Abstract
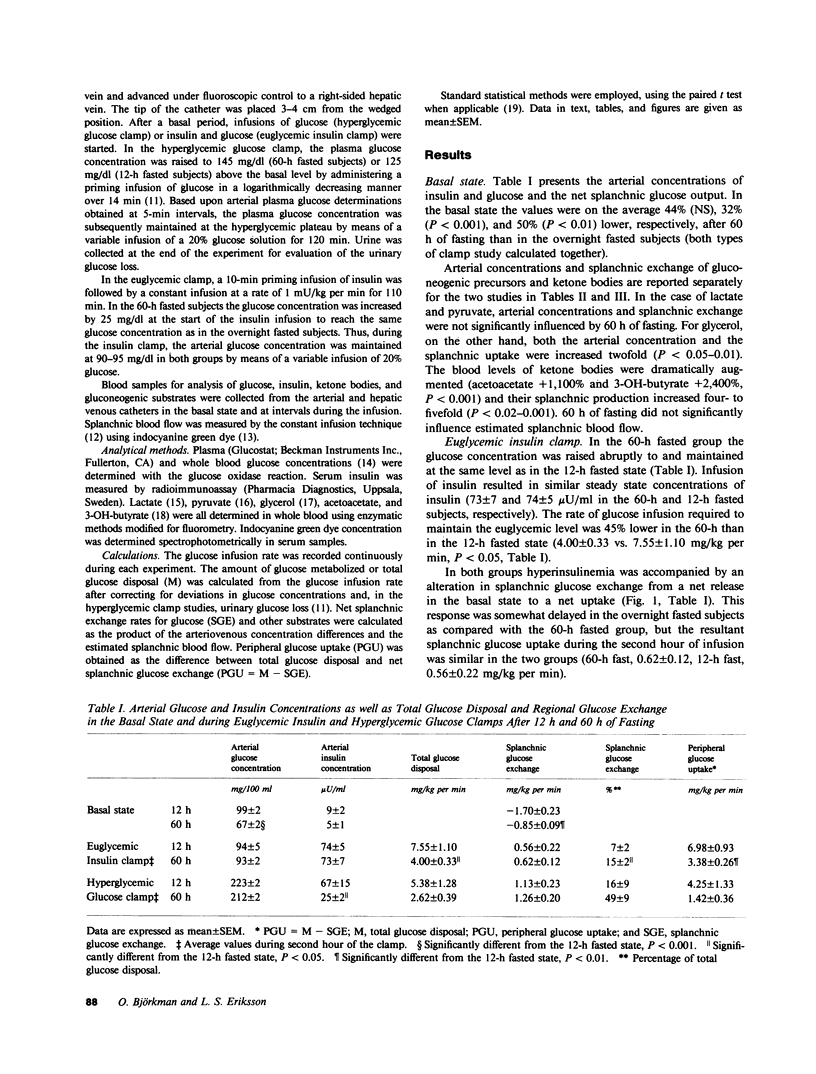
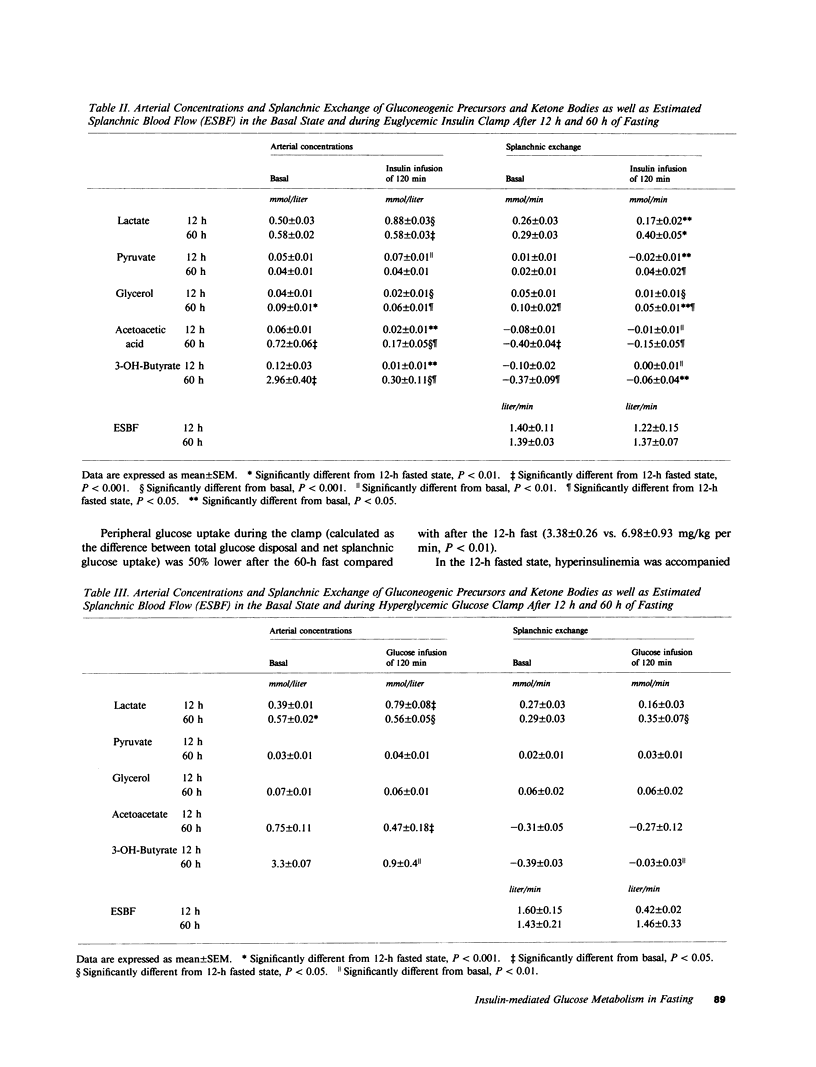
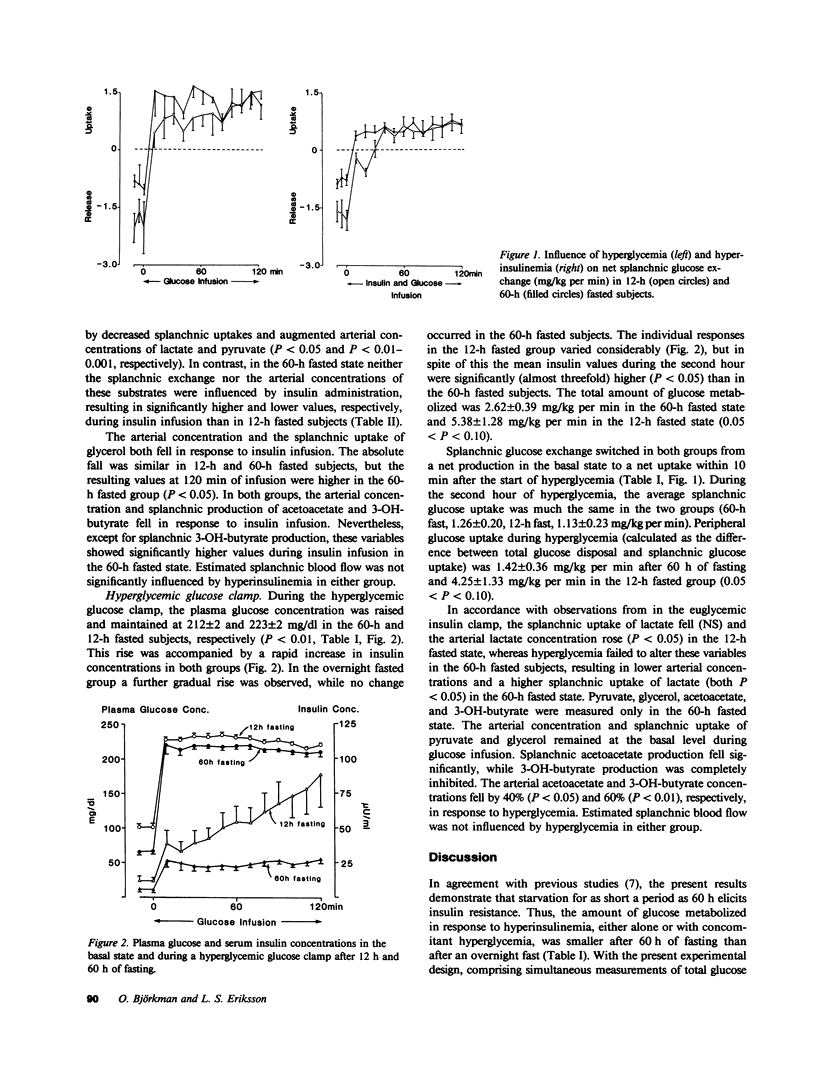
A brief period of starvation (2-3) depletes the hepatic glycogen stores but results in only a limited reduction of the muscle glycogen depots. In this situation insulin resistance contributes to the glucose intolerance, but it is not known which tissue or tissues are responsible for the decreased insulin sensitivity. The present study was therefore undertaken to examine the influence of a 60-h fast on insulin sensitivity in splanchnic and peripheral tissues in normal humans. Euglycemic (95 mg/dl) 1-mU insulin and hyperglycemic (215-225 mg/dl) glucose clamp studies were conducted for 2 h in overnight (12 h) and prolonged (60 h) fasted nonobese subjects. Splanchnic exchange of glucose and gluconeogenic precursors was measured using the hepatic vein catheter technique. During the euglycemic clamp, insulin infusion resulted in similar steady state insulin levels in 60-h and 12-h fasted subjects (73 +/- 7 vs. 74 +/- 5 microU/ml). Total glucose disposal was reduced by 45% after 60 h of fasting (4.0 +/- 0.3 vs. 7.6 +/- 1.1 mg/kg per min, P less than 0.05) and the splanchnic glucose balance reverted from a net release in the basal state (12 h fast, -1.7 +/- 0.2, and 60-h fast, -0.9 +/- 0.1 mg/kg per min, P less than 0.01) to a net uptake during the clamps that was similar after 60 h and 12 h of fasting (0.6 +/- 0.1 vs. 0.6 +/- 0.2 mg/kg per min). During the hyperglycemic clamp, insulin levels rose rapidly in all subjects. In the 12-h fasted group this rise was followed by a further gradual one, reaching significantly higher values than in 60-h fasted subjects during the second hour (67 +/- 15 vs. 25 +/- 2 microU/ml, P less than 0.05). Total glucose disposal was lower, though not significantly so, after the 60-h fast (2.6 +/- 0.4 vs. 5.4 +/- 1.3 mg/kg per min, 0.05 less than P less than 0.10), and as with the euglycemic clamp, the splanchnic glucose balance was altered from a basal net release to a net uptake during the clamp (1.3 +/- 0.2 vs. 1.1 +/- 0.2 mg/kg per min). After an overnight fast, splanchnic lactate uptake fell and the arterial lactate concentration rose in response to both hyperglycemia and hyperinsulinemia, whereas these variables were unchanged in the 60-h fasted subjects during both types of clamp studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley S. E., Ingelfinger F. J., Bradley G. P., Curry J. J. THE ESTIMATION OF HEPATIC BLOOD FLOW IN MAN. J Clin Invest. 1945 Nov;24(6):890–897. doi: 10.1172/JCI101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERRICK G. R., STEIN S. W., LEEVY C. M., DAVIDSON C. S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Finger F. E., Lacy W. W. Differential sensitivity of glycogenolysis and gluconeogenesis to insulin infusions in dogs. Diabetes. 1976 Apr;25(4):283–291. doi: 10.2337/diab.25.4.283. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Menzel P. H., Boden G., Owen O. E. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974 Oct;54(4):981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Beckmann J. On the relation of glucose and insulin secretion in the fasting state. Metabolism. 1980 Jan;29(1):23–27. doi: 10.1016/0026-0495(80)90093-1. [DOI] [PubMed] [Google Scholar]
- Lilavivathana U., Campbell R. G., Brodows R. G. Control of insulin secretion during fasting in man. Metabolism. 1978 Jul;27(7):815–821. doi: 10.1016/0026-0495(78)90216-0. [DOI] [PubMed] [Google Scholar]
- Maehlum S. Muscle glycogen synthesis after a glucose infusion during post-exercise recovery in diabetic and non-diabetic subjects. Scand J Clin Lab Invest. 1978 Jun;38(4):349–354. doi: 10.3109/00365517809108433. [DOI] [PubMed] [Google Scholar]
- Newman W. P., Brodows R. G. Insulin action during acute starvation: evidence for selective insulin resistance in normal man. Metabolism. 1983 Jun;32(6):590–596. doi: 10.1016/0026-0495(83)90029-x. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Fürst P., Hultman E. Carbohydrate metabolism of the liver in normal man under varying dietary conditions. Scand J Clin Lab Invest. 1973 Dec;32(4):331–337. doi: 10.3109/00365517309084356. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest. 1973 Dec;32(4):325–330. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- Ravussin E., Bogardus C., Schwartz R. S., Robbins D. C., Wolfe R. R., Horton E. S., Danforth E., Jr, Sims E. A. Thermic effect of infused glucose and insulin in man. Decreased response with increased insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Sep;72(3):893–902. doi: 10.1172/JCI111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch-Norlund A. E., Bergström J., Hultman E. Muscle glycogen and glycogen synthetase in normal subjects and in patients with diabetes mellitus. Effect of intravenous glucose and insulin administration. Scand J Clin Lab Invest. 1972 Sep;30(1):77–84. doi: 10.3109/00365517209081094. [DOI] [PubMed] [Google Scholar]
- Saccà L., Orofino G., Petrone A., Vigorito C. Differential roles of splanchnic and peripheral tissues in the pathogenesis of impaired glucose tolerance. J Clin Invest. 1984 Jun;73(6):1683–1687. doi: 10.1172/JCI111375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Efendić S., Luft R., Hagenfeldt L., Björkman O., Felig P. Influence of somatostatin on splanchnic glucose metabolism in postabsorptive and 60-hour fasted humans. J Clin Invest. 1977 Feb;59(2):299–307. doi: 10.1172/JCI108641. [DOI] [PMC free article] [PubMed] [Google Scholar]



