Abstract
Background
To investigate the inhibitory role of pterostilbene in pancreatic cancer, we conducted a genomic analysis of pterostilbene-treated pancreatic cancer cells. We also investigated the effect of pterostilbene upon the carcinogenic markers, manganese superoxide dismutase, cytochrome C, Smac/DIABLO, and STAT3 phosphorylation in vitro. The antiproliferative effects of pterostilbene were further evaluated in an in vivo model.
Methods
Pancreatic cancer cells were treated with pterostilbene and evaluated with DNA microarray analysis. Pterostilbenetreated cells were analyzed for cytochrome C, Smac/DIABLO, manganese superoxide dismutase (MnSOD)/antioxidant activity, and STAT3 phosphorylation using ELISA. Data were statistically analyzed using ANOVA. Pterostilbene was then administered to nude mice for 8 weeks, and tumor growth rates were recorded and statistically analyzed.
Results
Microarray analysis of pterostilbene-treated cells revealed upregulation of pro-apoptosis genes. In vitro, pterostilbene treatment altered levels of phosphorylated STAT3, MnSOD/antioxidant activity, cytochrome C, and Smac/DIABLO. In nude mice, oral pterostilbene inhibited tumor growth rates.
Conclusion
Pterostilbene alters gene expression in pancreatic cancer and increases the antiproliferative markers cytochrome C, Smac/DIABLO, and MnSOD/antioxidant activity. It was also shown to inhibit phosphorylated STAT3, a marker of accelerated tumorigenesis, and decrease pancreatic tumor growth in vivo. Further studies are warranted to elucidate the effects of pterostilbene in humans.
Keywords: Pancreatic cancer, Pterostilbene, Apoptosis
Introduction
Pancreatic cancer is the fourth leading cause of cancerrelated death in the USA with an overall estimated 5-year survival of less than 5 %.1,2 Poor prognosis in pancreatic cancer is attributed to late stage diagnosis, local tumor aggression, and high rates of chemoresistance.1,2 Surgical resection of pancreatic cancer can be curative; however, only 15–20 % of pancreatic cancer patients are surgical candidates.2 Chemotherapeutic options include gemcitabine, which is the only approved chemotherapeutic agent for treatment of pancreatic cancer, with a dismal response rate of 5 %.1,2 Recently, FOLFIRINOX has been shown to be efficacious for metastatic pancreatic cancer, but with significant side effects.3 Therefore, the quest for novel treatment options in the management of pancreatic cancer is of monumental significance.
We have previously demonstrated that pterostilbene (3, 5-dimethoxy-4-hydroxystilbene), an antioxidant found in blueberries, reduces proliferation in pancreatic cancer in vitro through mitochondrial membrane depolarization, caspase 3/7 activation, and cell cycle arrest.1,4 To further elucidate the mechanism of pterostilbene, we conducted a microarray genomic analysis of pterostilbene-treated pancreatic cancer cells to identify upregulated and downregulated genes. Manganese superoxide dismutase (MnSOD), an antioxidant located in the mitochondria, was found to be significantly upregulated by pterostilbene in the genomic microarray analysis. Hence, we further investigated the effect of pterostilbene upon this enzyme.
Interestingly, studies have shown that pancreatic cancer cells have decreased expression of MnSOD as compared to normal cells. MnSOD overexpression studies in pancreatic cancer show that increased MnSOD activity correlates with decreased rates of tumor growth.5-8 We hypothesized that pterostilbene would increase MnSOD enzymatic activity in a dose-dependent manner and decrease intracellular oxidative stress.
Additionally, we investigated the effect of pterostilbene upon two mitochondrial markers of intrinsic apoptosis, cytochrome C and second mitochondrially derived activator of caspase (Smac/DIABLO). During apoptosis and following the loss of outer mitochondrial membrane polarization, both cytochrome C and Smac/DIABLO are released from the intermembrane mitochondrial space into the cytosol.4 Upon its release into the cytosol, cytochrome C directly induces caspase activation leading to programmed cell death. Smac/DIABLO, however, indirectly induces apoptosis by blocking inhibitor of apoptosis proteins, which in turn allows apoptosis to proceed.4 In addition, in vivo pancreatic tumor models show that Smac mimetic proteins induce tumor regression and prolong survival in mouse models both alone and in combination with gemcitabine, making it a potential therapeutic target of pterostilbene.9 Based on our genomic analysis, we hypothesized that pterostilbene would increase cytosolic levels of cytochrome C and Smac/DIABLO in a dose-dependent fashion further signifying a mitochondrially derived mechanism of cell death.
We also investigated the effect of pterostilbene upon STAT3 phosphorylation, a marker of the constitutively active Janus kinase/signal transducer and activator of transcription-3 (JAK/STAT3) pathway, which is associated with pancreatic tumorigenesis, decreased apoptosis, and enhanced metastatic potential.10-12 We hypothesized that pterostilbene treatment would decrease phosphorylated STAT3, thereby indicating downregulation of the JAK/STAT3 pathway. The final component of our study was a mouse xenograft model used to determine whether the inhibitory effects of pterostilbene were applicable to human pancreatic tumors in vivo. We hypothesized that orally administered pterostilbene would inhibit pancreatic adenocarcinoma growth in a nude mouse model.
Materials and Methods
Pterostilbene
Pterostilbene (3, 5-dimethoxy-4-hydroxystilbene) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and reconstituted into dimethyl sulfoxide (DMSO) solution (Sigma-Aldrich, St. Louis, MO, USA). Experimental pterostilbene concentrations were formulated using sterile media dilutions.
Cell Culture
MIA PaCa-2 and PANC-1 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained as monolayers in T-25 flasks in Dulbecco’s modification eagle’s medium (Mediatech, Inc., Herndon, VA, USA) supplemented with 10 % fetal bovine serum (HyClone, Logan, UT, USA) and 1 % penicillin/streptomycin (Mediatech, Inc.). Flasks were kept at 37 °C in a water-jacketed 5 % CO2 incubator (Fisher Scientific, Houston, TX, USA). Cells were harvested from culture monolayers at 80–90 % confluency and rinsed with sterile phosphate-buffered saline (PBS; Mediatech, Inc.). Live cells were detached using 0.25 % trypsin in 0.1 % EDTA (Mediatech, Inc.), centrifuged at 1,000 rpm for 5 min, and resuspended in PBS to obtain specific concentrations for cytochrome C, Smac/DIABLO, STAT3, and MnSOD/antioxidant assays.
RNA Preparation and Microarray Analysis
MIA PaCa-2 cells were treated with 50 μM pterostilbene for 3, 6, 9, and 12 h. A final cell concentration of 6×106 per 200 μL was obtained, and RNA isolation was performed by direct extraction of adherent cells from the tissue culture plate at 70 % confluency using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The resulting RNA was quantified using the Nanodrop spectrophotometer and analyzed for integrity using the Agilent Bioanalyzer 2100. RNA amplification, fragmentation, and labeling were performed using the Ovation V2 and Encore reagents by NuGEN technologies.13,14
Microarray Data Analysis
Hybridization mixes were prepared using 4 μg of the final amplified sample and hybridized to the Human Genome U133 2.0 Plus GeneChip array (Affymetrix, Santa Clara, CA, USA) as per manufacturer’s recommendations for 16 h at 45 °C at 60 rpm, double stained with streptavidin-PE using the Affymetrix 450 fluidics station, and scanned with the Affymetrix 3000-G7 scanner. Data analysis was performed at the university Bioinformatic Core Facility using Bioconductor Software and analyzed using Robust Multichip Analysis.
Cytochrome C ELISA
MIA PaCa-2 and PANC-1 cells were plated at 106 cells per well into 100-mm culture dishes and allowed to adhere for 24 h. Cells were incubated with a DMSO control or 25-, 50-, and 75-μM concentrations of pterostilbene for 24 h. Cell lysates were prepared using a digitonin cell permeabilization assay protocol followed by centrifugation at 1,000×g for 5 min to obtain cytosolic and mitochondrial fractions. Cytosolic fractions were then evaluated for cytochrome C according to the ELISA protocol (Enzo Life Sciences, Plymouth Meeting, PA, USA). Protein analysis was performed, and cytochrome C was measured and expressed in picograms per milligram.
Smac/DIABLO ELISA
MIA PaCa-2 and PANC-1 cells were plated at 106 cells per well into 100-mm culture dishes and allowed to adhere for 24 h. Cells were incubated with a DMSO control or 25-, 50-, and 75-μM concentrations of pterostilbene for 24 h. Cell lysates were prepared using a digitonin cell permeabilization assay protocol followed by centrifugation at 1,000×g for 5 min to obtain cytosolic and mitochondrial fractions. Cytosolic fractions were then evaluated for Smac/DIABLO using a sandwich ELISA protocol (R&D Systems, Minneapolis, MN, USA). Protein analysis was performed, and Smac/DIABLO was measured and expressed in picograms per milligram.
MnSOD Activity
MIA PaCa-2 and PANC-1 cells were plated at 106 cells per well into 100-mm culture dishes and allowed to adhere for 24 h. Cells were incubated with a DMSO control or 25- and 50-μM concentrations of pterostilbene for 48 h to optimize enzymatic activity with minimal cell cytotoxicity. Cells were lysed with Triton X-100 cell lysis buffer and centrifuged at 12,000×g for 5 min. Supernatants were assayed for MnSOD activity after addition of 2 mM cyanide to block Cu/Zn-SOD activity (Cell Technology Inc., Mountain View, CA, USA). A protein analysis was performed, and MnSOD enzymatic activity was measured and expressed in picograms per milligram.
Antioxidant Activity
Detection of malondialdehyde (MDA), a by-product of lipid peroxidation, was used as a marker of antioxidant activity. MIA PaCa-2 and PANC-1 cells were plated at 3×105 cells per well into six-well plates and allowed to adhere for 24 h. Cells were then incubated with DMSO and 25-, 50-, and 75-μM concentrations of pterostilbene for 24 h. Cells were rinsed with media and incubated with 500 μM hydrogen peroxide for 1 h. Cell lysates were prepared according to the Oxiselect MDA Adduct ELISA protocol (Cell Biolabs, San Diego, CA).
STAT3 ELISA
STAT3 phosphorylation was used as a marker of JAK/STAT3 activation. MIA PaCa-2 and PANC-1 cells were plated in 100-mm dishes at 106 cells per dish and allowed to adhere overnight. Both cell lines were treated with 25, 50, and 75 μM of pterostilbene for 24 and 48 h. Cells were lysed, and analysis of STAT3 phosphorylation was performed using the pSTAT3 ELISA (Invitrogen, Carlsbad, CA, USA). Quantitative values of STAT3 phosphorylation were calculated based on the standard curve and adjusted for protein sample content using a standard protein assay.
Statistical Analysis
GraphPad Prism software using ANOVA and Tukey post hoc analysis was used to analyze experimental data.
Tumor Volume
Animals
The nude mice protocol was reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee. Forty Nu/Nu female mice were obtained from Charles River Laboratories, Canada, and housed in the health sciences research facility at the University of Vermont. The mice were allowed to acclimate for a period of 1 week before the start of the study. The mice were housed under barrier conditions in Lab Products Micro-Isolator Ventilated Racks and kept on a 12-h day/night cycle with constant temperature and environmental control (70–72°F, 30–70 % humidity).
Oral Pterostilbene Dosing
Pterostilbene concentrations were prepared by adding pterostilbene into DMSO solution and autoclaved water. Animals received oral pterostilbene or a DMSO control diet. Oral dosing of pterostilbene was grouped into three categories of 100, 500, and 1 mg/kg/day, via oral gavage using a stainless steel gavage needle attached to a sterile tuberculin syringe.
Tumor Inoculation
Mice were anesthetized with 3 % isofluorane, purchased from the Veterinary Department at the University of Vermont and supplied by Webster Veterinary Supply (Sterling, MA), while in a laminar flow hood with appropriate sterile precautions and conditions. After ensuring that proper anesthesia was achieved, the right flank of each animal was prepped in the usual sterile fashion, and 6×106 MIA PaCa-2 cells with PBS, total volume of 200 μL, was injected into the subcutaneous tissue. The mice were monitored for adverse reactions and allowed to recover for 1 week after the inoculation before undergoing tumor volume assessment.
Tumor Measurement/Animal Weight
Tumor volume was measured in cubic millimeters three times weekly using the formula TV 0[L ×(W2)]/2. The smallest tumor volume acceptable for initiation of treatment was 150 mm3. The length (caudal–cranial axis) and width (ventral–dorsal axis) were measured using digital display calipers (Fisher Scientific, Houston, TX, USA). If variations in tumor dimensions were observed, repeat measurements were performed, and the average was recorded. The mice were weighed three times weekly on a Mettler PM3000 digital scale (Hightstown, NJ) to monitor potential undue weight loss of the animals and to observe any interactions between tumor burden and overall health. If multiple tumors developed on one mouse, the combined total volume of the tumors was used as the recorded tumor volume. Animals underwent a total of 8 weeks of oral gavage with the appropriate concentration solution before they were eligible for removal from the protocol via euthanasia. Mice that completed 8 weeks of treatment were euthanized, and tumors were procured and immediately stored in formalin.
Statistical Analysis
In conjunction with the Medical Biostatistics Department at the University of Vermont, a standard exponential growth model was constructed. The following formula was used: V0P0×Exp (P1 × T), where P00tumor volume at T00 and P10growth rate parameter since the rate of change in the tumor volume is dV/dT0P1 × V. The parameter estimates for P0 and P1 were obtained for each group, along with the 95 % confidence intervals, using a nonlinear regression approach.
Tumor Histology
Xenograft tumors were sectioned by the Pathology Department at the University of Vermont/Fletcher Allen Health Care. Tumors were stained with hematoxylin and eosin (H&E) for histologic evaluation and examined under light microscopy. Morphologic features were compared between pterostilbene treatment and control groups.
Results
Pterostilbene Genomic Profile
After 12 h, the samples demonstrated numerous upregulated (greater than fourfold) and downregulated (greater than threefold) genes (Table 1). Upregulated genes included heme oxygenase, DNA-damage-inducible transcript 3 (DDIT-3), growth differentiation factor 15, and mitochondrial superoxide dismutase. Downregulated genes included beta 8 integrin, cytochrome P450, and dehydrogenase/reductase (SDR Family).
Table 1.
List of genes up- or downregulated by pterostilbene by at least threefold with associated p values
| Gene name | Ratio | p value | Identifier |
|---|---|---|---|
| Upregulated | |||
| Heme oxygenase (decycling) 1 | 16.96 | 0.00444 | NM_002133 |
| DNA-damage-inducible transcript 3 | 6.3 | 0.00622 | BC003637 |
| Chemokine (C-X-C motif) ligand 3 | 6.08 | 0.03221 | NM_002090 |
| Growth differentiation factor 15 | 4.66 | 0.00273 | AF003934 |
| Superoxide dismutase 2, mitochondrial | 4.53 | 0.03353 | AL050388 |
| Heat shock 70 kDa protein 9 (mortalin) | 4.33 | 0.00124 | AK023317 |
| Seryl-tRNA synthetase | 4.33 | 0.00251 | BF111108 |
| Fucosyltransferase 1 | 4.05 | 0.00313 | NM_000148 |
| Integrin, beta 8 | 4.03 | 0.01345 | BF513121 |
| Heterogeneous nuclear ribonucleoprotein | 4.01 | 0.01924 | AL713781 |
| Downregulated | |||
| Zinc finger protein 488 | 4.32 | 0.04088 | AIO56483 |
| Integrin, beta 8 | 4.03 | 0.01345 | BF513121 |
| Cytochrome P450, family 1, subfamily A | 3.58 | 0.00029 | NM_000499 |
| Dehydrogenase/reductase (SDR family) | 3.15 | 0.01714 | BC015030 |
Pterostilbene Increases Cytosolic Cytochrome C
In both MIA PaCa-2 and PANC-1 cells, pterostilbene treatment produced an increase in cytosolic cytochrome C levels. In the MIA PaCa-2 cell line, 25 and 50 μM pterostilbene increased cytosolic levels of cytochrome C (p<0.001), and in PANC-1 cells, pterostilbene treatment produced a doserelated increase in cytosolic cytochrome C levels at concentrations of 25 μM (p<0.001), 50 μM (p<0.01), and 75 μM (p<0.05) (Fig. 1).
Fig. 1.
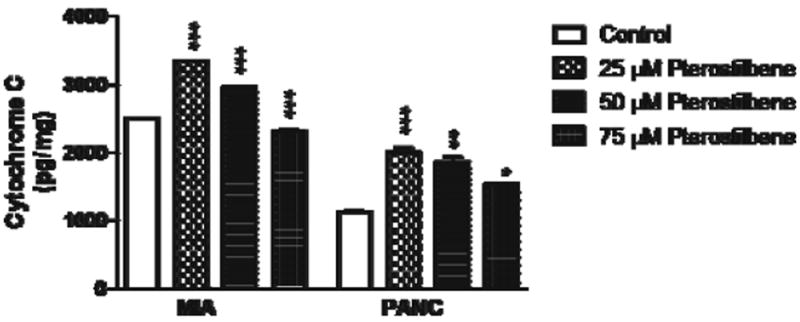
MIA PaCa-2 cells treated with 25 and 50 μM pterostilbene had an increase in cytosolic cytochrome C when compared to control (***p< 0.001). However, 75 μM decreased cytosolic cytochrome C. PANC-1 cells treated with 25, 50, and 75 μM showed a dose-related increase in cytosolic cytochrome C release when compared to control (***p<0.001; **p<0.01; *p<0.05)
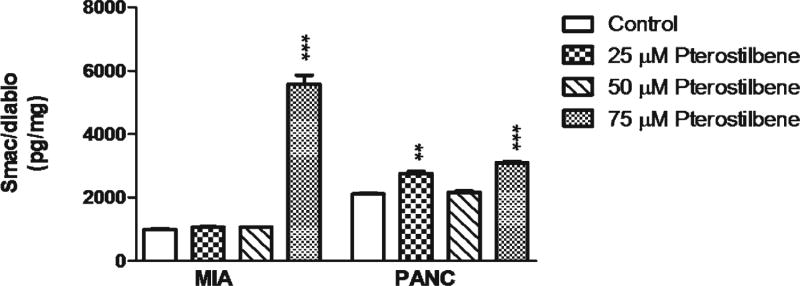
Pterostilbene Increases Cytosolic Smac/DIABLO
In both MIA PaCa-2 and PANC-1 cells, pterostilbene treatment produced an increase in cytosolic Smac/DIABLO. In the MIA PaCa-2 cell line, 75 μM pterostilbene increased (p< 0.001) Smac/DIABLO. In PANC-1 cells, pterostilbene treatment increased cytosolic Smac/DIABLO at concentrations of 25 μM (p<0.01) and 75 μM (p<0.001) (Fig. 2).
Fig. 2.
MIA PaCa-2 cells treated with 75 μM pterostilbene had an increase in Smac/DIABLO when compared to control (***p<0.001). PANC-1 cells treated with 25 and 75 μM pterostilbene showed a dose-related increase in Smac/DIABLO when compared to control (***p<0.001; **p<0.01)
Pterostilbene Increases MnSOD Activity
Pterostilbene treatment upregulated MnSOD activity in MIA PaCa-2 and PANC-1 cells at concentrations of 25 and 50 μM (p<0.01), demonstrating pterostilbene’s effect upon inducible mitochondrial enzymatic activity (Fig. 3).
Fig. 3.

MIA PaCa-2 and PANC-1 cells treated with 25 and 50 μM pterostilbene had a significant increase in MnSOD enzymatic activity when compared to controls (**p<0.01)
Pterostilbene Decreases Oxidative Stress
Pterostilbene treatment for 24 h reduced the amount of MDA protein adducts detected after treatment with hydrogen peroxide, indicating an increase in antioxidant activity (Fig. 4). In the MIA PaCa-2 cell line, all concentrations of pterostilbene decreased MDA protein adducts (p<0.05, p<0.01); however, in PANC-1, the effect reached statistical significance only with the 75-μM dose of pterostilbene (p<0.01).
Fig. 4.
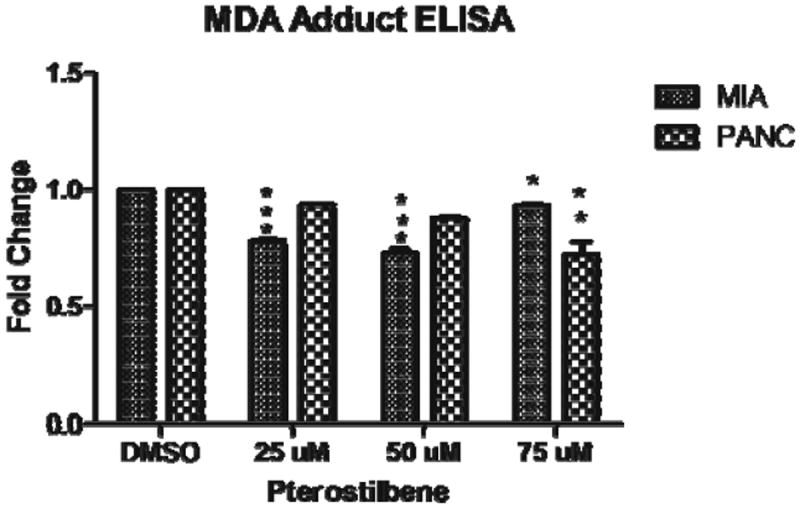
In MIA PaCa-2 cells, pterostilbene decreased MDA adducts (*p<0.05; **p<0.01). In PANC-1, MDA protein adducts were decreased with 75 μM pterostilbene (**p<0.01)
Pterostilbene Inhibits Constitutive STAT3 Phosphorylation
Treatment with 25, 50, and 75 μM of pterostilbene produced a dose- and time-dependent decrease in constitutive STAT3 phosphorylation at 24- and 48-h time points in both MIA PaCa-2 (p<0.05, p<0.01) and PANC-1 cells (p<0.001) (Fig. 5).
Fig. 5.
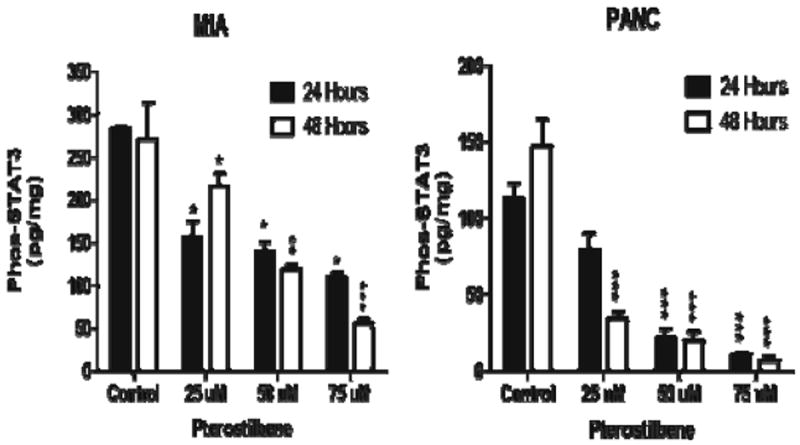
Inhibition of phosphorylated STAT3 was seen in both MIA PaCa-2 (*p< 0.05; **p<0.01; ***p<0.001) and PANC-1 cells (*** p<0.001) with pterostilbene treatment
Oral Pterostilbene Decreases Pancreatic Tumor Volume in Mice
There was a 100 % survival rate amongst mice receiving pterostilbene treatment and a 66 % survival rate in the control group (p<0.01). The calculated growth rate parameter for the control group was 0.0522. For pterostilbene-fed mice, the calculated growth rate parameters were 0.0260 for the 100-μg/kg/day group, 0.0264 for the 500-μg/kg/day group, and 0.0371 for the 1-mg/kg/day group. Oral administration of pterostilbene significantly inhibited tumor growth in the 100- and 500-μg/kg/day groups versus controls, based on the calculated 95 % confidence interval (Table 2).
Table 2.
Effect of oral pterostilbene on pancreatic tumor growth in nude mice
| Animal group | Number of animals | Growth parameter | Standard deviation | Confidence interval (95 %) | Doubling time |
|---|---|---|---|---|---|
| Control (DMSO + water) | n06 | P0067.08 | 25.31 | 17.20/116.96 | 10.55–17.91 (D) |
| P100.0522 | 0.0069 | 0.0387/0.0657 | |||
| Group 1 (100 μg/kg/day) | n06 | P00313.18 | 79.61 | 155.98/470.37 | 19.42–42.27 (D) |
| P100.0260 | 0.0049 | 0.0164/0.0357 | |||
| Group 2 (500 μg/kg/day) | n09 | P00407.61 | 76.66 | 256.67/558.55 | 20.51–36.67 (D) |
| P100.0264 | 0.0038 | 0.0189/0.0338 | |||
| Group 3 (1 mg/kg/day) | n07 | P00253.72 | 40.68 | 173.45/333.98 | 16.01–22.50 (D) |
| P100.0371 | 0.0032 | 0.0308/0.0433 |
P0 tumor volume at time 00; P1 tumor growth rate parameter, calculated by exponential growth model V0P0×Exp (P1×T)
In Vivo Tumor Histology
Histologic evaluation of the mouse tumors found poorly differentiated adenocarcinoma consisting of markedly pleomorphic round to ovoid cells with abundant eosinophilic cytoplasm and eccentric nuclei (Fig. 6b and d). The nuclei had vesicular chromatin and prominent nucleoli. Glandular formation was not seen as the tumors had a solid nested pattern with pushing borders. Rare foci of skeletal muscle invasion were evident in the control tumors and the 500-μg/kg treated tumors. Perineural invasion, a typical finding in pancreatic adenocarcinoma specimens, was seen at the edge of the 500-μg/kg treated tumors. The morphologic features of the tumors were similar throughout the treatment groups and did not vary significantly from the control tumors (Fig. 6a–d). All tumors showed prominent central necrosis comprising 60–80 % of the tissue, which did not significantly differ among treatment groups.
Fig. 6.
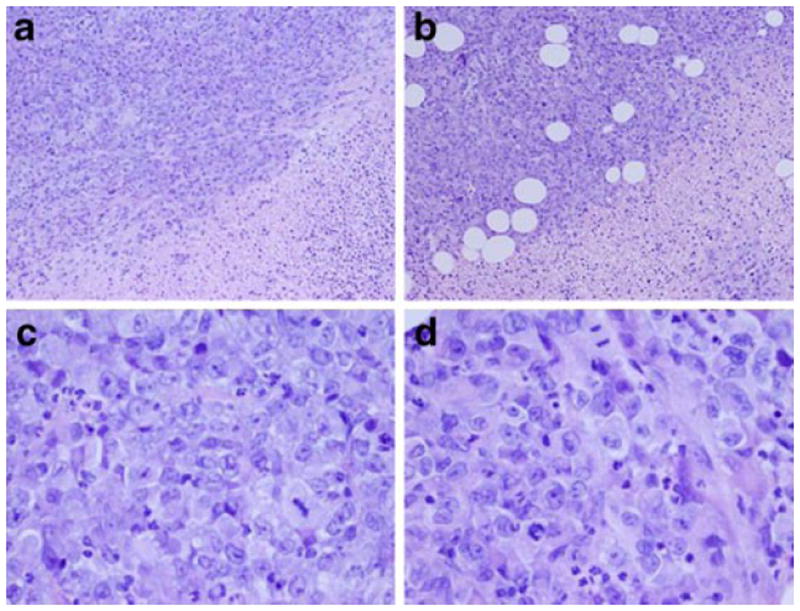
a Control ×100, viable tumor in upper left, necrosis in lower right. b Treatment group ×100, viable tumor in upper left, necrosis in lower right. The volume of necrosis was approximately the same for all treatment doses and the control. c Control ×400, cell morphology and cytology. d Treatment group ×400, cell morphology and cytology were similar for all treatment doses and the control
Discussion
Resveratrol has been considered an important component of the Mediterranean diet as it is found in red wine. Unfortunately, a liter bottle of red wine contains an average of 3 mg of resveratrol, which has only an approximate 30 % bioavailability.15 Pterostilbene, an analog of resveratrol (trans-3, 5-dimethoxy-4-hydroxystilbene), is a phenylpropanoid-derived plant compound found in blueberries, several types of grapes, and tree wood.16 In nature, pterostilbene acts as a phylotaxin and is significantly increased during times of plant stress in response to microbial attack, ultraviolet light, and irradiation mainly as a protective mechanism.17 Additionally, pterostilbene has nearly three times the bioavailability of resveratrol.18
First, we have demonstrated in the pancreatic cancer cell line MIA PaCa-2 that pterostilbene causes alteration of antiapoptosis and pro-apoptosis gene expression using DNA microarray analysis, its net effect supporting pro-apoptosis. Our results show for the first time that pterostilbene significantly upregulates genomic expression of DDIT-3, growth differentiation factor 15, also known as macrophage inhibitory cytokine 1, and MnSOD, which are all associated with induction of pancreatic cancer cell death.5-8,19,20 Pterostilbene also significantly upregulated heme oxygenase-1 (HO-1), an inducible stress-response protein. Enhanced HO-1 expression is thought to increase resistance to cell death; however, in pancreatic cancer cells, HO-1 is also upregulated in response to radiation, gemcitabine, and oxidative stress suggesting an anticancer effect.21
Based on the results of the genomic analysis, we performed a MnSOD enzymatic activity assay in two pterostilbene-treated pancreatic cancer cell lines to determine if increased genomic expression of MnSOD translated into increased mitochondrial enzymatic activity. MnSOD was chosen as a gene to further investigate because numerous studies demonstrate a significant relationship between MnSOD expression and cancer growth. The results confirm that pterostilbene’s upregulation of MnSOD at the genomic level also translates into increased enzymatic activity, indicating that the mitochondrially derived enzyme plays a significant role in promoting pterostilbene’s effect on pancreatic cancer cells. Likewise, pterostilbene treatment decreased MDA adduct production indicating increased antioxidant activity. In addition to upregulating MnSOD activity, pterostilbene also significantly increased cytosolic levels of the apoptotic markers cytochrome C and Smac/DIABLO which may suggest mitochondrial release of both proteins from the inner mitochondrial membrane. Based on these findings, we may infer that pterostilbene promotes cell death and decreased proliferation in pancreatic cancer in vitro through a mitochondrially derived mechanism. Moreover, we have shown that oral pterostilbene administration in an in vivo model inhibits pancreatic cancer growth, which further supports the results obtained in our in vitro model. In our present study, in vitro and in vivo pterostilbene doses were determined based on prior pharmacokinetic studies in rats; however, the exact relationship between in vitro and in vivo dosing is currently unknown.
In conclusion, pancreatic cancer is associated with high rates of chemoresistance and low chances of 5-year survival.1,2 We have now shown that the natural dietary compound pterostilbene inhibits pancreatic cancer both in vitro and in vivo. Further studies are needed to elucidate the chemotherapeutic and chemo-preventative potential of pterostilbene in the treatment of pancreatic cancer in preclinical trials.
Acknowledgments
Microarray analysis was conducted by the Vermont Genetics Network microarray facility through grant number 2P20RR016462 from the INBRE Program of the National Center for Research Resources (NCRR). Bioinformatic support and data analysis was conducted by Dr. Jeff Bond at the University of Vermont Bioinformatics shared resource facility.
Contributor Information
Denise Elizabeth McCormack, Email: Denise.McCormack@wcthealthnetwork.org, Department of Surgery, Danbury Hospital, 111 Osborne Street, Danbury, CT 06810, USA.
Patrick Mannal, Email: Patrick.Mannal@vtmednet.org, Department of Surgery, Fletcher Allen Health Care, University of Vermont, 111 Colchester Ave, Burlington, VT 05401, USA.
Debbie McDonald, Email: Debbie.McDonald@vtmednet.org, Department of Surgery, Fletcher Allen Health Care, University of Vermont, 89 Beaumont Ave, Given Building D, Room 319C, Burlington, VT 05405, USA.
Scott Tighe, Email: Scott.Tighe@uvm.edu, Advanced Genome Technology Lab, University of Vermont, 149 Beaumont Avenue, Health Science Research Building Room 305, Burlington, VT 05405, USA.
Joshua Hanson, Email: Joshua.Hanson@vtmednet.org, Department of Pathology, Fletcher Allen Health Care, University of Vermont, 111 Colchester Ave, Burlington, VT 05401, USA.
David McFadden, Email: DMcFadden@uchc.edu, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA.
References
- 1.Mannal P, Alosi J, Schneider J, et al. Pterostilbene inhibits pancreatic cancer in vitro. J Gastrointest Surg. 2010;14(5):873–9. doi: 10.1007/s11605-010-1164-4. [DOI] [PubMed] [Google Scholar]
- 2.Sharma C, Eltawil K, Renfrew P, et al. Advances in diagnosis treatment and palliation of pancreatic clinical carcinoma: 1990–2010. World J Gastroenterol. 2011;17(7):867–97. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. NEJM. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen J, Weydert C, Hinkhouse M, et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63(6):1297–303. [PubMed] [Google Scholar]
- 6.Ough M, Lewis A, Zhang Y, et al. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic Res. 2004;38(11):1223–33. doi: 10.1080/10715760400017376. [DOI] [PubMed] [Google Scholar]
- 7.Weydert C, Roling B, Liu J, et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2(4):361–9. [PubMed] [Google Scholar]
- 8.Hurt E, Thomas S, Peng B, et al. Molecular consequences of SOD2 expression in epigenetically silenced pancreatic carcinoma cell lines. Br J Cancer. 2007;97(8):1116–23. doi: 10.1038/sj.bjc.6604000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dineen S, Roland C, Greer R, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–61. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Cao J, Huang KJ, et al. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006;97(12):1417–23. doi: 10.1111/j.1349-7006.2006.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholz A, Heinze S, Detjen KM, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125(3):891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 12.Toyonaga T, Nakano K, Nagano M, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201(1):107–16. doi: 10.1016/s0304-3835(03)00482-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Chen P, Dafforn A, et al. Macroresults for Microarrays/Genomic and Proteomic Sample Preparation. World Trade Center; Boston, Massachusetts: 2003. Ribo-SPIA™: A Novel Isothermal Linear Amplification Method for Transcriptome Analysis of Very Small Samples. [Google Scholar]
- 14.Chen P, Deng G, Iglehart D, et al. A Novel Global mRNA Amplification Method For Gene Expression Analysis in Very Small Total RNA Samples. Molecular profiling of normal development and pathology in tissue integrating laser microdissection and microanalysis. 2002 [Google Scholar]
- 15.Athar M, Back JH, Tang X, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224(3):274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roupe K, Remsberg C, Yáñez J, et al. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr Clin Pharmacol Curr Clin Pharmacol. 2006;1(1):81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 17.Douillet-Breuil A, Jeandet P, Adrian M, et al. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J Agric Food Chem. 1999;47(10):4456–61. doi: 10.1021/jf9900478. [DOI] [PubMed] [Google Scholar]
- 18.Kapetanovic IM, Muzzio M, Huang Z, et al. Pharmacokinetics, oral bioavailability and metabolic profile of resveratrol and its dimethylether analog pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68(3):593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golkar L, Ding X, Ujiki M, et al. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res. 2007;138(2):163–9. doi: 10.1016/j.jss.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 20.Holtrup F, Bauer A, Fellenberg K, et al. Microarray analysis of nemorosone-induced cytotoxic effects on pancreatic cancer cells reveals activation of the unfolded protein response (UPR) Br J Pharmacol. 2011;162(5):1045–59. doi: 10.1111/j.1476-5381.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berberat PO, Dambrauskas Z, Gulbinas A, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11(10):3790–8. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]