2012). Furthermore, these VOCs can be perceived by neighbouring plants, in which they can induce priming (Kim & Felton 2013). In potato plants, an attack by the CPB induces the release of a complex blend of VOCs (Bolter et al. 1997; Schütz et al. 1997). However, although plant viruses have been shown to influence VOC release (Ponzio et al. 2013), PVY infection of potato causes only minor changes in VOC release (Eigenbrode et al. 2002). Potato VOC release in a multi-attacker situation has not been previously explored.
In this study, we investigated the responses of PVYNTN-infected potato plants to infestation by CPB larvae. We have first shown that the CPB larvae grow faster on PVYNTN-infected potato plants than on healthy potato plants. We further examined the following hypotheses: (i) In PVYNTN-infected potato plants, induced JA-signalling-dependent defence against herbivores is attenuated due to the proposed SA–JA antagonism; (ii) plant defence signalling perturbation causes a decrease in production of antinutritional compounds in virus-infected plants; (iii) CPB larvae reared on PVYNTN-infected plants face lower inhibition of digestive enzymes, therefore their midgut transcriptional response is attenuated; and (iv) PVYNTN infection alters the release of VOCs which can impact the priming of neighbouring plants.
Materials and methods
Plant growth, larval feeding assays and tissue sampling
The study was designed to initially measure the CPB larval weight gain and included collection of potato leaf tissue and sampling of the CPB larva midgut during differential feeding assays. Potato plants of cv. ‘Igor’ (healthy and secondary PVYNTN infected, i.e. plants grown from infected tubers) and cv. ‘Désirée’ (nontransgenic and coi1 plants; Halim et al. 2009) were grown in separate glass containers and used for the larval feeding assays. The leaves from the healthy, infested and neighbouring but noninfested plants were sampled at three time points [0, 3, and 24 h post-infestation (hpi)] in three replicates. Noninfested plants of cv. ‘Igor’ (healthy and secondary PVYNTN infected) and noninfested plants of cv. ‘Désirée’ (nontransgenic and coi1 plants) grown outside the glass containers were sampled as controls at the same time points.
For the differential feeding assays, a total of 20 first instar CPB larvae were placed on the upper developed leaves of four potato plants in each glass container (five larvae per plant; for both cv. ‘Igor’ and cv. ‘Désirée’ plants). A further control of detached leaves-reared CPB larvae was supplied with fresh potato leaves of cv. ‘Igor’ every 24 h (to avoid activation of the plant defence system). The larvae were weighed as groups at 0 days postinfestation (dpi) (immediately before infestation), 2 and 4 dpi, and individually every day from 5 to 9 dpi (see Fig. S1, Supporting information for experimental scheme). The larval midgut sampling for transcriptional analysis was performed on 4th instar larvae at 10 dpi, as described in Petek et al. (2012). Four pooled midgut samples (each containing 3–5 midguts) were prepared for each of the five differential feeding assay groups: larvae reared on healthy and PVYNTN-infected cv. ‘Igor’ plants, larvae reared on nontransgenic and coi1 plants cv. ‘Désirée’ and control CPB larvae reared on detached leaves. For a more detailed description, see Supporting Information.
RNA isolation and quantitative PCR
Potato leaf samples (∼150 mg) were homogenized using a TissueLyzer (Qiagen, Germany) and the total RNA was extracted using RNeasy Plant Mini kits (Qiagen), as described by Baebler et al. (2009). The total RNA from the larval midgut tissue was isolated using TRIzol (Invitrogen, USA), as described by Petek et al. (2012). The RNA concentrations and integrity were validated using a NanoDrop ND-1000 spectrometer (NanoDrop Products, USA) and agarose gel electrophoresis. DNase treatment (DNase I; Invitrogen) and reverse transcription (High Capacity cDNA RT kits; Invitrogen) were performed as described by Baebler et al. (2009). The samples were analysed using a LightCycler 480 real-time PCR system (Roche Applied Science, USA), as described by Petek et al. (2010).
For the potato plants, the analysis included the expression of 26 genes involved in JA biosynthesis and signalling, ET, SA and auxin signalling, phenylpropanoid biosynthesis, gene silencing, photosynthesis, sugar metabolism and genes regulated by phytohormonal pathways. Cytochrome oxidase (COX), elongation factor 1 (EF-1) and 18S rRNA were used as the reference genes for data normalization. In the CPB larvae, 11 midgut-expressed genes involved in larval response to potato defences were assayed, and Ld_smt3 and 18S rRNA were used as the reference genes for data normalization. Detailed descriptions of the assay design and analysis are given in the Supporting Information and in Table S1 (Supporting information). The standard curve approach described by Petek et al. (2012) was used for quantification.
RNAseq analysis
RNA samples from potato leaves infested with CPB larvae and leaves from noninfested control plants were collected 24 hpi and pooled to obtain four samples for each cultivar. These pooled samples were treated with DNase I. The enrichment of the mRNA using oligo (dT) beads, the RNAseq library preparation following standard Illumina protocols, and the high-throughput sequencing on the Illumina HiSeq2000 platform were performed at BGI Tech. After removing the adapter sequences and low-quality reads, ∼49 mio 90 bp paired-end reads (insert size 200 bp) per sample were obtained (Table S2, Supporting information).
Quality trimming, merging of overlapping pairs, mapping the reads to the potato transcriptome (Ramšak et al. 2014) and read counting were performed using CLC Genomics Workbench 6. The read counts for the transcripts belonging to the same StNIB putative paralogue group were summarized, and the reads per kilobase per million mapped reads (RPKM) were calculated using representative transcript lengths. The log fold change (logFC) was calculated as log2(CPB-larvae-infested sample RPKM) − log2(noninfested control sample RPKM). More detailed descriptions of the RNAseq analysis are available in the Supporting Information.
Detection and analysis of volatile compounds
The trapping of the VOCs was performed on intact plants using a ‘push’ headspace collection system in hermetically sealed glass containers, as described previously (Arimura et al. 2008). Ten biological replicates of potato cv. ‘Igor’ and six biological replicates of potato cv. ‘Désirée’ were sampled. The headspace VOCs from the same plants were sampled before infestation and during infestation with two 4th instar CPB larvae, 24 h for cv. ‘Désirée’ and 48 h for cv. ‘Igor’. Because neighbouring receiver plants were of the same treatment group as the focal emitter plants, any effects of treatment on volatile emissions in the focal emitter plant cannot be distinguished from the effects of treatment on volatile perception and downstream processes in the neighbouring receiver plant. Gas chromatography–mass spectrometry (GC-MS) analysis was performed as described by Arimura et al. (2008), using a Zebron ZB-5 capillary column (0.25 mm i.d. × 15 m, with 0.25 μm d.f.; Phenomenex, Torrance, CA, USA). AMDIS (NIST, USA) and LCquan (Thermo Scientific, USA) were used for the relative quantification of the VOCs.
Statistical analysis
The differences in the larval weight gains and gene expression differences between the potato plant groups used in the feeding assays and their noninfested control plants, and between the larvae reared on these different potato plant groups, were evaluated using Student's t-tests, with the assumption of equal sample variance. Enrichment of the RNAseq differentially expressed genes in biological processes present in MapMan gene ontology was determined using Wilcoxon rank-sum tests implemented in mapman software (Usadel et al. 2005). Only genes with absolute logFC > 0.5 were used for the enrichment analysis. Multifactorial statistical model was set for analysis of VOC release (for detailed description, see Supporting Information).
Results
Rearing beetle larvae on PVYNTN-infected potato positively affects their growth
The PVYNTN-infected potato plants of cv. ‘Igor’ showed systemic symptoms, including leaf mosaics, curling, senescence and stunted growth, which indicated the severe disease status of these plants. Independent feeding assays were performed to examine the effects of the PVYNTN infection of these potato plants on the growth of the CPB larvae, and to compare this to the effects of the disruption of JA signalling in the coi1 plants of cv. ‘Désirée’. From the eighth day onwards, the larvae reared on the PVYNTN-infected plants gained significantly more weight than those reared on the healthy plants, and reached an ∼20% greater mean larval weight by 9 dpi (Fig.1). This increase in weight of the CPB larvae feeding on the PVYNTN-infected plants suggests that PVYNTN attenuates the plant defence responses against these herbivores. The CPB larvae reared on the coi1 plants gained ∼60% more weight by 9 dpi than those reared on the nontransgenic plants (Fig.1). Because of the different genetic backgrounds used in our viral infection and COI1 silencing treatments, we cannot compare the impacts of these treatments directly. With this caveat in mind, our data suggest that the PVYNTN infection may have less impact on larva weight gain improvement than the COI1 silencing. At the same time, the control CPB larvae reared on the detached leaves, and thus with plant defences induced to minor extent (Gruden et al. 1998), gained about 35% more weight than those reared on either the PVYNTN-infected or the coi1 plants (Fig.1).
Figure 1.
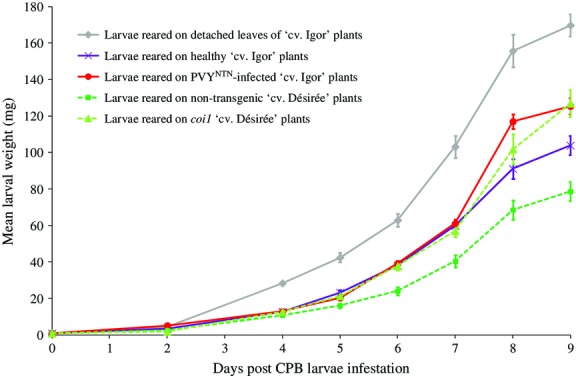
Growth of CPB larvae in the differential feeding assays. The CPB larvae were fed on Potato virus YNTN (PVYNTN)-infected and healthy plants of cv. ‘Igor’, and on nontransgenic and coi1-plants of cv. ‘Désirée’. Fresh potato leaves of cv. ‘Igor’ were provided for the ‘control’ larval group every 24 h. Larvae were weighted as whole groups at 0, 2 and 4 dpi, and individually every day from 5 to 9 dpi. Error bars (5–9 dpi) show standard errors of the mean (n = 20). The statistical evaluation of differences in larval weight throughout the feeding assays is presented in Table S3 (Supporting information).
PVYNTN infection attenuates herbivore-induced defences in potato leaves
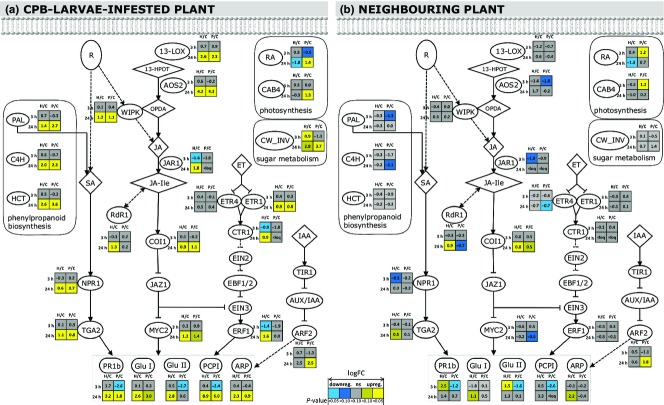
To examine how the PVYNTN infection affects the plant defences when exposed to herbivory, gene expression changes in the pathways associated to biotic stress were examined in potato leaves using qPCR. The genes examined were those involved in JA-Ile biosynthesis (13-LOX,AOS2,JAR1) and in the JA, SA and ET signalling pathways (WIPK,NPR1,TGA2,COI1,MYC2,ETR1,ETR4,CTR1,ERF1), and also genes downstream of these pathways (PR1b,Glu I,Glu II,PCPI). Additionally, this included an examination of genes related to auxin signalling (ARF2,ARP), phenylpropanoid biosynthesis (PAL,C4H,HCT), photosynthesis (RA,CAB4), sugar metabolism (CW_INV) and gene silencing (RdR1). Gene expression was examined in the healthy plants and in the corresponding PVYNTN-infected plants, at 3 and 24 hpi (Table S4A, Supporting information).
Three hours after CPB larvae infestation, not many significant differences in gene expression were observed in the PVYNTN infected when compared to their respective noninfested control plants. In the healthy plants, only CW_INV was significantly upregulated, whereas JAR1,ARF2,CTR1 and ERF1 were significantly downregulated (Fig.2A). In the PVYNTN-infected plants, there was significant downregulation of the SA-regulated and JA-regulated genes PR1b,Glu II and PCPI, and a trend towards downregulation of the photosynthesis activity marker RA (Fig.2A).
Figure 2.
Differential expression of genes involved in plant defence responses in Potato virus YNTN (PVYNTN)-infected and healthy cv. ‘Igor’ potato plants after infestation with CPB larvae. In the scheme, the proteins involved in plant defence are shown as ellipses and the metabolites as deltoids. Dashed lines show indirect regulation. The small tables beside the proteins show the gene expression changes in (A) CPB-larvae-infested plants and (B) neighbouring plants, at 3 and 24 hpi. H/C, comparison between CPB-larvae-infested healthy and noninfested healthy control plants; P/C, comparison between CPB-larvae-infested PVYNTN-infected and noninfested PVYNTN-infected control plants. The fold changes in gene expression were calculated by dividing the average relative copy number of healthy and PVYNTN-infected samples by the average relative copy number of their respective control samples, and then applying the log2 transformation. Statistical significances of expression differences (t-test P-values) are represented by the colours of cells in the table (see Table legend). <loq, comparisons in which the expression of all of the samples from both groups were below the limit of quantification. The relative copy number data are available in Table S4A (Supporting information). The gene names are abbreviated follows: PR1b,basic pathogenesis-related protein 1;GluII,β-1,3-glucanase II;CAB4,chlorophyll a/b-binding protein;RA,Rubisco activase;ARF2,auxin response factor 2;13-LOX,13-lipoxygenase H3;AOS2,allene oxide synthase 2;WIPK,wound-induced protein kinase;JAR1,jasmonate resistant 1;COI1,coronatine insensitive 1;JAZ1,jasmonate-ZIM-domain protein 1;MYC2,transcription factorMYC2;ETR1,ethylene receptor 1;ETR4,ethylene receptor 4;CTR1,constitutive triple response 1;ERF1,ethylene-responsive transcription factor 1;ARP,auxin-repressed protein;CW_INV,cell wall invertase;PAL,phenylalanine ammonia-lyase;C4H,cinnamic acid 4-hydroxylase;HCT,hydroxycinnamoyl transferase;NPR1,nonexpressor ofPR1;TGA2,leucine zipper transcription factor TGA2;RdR1, RNA-dependentRNA-polymerase 1;PCPI,potato cysteine proteinase inhibitors.
After 24 h of infestation, the gene expression changes in the CPB-larvae-infested plants were much more pronounced. In both the healthy and the PVYNTN-infected plants, the JA-pathway-related genes (WIPK,13-LOX,AOS2,COI1) were strongly and significantly upregulated, and the SA-pathway-related genes (NPR1,TGA2) were moderately, but significantly, upregulated, when compared to their respective noninfested control plants (Fig.2A). The MYC2 transcription factor was upregulated to approximately twofold upon CPB larvae infestation in both healthy and PVYNTN-infected plants, although this upregulation was statistically significant (P < 0.05) only for the healthy plants (Fig.2A). Some genes were upregulated only in the healthy plants, namely CTR1,ERF1,JAR1 and RdR1, and some genes were upregulated only in the PVYNTN-infected plants, namely RA and CAB4 (Fig.2A). For many of the assayed genes, the responses in the healthy and PVYNTN-infected plants were quantitatively different. These genes were either more strongly upregulated in the healthy plants (PR1b,Glu II,PCPI,ARP; Fig.2A) or in the PVYNTN-infected plants (PAL,HCT,CW_INV; Fig.2A).
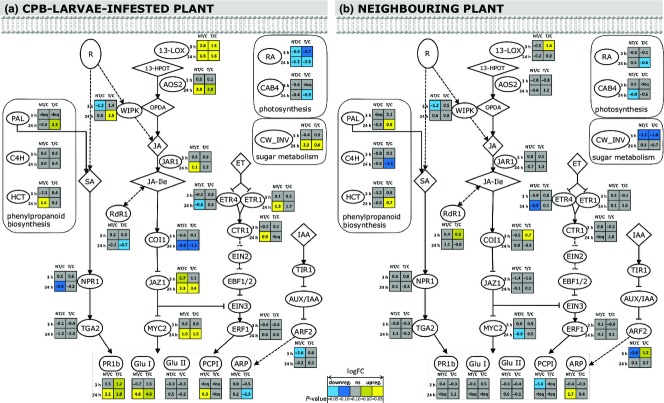
Jasmonic acid signalling is known to be a major contributor to defence against herbivores, and thus we hypothesized that the responses observed in the potato–PVYNTN–CPB larvae interaction were the final outcome of attenuation of this pathway. To test this hypothesis, the transcriptional response to CPB larvae infestation was investigated using the same qPCR assays in the control nontransgenic and coi1 cv. ‘Désirée’ plants. In the coi1 plants, COI1 expression was ∼35% of that in the control nontransgenic plants (Table S4B, Supporting information). Gene expression differences in cv. ‘Désirée’ were again relatively low at 3 hpi, while more pronounced changes were detected at 24 hpi. JA biosynthesis and signalling was induced at 24 hpi in the nontransgenic plants, and to a lesser extent also in the coi1 plants (Fig.3A). In the coi1 plants, however, this did not result in induced expression of genes regulated by the JA signalling cascade, such as PCPI and HCT, as was seen in the nontransgenic plants. Contrary to the upregulation in the PVYNTN-infected plants, the photosynthesis genes were downregulated in these coi1 plants. Similarly, the cell wall invertase gene (CW_INV) that was induced in the PVYNTN-infected plants was induced to a lesser extent in the coi1 plants than in the nontransgenic plants (Fig.3A). On the other hand, suppression of CTR1 kinase involved in ET signalling and auxin-repressed protein (ARP) expression was observed in the coi1 and the PVYNTN-infected plants (Figs2A and 3A).
Figure 3.
Differential expression of genes involved in the plant defence response in the nontransgenic and coi1 potato cv. ‘Désirée’ plants after infestation with CPB larvae. For the details of the scheme, see legend to Fig.2. NT/C, comparison between infested nontransgenic and control noninfested nontransgenic plants; T/C, comparison between infested coi1 and control noninfested coi1 plants. Relative copy numbers data are available in Table S4B (Supporting information). For gene name abbreviations, see legend to Fig.2.
We also performed RNAseq to provide an overview of the transcriptome changes in the CPB-larvae-infested plants after 24 h of infestation, and this showed missing links in our understanding of the defence signalling in multi-attacker systems. The RNAseq differential expression (|logFC| > 1) correlated highly with the qPCR (r = 0.93). Analysis at the biological process level (Table1) showed that upregulation of photosynthesis, metabolism of phenylpropanoids and cell wall components was specific for potato plants infected with PVYNTN and exposed to the CPB larvae. In addition, PVYNTN infection showed specific upregulation of gibberellin signalling components and downregulation of auxin response transcription factors. Proteinase inhibitors were upregulated in all of the plants infested with the CPB larvae except in coi1 plants; the induction was albeit attenuated in PVYNTN-infected plants compared to the induction in healthy plants (up to 32-fold lower induction). More detailed evaluation of the RNAseq data revealed that besides the proteinase inhibitors, other antinutritional proteins, such as α-amylase inhibitors, arginase and polyphenol oxidases, were suppressed in the PVYNTN-infected plants when compared to the induction in CPB-larvae-infested healthy plants, although to a much lower extent than in the coi1 plants (Table S5, Supporting information). In addition, the expression of several key genes involved in ET biosynthesis and signalling (ACS, ACO, EBF1/2, individual ERFs) was attenuated in the PVYNTN-infected plants, which was not the case in COI1-silenced plants, while expression of main SA biosynthesis gene ICS1 and JA methyltransferase (JMT) was enhanced (Table S5, Supporting information). However, as both treatments were performed in different genetic backgrounds, comparison between the treatments has to be treated with caution (see Supporting Information Supplementary results for details).
Table 1.
MapMan bins significantly enriched for upregulated (+) and downregulated (−) genes. MapMan bins identified as significantly enriched (Wilcoxon rank-sum test, Benjamini–Hochberg corrected P < 0.01) in cv. ‘Igor’ PVYNTN-infected plants 24 h after CPB larvae infestation are presented, along with the significant enrichment of the bins in healthy cv. ‘Igor’ and nontransgenic and coi1 cv. ‘Désirée’ potato plants. Bin description and sizes are shown
| MapMan bin description | Elements in bin | cv. ‘Igor’ plants | cv. ‘Désirée’ plants | ||
|---|---|---|---|---|---|
| Healthy | PVYNTN infected | Nontransgenic | coi1 | ||
| Cell wall | 364 | + | |||
| Photosynthesis | 247 | − | + | − | − |
| Phenylpropanoids/lignin biosynthesis | 73 | + | + | ||
| Proteinase inhibitors | 57 | + | + | + | |
| Amino acid synthesis/aromatic | 47 | + | |||
| Auxin response transcription factors | 21 | − | |||
| Gibberellin-regulated genes | 14 | + | |||
PVYNTN, Potato virus YNTN.
PVYNTN infection moderately alters the transcriptional response in the larval gut
The feeding assay data suggested that PVYNTN infection attenuates the plant defence that is induced upon CPB larvae infestation. We showed that the majority of the proteinase inhibitor genes were induced to a lesser extent in the PVYNTN-infected plants (Fig.2A, Table S5, Supporting information). To determine whether these differences were reflected in the transcriptional response in the CPB larval gut, we sampled the midgut from the last instar larvae in the feeding assays and examined the differences in the expression of the intestain family genes, and of other genes previously associated with larval response (Gruden et al. 2004; Petek et al. 2012).
As expected, all of the assayed genes were significantly differentially expressed in the larval response to plant defences (Fig.4A). Rearing larvae on PVYNTN-infected plants did not result in major changes in the expression of these genes (Fig.4B). Slightly weaker response was detected for the genes Ld_GH45-6 and Ld_GH48-2 (Fig.4B), which encode cellulases from the families of glycoside hydrolase 45 (GH45) and glycoside hydrolase 48 (GH48), respectively. The differences in larval response were more pronounced for the larvae reared on the coi1 plants. The intestain D,serine protease,cellulase,pectinase and JHBP-like genes were expressed at significantly higher levels in the larvae reared on the nontransgenic plants than in the larvae reared on the coi1 plants, whereas the intestain C genes were regulated in the opposite direction (Fig.4C).
Figure 4.
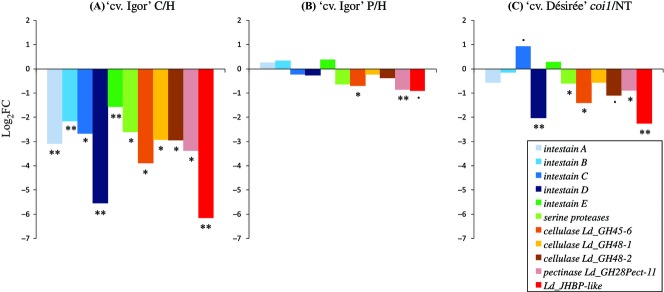
Effects of different diets on the response of CPB larvae. Comparisons of gene expression in (A) larvae reared on detached potato plants exchanged every 24 h and larvae reared on healthy cv. ‘Igor’ potato plants (C/H), (B) larvae reared on Potato virus YNTN (PVYNTN)-infected plants and larvae reared on healthy cv. ‘Igor’ potato plants (P/H), and (C) larvae reared on coi1 plants and larvae reared on nontransgenic cv. ‘Désirée’ potato plants (coi1/NT). Expression fold changes (log2FC) were calculated by comparison of the average relative copy number of larval samples (Table S6, Supporting information) from the same groups, and then applying the log2 transformation. Statistically significant differences (t-tests) are designated as (**)P < 0.01, (*)P < 0.05 and (.)P < 0.10. JHBP-like,Juvenile hormone binding protein-like genes.
Herbivore-induced volatile emissions in potato are to a large extent COI1 dependent
After observing virus-induced and CPB-larval-induced transcriptional changes in potato defence signalling, we investigated whether VOC release was also affected in these interactions. We measured the release of 68 compounds from the potato plants of cv. ‘Igor’ and 59 compounds from the potato plants of cv. ‘Désirée’ (Table S7, Supporting information). Upon CPB larvae infestation, we detected significantly enhanced release of several VOCs that belong to different chemical groups; for example, the aliphatic undecane, several sesquiterpenoids, and the monoterpenoid shyobunol in the healthy and PVYNTN-infected potato plants of cv. ‘Igor’ (Table2). Moreover, there was a trend to an increase in methyl salicylate (MeSA) release detected after the CPB larvae infestation (Table2).
Table 2.
Volatile organic compounds (VOCs) that show release that is significantly affected by secondary PVYNTN infection, CPB larvae infestation or the interaction of both of these factors in cv. ‘Igor’ potato plants. Average VOC response factors are given as log10 values relative to release from healthy plants before CPB larvae infestation
| Compound group | Identification | Retention index | PVYNTN infected | CPB larvae infested | PVYNTN infected and CPB larvae infested |
|---|---|---|---|---|---|
| Aliphatics | Undecane | 1100 | 0.29 | 1.34** | 1.23 |
| Heptadecane‡ | 1699 | −0.55 | −0.70† | −1.34 | |
| Aliphatic aldehydes | Decanal | 1205 | 0.14 | −0.82* | −1.08 |
| Benzenoids | MeSA | 1193 | 0.03 | 0.63† | 0.68 |
| Benzyl alcohol | 1034 | 0.82* | 0.72† | 2.75 | |
| 2-Ethylhexyl salicylate‡ | 1805 | 0.07 | −0.61** | −0.53 | |
| Monoterpenoids | Shyobunol | 1691 | −0.47 | 7.75** | 10.11 |
| Sesquiterpenoids | α-Copaene | 1376 | −0.09 | 1.47** | 1.76 |
| α-Gurjunene | 1410 | −0.57 | 3.38*** | 3.78 | |
| (E)-β-Caryophyllene | 1421 | −0.19 | 6.19*** | 7.16 | |
| β-Barbatene | 1442 | −1.80** | 1.56** | 0.09 | |
| α-Humulene‡ | 1454 | 0.10 | 4.33*** | 5.43 | |
| (E)-β-Farnesene | 1458 | −0.24 | 8.23*** | 9.63 | |
| Germacrene A‡ | 1505 | −0.06 | 5.85*** | 6.48 | |
| Sesquiphellandrene | 1523 | 0.40 | 0.66* | 0.81 | |
| Caryophyllene oxide | 1584 | −0.32 | 12.02*** | 11.37 |
PVYNTN, Potato virus YNTN.
Tentative identification.
Significant differences in VOC release are indicated as:
P < 0.0005;
P < 0.005;
P < 0.05
P < 0.10.
Intriguingly, the PVYNTN infection itself significantly suppressed only the release of the sesquiterpenoid β-barbatene and induced the release of benzyl alcohol. Nevertheless, there was a clear reducing trend in the terpenoid release levels in these PVYNTN-infected plants after CPB larvae infestation (Table2).
In the combination of PVYNTN infection with CPB larval feeding, VOC release related to the CPB larvae dominated over that associated with the PVYNTN infection. Interestingly, β-barbatene release was induced by the CPB larval feeding and was completely abolished in the PVYNTN-infected plants, while the induction of benzyl alcohol was enhanced (Table2). The dominance of the herbivory for the release of terpenoids was also evident from the RNAseq data, which showed that JA-pathway-responsive terpene synthase genes were induced in healthy and in PVYNTN-infected plants upon CPB larval attack (Table S5, Supporting information).
The nontransgenic and coi1-plants of cv. ‘Désirée’ were also analysed. Several sesquiterpenoid volatiles [(E)-β-caryophyllene, (E)-β-farnesene, germacrene D-4-ol] were released in significantly smaller amounts from the infested coi1 plants than from the infested nontransgenic plants (Table3). Supporting these data, 13 of 18 terpene synthase genes induced by infestation in the nontransgenic plants (logFC > 1) had markedly reduced expression, or were expressed below the limit of quantification in coi1 plants (Table S5, Supporting information). A similar decrease in release was observed for MeSA, and for an unknown phenyl ester, decanal and compounds tentatively identified as dodecane, heptadecane and nonanal (Table3).
Table 3.
Volatile organic compounds (VOCs) that show release that is significantly affected by CPB larvae infestation, COI1 silencing (coi1) or the interaction of both of these factors in cv. ‘Désirée’ potato plants. Average VOC response factors are given as log10 values relative to release from nontransgenic plants before CPB larvae infestation. For coi1 and CPB larvae-infested interaction, only the VOCs with significantly different release compared to both factors alone are indicated
| Compound group | Identification | Retention index | CPB larvae infested | coi1 | coi1 and CPB larvae infested |
|---|---|---|---|---|---|
| Aliphatics | Undecane | 1100 | 3.41*** | −0.73 | −0.21* |
| Dodecane‡ | 1200 | 0.31** | −1.02** | −1.28 | |
| Pentadecane‡ | 1496 | 0.79* | −0.62 | −0.01 | |
| Heptadecane‡ | 1699 | −0.33 | −1.88† | −3.30 | |
| Aliphatic aldehydes | Nonanal‡ | 1104 | −0.08 | −1.45* | −1.51 |
| Decanal | 1205 | 0.02 | −0.98† | −0.96 | |
| Benzenoids | Unknown phenyl ester | 959 | −0.03 | −0.56* | −0.48 |
| MeSA | 1193 | 1.41*** | −0.28 | 0.19† | |
| Monoterpenoids | Shyobunol | 1691 | 4.23*** | −7.18 | −2.60 |
| Sesquiterpenoids | α-Copaene | 1376 | 1.29*** | −0.96 | 0.12* |
| (E)-β-Caryophyllene | 1421 | 1.65*** | −0.70* | 0.47 | |
| α-Humulene‡ | 1454 | 2.25*** | −0.90† | 0.81 | |
| (E)-β-Farnesene | 1458 | 7.47*** | −1.01* | 3.62 | |
| Germacrene D-4-ol | 1576 | 2.17*** | −1.68*** | −0.27 |
Tentative identification.
Significant differences in VOC release are indicated as:
P < 0.0005;
P < 0.005;
P < 0.05;
P < 0.10.
Priming of neighbouring plants after herbivore infestation
In addition to the CPB-larvae-infested leaves, gene expression changes that might be associated to elicitation by VOCs were analysed at the same time points in the leaves of neighbouring noninfested plants. The neighbouring plants (the VOC receivers) had the same disease status (healthy, PVYNTN infected, with cv. ‘Igor’) and were of the same genotype (nontransgenic, coi1, with cv. ‘Désirée’).
Compared to the responses in the CPB-larvae-infested plants, the transcriptional changes in neighbouring plants were in general much more subtle and occurred for fewer genes. The photosynthesis-related genes RA and CAB4 were upregulated at 3 hpi only in the PVYNTN-infected leaves. Additionally, the SA-regulated genes PR1b and Glu II were significantly upregulated in healthy plants, and downregulated in PVYNTN-infected plants (Fig.2B). Interestingly, 13-LOX and COI1 were upregulated at 3 hpi in the coi1 plants only (Fig.3B). In addition, in the neighbouring coi1 plants, phenylpropanoid biosynthesis was significantly affected after infestation, whereby PAL and HCT were upregulated, and C4H was downregulated (Figs2B and 3B).
Discussion
Most studies of plant–herbivore interactions have investigated the responses of plants to single stressors. Due to the inherent complexity of the plant defence pathways, the responses to combined biotic stressors are difficult to predict from single attacker responses (Pieterse et al. 2012). To provide answers with infield relevance, more investigations into multi-attacker settings are needed. We present here a study of the interactions between potato plants and two of its economically most important attackers: the CPB and an aggressive strain of PVY, PVYNTN.
Interference between PVYNTN infection and defence signalling against herbivores
Potato virus Y infection is known to interfere with the SA and JA defence pathways in potato (Pompe-Novak et al. 2006; Baebler et al. 2009, 2014), and in the present study, we tried to determine how this further affects the defence responses against herbivores. The SA–JA antagonism hypothesis was tested by investigating the response of the coi1 plants. Direct comparisons of both viral infection and COI1 depletion need to be taken with caution as they were performed in different genetic backgrounds of potato (see Supporting Information Supplementary results for details). The SA signalling genes NPR1 and TGA2 were upregulated in both healthy and PVYNTN-infected plants, while the downstream targets of SA signalling, PR1b and Glu II, and the JA-regulated PCPI genes had lower induction of expression in the PVYNTN-infected plants after CPB larvae infestation (Fig.2A). Together with the data from the coi1 plants, this shows that the plant defence in a multi-attacker system is modulated by a complex signalling network, and not only by the combination of JA and SA signalling. ET biosynthesis and signalling components were also differentially regulated in the healthy and PVYNTN-infected plants, including some specific ERF transcription factors (Table S5, Supporting information). In Arabidopsis, the ERF transcription factor ORA59 was shown to be suppressed by SA signalling, which in turn suppressed some JA-regulated genes (Van der Does et al. 2013). Similarly, individual potato ERFs might also be involved in the modulation of the SA–JA crosstalk, and thus the attenuation of ERF expression by PVYNTN might influence this crosstalk and result in potato plants that are less protected against herbivore attack. Another group of transcription factors that were regulated specifically in these PVYNTN-infected plants after CPB infestation are the ARFs. The ARFs have important roles in plant defence against pathogens. ARF2 is established as a signalling hub that integrates responses from the ET, auxin, brassinosteriod and abscisic acid pathways in Arabidopsis (Wang et al. 2011). Lower amounts of ARF transcripts were detected in the potato leaves here after CPB larvae infestation, and this effect was strongest in the PVYNTN-infected plants (Tables1 and S5, Supporting information).
Another signalling module where the PVYNTN infection was shown to interfere with the responses to herbivore infestation was gibberellic acid (GA) signalling. Comparing the healthy and PVYNTN-infected plants that were CPB–larvae-infested, several components involved in GA metabolism and signalling were regulated in a different manner (Table S5, Supporting information). GA is known to cause accumulation of reactive oxygen species and SA, and attenuation of JA signalling in Arabidopsis (Navarro et al. 2008). A positive correlation between SA and GA, and negative feedback between JA/ET and GA in potato after PVY infection was shown previously (Baebler et al. 2014). A possible mechanism for this action suggested for Arabidopsis is via the DELLA proteins, which are growth repressor proteins that are degraded by a GA-dependent mechanism (Navarro et al. 2008; Yang et al. 2012).
Why would herbivores benefit from PVY infection?
Our initial investigation into the feeding of CPB larvae showed that PVYNTN infection promotes the potato leaves as a better food source for these CPB larvae (Fig.1). Our hypothesis was that the defence response to herbivory is attenuated in PVYNTN-infected plants and consequently lower amounts of antinutritional compounds are produced. Such indirect effects have already been reported from studies of plant interactions with several attackers. Myzus persicae, the aphid vector of PVY, showed increased growth when feeding on PVY-infected plants (Castle & Berger 1993; Boquel et al. 2011). While such a positive interaction between virus and virus vectors might have evolved to promote viral spreading (Castle & Berger 1993; Jiu et al. 2007), it would be difficult to interpret the positive growth influence of PVYNTN on the CPB larvae identified here as beneficial for the PVYNTN, as the severity of infestation can only reduce the chances of transmission. Similarly, there are no apparent benefits of this interaction between aphids and the CPB. However, aphid infestation has been shown previously to have growth benefits for caterpillars feeding on the same plants (Stout et al. 1998; Heidel & Baldwin 2004; Rodriguez-Saona et al. 2010). The virus-induced susceptibility to CPB may just be a side-effect of the potentially adaptive-induced susceptibility to aphid vectors.
The reduced antinutritional potential of PVYNTN-infected potato plants arises as a result of changes on several levels. Most notably, the PCPI genes in these PVYNTN-infected plants were induced to a much lower level than in the healthy plants (Fig.2A and Table S5, Supporting information). The role of proteinase inhibitors as antinutritional compounds is supported by several studies which show that the heterologous expression of proteinase inhibitors can make plants more resistant to herbivores (Schlüter et al. 2010; Carrillo et al. 2011). The leaves of the PVYNTN-infected plants should therefore be easier to digest by the CPB larvae. Feeding CPB larvae on high doses of cysteine protease inhibitors was previously shown to induce the expression of intestain genes to compensate for the loss of gut protease activity (Bolter & Jongsma 1995; Gruden et al. 2004). We hypothesized that lower induction of PCPI in PVY-infected leaves would be reflected also in the gene expression profile of the larval digestive proteases. The expression of intestain genes did however not change to any great extent in the CPB larvae reared on the PVYNTN-infected plants (Fig.4). This might be due to yet another set of digestive protease genes that is regulated in such multi-attacker systems, or it might be because the differences in the proteinase inhibitor levels did not reach certain threshold to be reflected in the changes in the larval gut gene expression. In addition, attenuation of the biosynthesis of α-amylase inhibitors, arginase and polyphenol oxidases (Table S5, Supporting information) with proposed antinutritional properties (Chung & Felton 2011) was detected, which might have contributed to a higher nutritional value of virus-infected plants. Tomato odorless-2 mutant plants with reduced trichome density and VOC production supported faster CPB larval growth than nontransgenic plants, which suggested that VOCs have antinutritional or deterrent activities (Kang et al. 2010). Here, in PVYNTN-infected potato plants, a lower production of the sesquiterpenoid β-barbatene was detected (Table2), which might have again contributed to the higher larval weight gain on the infected potato plants.
Another aspect of the PVYNTN effect on CPB growth might be modulation of the nutrient contents. Herbivore attack and other biotic stresses generally cause downregulation of photosynthesis genes (Nabity et al. 2009; Bilgin et al. 2010). Such herbivore damage-related downregulation of photosynthetic gene expression was observed also in the present study (e.g. RA and CAB4; see Figs2A and 3A and Table1). Suppression of photosynthesis-related genes in wounded tissue has been speculated to be the consequence of the metabolic switch from source to sink for carbohydrates regulated by cell wall invertase (CW_INV), which was shown in Solanum lycopersicum,Solanum peruvianum and Pisum sativum (Schwachtje & Baldwin 2008). We detected an increase in transcriptional activity of CW_INV after CPB larvae infestation of the potato leaves in both of the studied cultivars (Figs2A and 3A). However, an unexpected and intriguing aspect was the upregulation of photosynthetic genes in PVYNTN-infected plants at 24 hpi in parallel with the upregulation of CW_INV (Fig.2A and Table1). This might mean that the photosynthetic primary metabolites (monosaccharide sugars) accumulate to a greater extent in virus-infected leaves. Transcriptome profiling also showed significant enrichment of upregulated genes in amino acid biosynthesis processes (Table1), which might lead to increased levels of amino acids. As sugars and amino acids are the primary dietary carbon and nitrogen sources, respectively, for the larvae, these changes might additionally contribute to the higher weight gain observed for the larvae reared on the PVYNTN-infected plants.
Priming of neighbouring plants by volatiles from infested plants
Besides for their antinutritional or insect deterrent properties, plants also use VOCs to communicate with each other and to allow the priming of neighbouring plants for insect attack (Arimura et al. 2009; Ueda et al. 2012). Our hypothesis was that PVYNTN infection alters the release of VOCs which can impact the priming of neighbouring plants. Several terpenoids and aliphatic aldehydes were released in higher amounts from the CPB-larvae-infested plants (Tables2 and 3). No plant receptors for these VOCs have been identified yet, and the influence of VOCs on plant-to-plant communication through defence signalling is mainly unknown. Methyl jasmonate, MeSA and ethylene are the other potential candidates for elicitation of neighbouring plant defences (Farmer & Ryan 1990; Shulaev et al. 1997; Baldwin et al. 2006). MeSA release from CPB-larvae-infested plants was induced in the present study in both of the potato cultivars (Tables2 and 3), while methyl jasmonate and ethylene were not detected in our analytical set-up. In agreement with previous data, we observed transcriptional upregulation of some defence pathway genes and downregulation of photosynthesis genes in neighbouring healthy cv. ‘Igor’ plants (Fig.2B). Similarly, increased emission of VOCs from herbivore-attacked plants has caused upregulation of defence-related genes in several other experimental systems (Kim & Felton 2013). Nicotiana attenuata plants exposed to VOCs from clipped sagebrush were shown to downregulate photosynthesis-related genes as well (Kessler et al. 2006). On the other hand, the neighbouring PVYNTN-infected plants responded to infestation by downregulation of some defence pathway genes and upregulation of photosynthesis genes (Fig.2B). The release of only two of the VOCs was shown to be affected by PVYNTN infection; namely the release of β-barbatene was reduced, and the release of benzyl alcohol was increased (Table2). This shows that long-distance priming can be affected when potato plants are exposed to different attackers simultaneously and that the combination of different biotic stressors can have broader agro-ecological implications and consequences. Additional work in a full factorial set-up would be needed to disentangle the effects of the treatment on the emitter plant from the effects of the treatment on the receiver plant to adequately explain the differences observed.
The present study contributes to our understanding of plant responses in agro-ecosystems through an examination of the responses of potato plants exposed to two biotic stressors. We showed that susceptible potato plants activated the responses to viral infection that counteracted their antiherbivore defences. Attenuated responses through the ERF and ARF signalling hubs occurred when PVYNTN-infected plants were exposed to CPB larvae. In accordance, lower induction of antinutritional compounds was observed (e.g. proteinase inhibitors, sesquiterpenoids). PVYNTN infection therefore rendered these plants more vulnerable to subsequent CPB attack. Additionally, PVYNTN infection affected the release of VOCs, which might contributed to the priming of the neighbouring plants.
Acknowledgments
The authors thank Daniel J. Palmer and Oxana Habuštová for providing CPB eggs, and Sabine Rosahl for providing the cv. ‘Désirée’ potato plants. We also thank Neža Turnšek, Lidija Matičič, Tina Demšar and Andrea Lehr for technical support in the laboratory, and Christopher Berrie for scientific English language editorial assistance. This study was supported financially by the Slovenian Research Agency Program P4-0165 and Project J4-4165, COST actions FA0806 and BM1006, and Ad Futura grant (Slovene Human Resources Development and Scholarship Fund).
M.P. conducted the experiments, designed the qPCR assays, analysed the qPCR, RNAseq and GC-MS data, and drafted the manuscript. Š.B. and P.K. designed the qPCR assays and helped to draft the manuscript. A.R. performed the statistical analysis of the VOC data. K.G. and A.M. participated in the design of the experiments, the biological interpretation and the drafting of the manuscript. All of the authors have read and approved the final manuscript.
Data accessibility
The normalized qPCR expression matrices, the VOC release data matrix and detailed descriptions of the Materials and methods are part of the online Supporting Information. Illumina RNAseq reads have been deposited at the Sequence Read Archive under accession no. SRP040682, and the RNAseq results and RPKM table are available at GEO under the accession no. GSE56333.
Supporting Information
Additional supporting information may be found in the online version of this article.
qPCR assays used in this study.
Experimental set-up for the collection and analysis of the potato leaves and VOCs, and the CPB midgut tissue.
Table S2 Potato RNAseq mapping statistics.
Table S3 Statistical evaluation of larval weight gain in feeding assays.
Potato qPCR gene expression matrix.
Potato RNAseq defence pathway expression matrix.
CPB gene expression matrix.
VOC release data matrix.
References
- Alyokhin A, Baker M, Mota-Sanchez D, Dively G, Grafius E. Colorado potato beetle resistance to insecticides. American Journal of Potato Research. 2008;85:395–413. [Google Scholar]
- Arimura G, Garms S, Maffei M, et al. Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta. 2008;227:453–464. doi: 10.1007/s00425-007-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G-I, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant and Cell Physiology. 2009;50:911–923. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- Baebler Š, Krečič-Stres H, Rotter A, et al. PVYNTN elicits a diverse gene expression response in different potato genotypes in the first 12 h after inoculation. Molecular Plant Pathology. 2009;10:263–275. doi: 10.1111/j.1364-3703.2008.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baebler Š, Witek K, Petek M, et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. Journal of Experimental Botany. 2014;65:1095–1109. doi: 10.1093/jxb/ert447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. Herbivore arthropods benefit from vectoring plant viruses. Ecology Letters. 2005;8:70–79. [Google Scholar]
- Bilgin DD, Zavala JA, Zhu J, et al. Biotic stress globally downregulates photosynthesis genes. Plant, Cell and Environment. 2010;33:1597–1613. doi: 10.1111/j.1365-3040.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- Bolter CJ, Jongsma MA. Colorado potato beetles (Leptinotarsa decemlineata) adapt to proteinase inhibitors induced in potato leaves by methyl jasmonate. Journal of Insect Physiology. 1995;41:1071–1078. [Google Scholar]
- Bolter CJ, Dicke M, Van Loon JJA, Visser J, Posthumus MA. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. Journal of Chemical Ecology. 1997;23:1003–1023. [Google Scholar]
- Boquel S, Giordanengo P, Ameline A. Divergent effects of PVY-infected potato plant on aphids. European Journal of Plant Pathology. 2011;129:507–510. [Google Scholar]
- Carrillo L, Martinez M, Alvarez-Alfageme F, et al. A barley cysteine-proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transgenic research. 2011;20:305–319. doi: 10.1007/s11248-010-9417-2. [DOI] [PubMed] [Google Scholar]
- Castle S, Berger P. Rates of growth and increase of Myzus persicae on virus-infected potatoes according to type of virus-vector relationship. Entomologia Experimentalis et Applicata. 1993;69:51–60. [Google Scholar]
- Castle S, Mowry T, Berger P. Differential settling by Myzus persicae (Homoptera: Aphididae) on various virus infected host plants. Annals of the Entomological Society of America. 1998;91:661–667. [Google Scholar]
- Chung SH, Felton GW. Specificity of induced resistance in tomato against specialist lepidopteran and coleopteran species. Journal of Chemical Ecology. 2011;37:378–386. doi: 10.1007/s10886-011-9937-0. [DOI] [PubMed] [Google Scholar]
- Eigenbrode SD, Ding H, Shiel P, Berger PH. Volatiles from potato plants infected with Potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae) Proceedings of the Royal Society of London B Biological Sciences. 2002;269:455–460. doi: 10.1098/rspb.2001.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden K, Štrukelj B, Popovič T, et al. The cysteine protease activity of Colorado potato beetle (Leptinotarsa decemlineata Say) guts, which is insensitive to potato protease inhibitors, is inhibited by thyroglobulin type-1 domain inhibitors. Insect Biochemistry and Molecular Biology. 1998;28:549–560. doi: 10.1016/s0965-1748(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Gruden K, Kuipers AGJ, Gunčar G, et al. Molecular basis of Colorado potato beetle adaptation to potato plant defence at the level of digestive cysteine proteinases. Insect Biochemistry and Molecular Biology. 2004;34:365–375. doi: 10.1016/j.ibmb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Halim VA, Altmann S, Ellinger D, et al. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant Journal. 2009;57:230–242. doi: 10.1111/j.1365-313X.2008.03688.x. [DOI] [PubMed] [Google Scholar]
- Heidel AJ, Baldwin IT. Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant, Cell and Environment. 2004;27:1362–1373. [Google Scholar]
- Jiu M, Zhou X-P, Tong L, et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE. 2007;2:e182. doi: 10.1371/journal.pone.0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-H, Liu G, Shi F, et al. The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiology. 2010;154:262–272. doi: 10.1104/pp.110.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia. 2006;148:280–292. doi: 10.1007/s00442-006-0365-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Felton GW. Priming of antiherbivore defensive responses in plants. Insect Science. 2013;20:273–285. doi: 10.1111/j.1744-7917.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- Kogovšek P, Ravnikar M. Physiology of the potato-potato virus Y interaction. In: Lüttge U, Beyschlag W, Francis D, Cushman J, editors. Progress in Botany. Berlin, Heidelberg: Springer; 2013. pp. 101–133. [Google Scholar]
- Kruzmane D, Jankevica L, Ievinsh G. Effect of regurgitant from Leptinotarsa decemlineata on wound responses in Solanum tuberosum and Phaseolus vulgaris. Physiologia Plantarum. 2002;115:577–584. doi: 10.1034/j.1399-3054.2002.1150412.x. [DOI] [PubMed] [Google Scholar]
- Lawrence SD, Novak NG, Ju CJ-T, Cooke JEK. Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. Journal of Chemical Ecology. 2008;34:1013–1025. doi: 10.1007/s10886-008-9507-2. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annual Review of Plant Biology. 2012;63:431–450. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Annals of Botany. 2009;103:655–663. doi: 10.1093/aob/mcn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Bari R, Achard P, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Current Biology. 2008;18:650–655. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Petek M, Baebler Š, Kuzman D, et al. Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiology. 2010;10:159. doi: 10.1186/1471-2180-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petek M, Turnšek N, Buh Gašparič M, et al. A complex of genes involved in adaptation of Leptinotarsa decemlineata larvae to induced potato defense. Archives of Insect Biochemistry and Physiology. 2012;79:153–181. doi: 10.1002/arch.21017. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental Biology. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- Pompe-Novak M, Gruden K, Baebler Š, et al. Potato virus Y induced changes in the gene expression of potato (Solanum tuberosum L.) Physiological and Molecular Plant Pathology. 2006;67:237–247. [Google Scholar]
- Ponzio C, Gols R, Pieterse CMJ, Dicke M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Functional Ecology. 2013;27:587–598. [Google Scholar]
- Ramšak Ž, Baebler Š, Rotter A, et al. GoMapMan: integration, consolidation and visualization of plant gene annotations within the MapMan ontology. Nucleic Acids Research. 2014;42:D1167–D1175. doi: 10.1093/nar/gkt1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard D, Cloutier C, Michaud D. Colorado potato beetles show differential digestive compensatory responses to host plants expressing distinct sets of defense proteins. Archives of Insect Biochemistry and Physiology. 2004;55:114–123. doi: 10.1002/arch.10136. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona CR, Musser RO, Vogel H, Hum-Musser SM, Thaler JS. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. Journal of Chemical Ecology. 2010;36:1043–1057. doi: 10.1007/s10886-010-9854-7. [DOI] [PubMed] [Google Scholar]
- Schlüter U, Benchabane M, Munger A, et al. Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. Journal of Experimental Botany. 2010;61:4169–4183. doi: 10.1093/jxb/erq166. [DOI] [PubMed] [Google Scholar]
- Scholthof K-BG, Adkins S, Czosnek H, et al. Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology. 2011;12:938–954. doi: 10.1111/j.1364-3703.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz S, Weißbecker B, Klein A, Hummel HE. Host plant selection of the Colorado potato beetle as influenced by damage induced volatiles of the potato plant. Naturwissenschaften. 1997;84:212–217. [Google Scholar]
- Schwachtje J, Baldwin IT. Why does herbivore attack reconfigure primary metabolism? Plant Physiology. 2008;146:845–851. doi: 10.1104/pp.107.112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P. Differential contribution of transcription factors to Arabidopsis thaliana defense dgainst Spodoptera littoralis. Frontiers in Plant Science. 2013;4:13. doi: 10.3389/fpls.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Stout MJ, Brovont RA, Duffey SS. Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. Journal of Chemical Ecology. 1998;24:945–963. [Google Scholar]
- Thaler JS, Agrawal AA, Halitschke R. Salicylate-mediated interactions between pathogens and herbivores. Ecology. 2010;91:1075–1082. doi: 10.1890/08-2347.1. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kikuta Y, Matsuda K. Plant communication: mediated by individual or blended VOCs? Plant Signaling and Behavior. 2012;7:222–226. doi: 10.4161/psb.18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Thimm O, et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiology. 2005;138:1195–1204. doi: 10.1104/pp.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D, Leon-Reyes A, Koornneef A, et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. The Plant Cell. 2013;25:744–761. doi: 10.1105/tpc.112.108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage A, Vlaardingerbroek I, Raaymakers C, et al. Rewiring of the jasmonate signaling pathway in Arabidopsis during insect herbivory. Frontiers in Plant Science. 2011;2:47. doi: 10.3389/fpls.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hua D, He J, et al. Auxin Response Factor2(ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genetics. 2011;7:e1002172. doi: 10.1371/journal.pgen.1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bing X-L, Li M, Ye G-Y, Liu S-S. Infection of tobacco plants by a begomovirus improves nutritional assimilation by a whitefly. Entomologia Experimentalis et Applicata. 2012;144:191–201. [Google Scholar]
- Yang D, Yao J, Mei C, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proceedings of the National Academy of Sciences of the USA. 2012;109:E1192–E1200. doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Luan J-B, Qi J-F, et al. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Molecular Ecology. 2012;21:1294–1304. doi: 10.1111/j.1365-294X.2012.05457.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qPCR assays used in this study.
Experimental set-up for the collection and analysis of the potato leaves and VOCs, and the CPB midgut tissue.
Table S2 Potato RNAseq mapping statistics.
Table S3 Statistical evaluation of larval weight gain in feeding assays.
Potato qPCR gene expression matrix.
Potato RNAseq defence pathway expression matrix.
CPB gene expression matrix.
VOC release data matrix.
Data Availability Statement
The normalized qPCR expression matrices, the VOC release data matrix and detailed descriptions of the Materials and methods are part of the online Supporting Information. Illumina RNAseq reads have been deposited at the Sequence Read Archive under accession no. SRP040682, and the RNAseq results and RPKM table are available at GEO under the accession no. GSE56333.