Abstract
Background
Schizophrenia is associated with deficits in the ability to discriminate auditory features such as pitch and duration that localize to primary cortical regions. Lesions of primary vs. secondary auditory cortex also produce differentiable effects on ability to localize and discriminate free-field sound, with primary cortical lesions affecting variability as well as accuracy of response. Variability of sound localization has not previously been studied in schizophrenia.
Methods
The study compared performance between patients with schizophrenia (n=21) and healthy controls (n=20) on sound localization and spatial discrimination tasks using low frequency tones generated from seven speakers concavely arranged with 30 degrees separation.
Results
For the sound localization task, patients showed reduced accuracy (p=0.004) and greater overall response variability (p=0.032), particularly in the right hemifield. Performance was also impaired on the spatial discrimination task (p=0.018). On both tasks, poorer accuracy in the right hemifield was associated with greater cognitive symptom severity. Better accuracy in the left hemifield was associated with greater hallucination severity on the sound localization task (p=0.026), but no significant association was found for the spatial discrimination task.
Conclusion
Patients show impairments in both sound localization and spatial discrimination of sounds presented free-field, with a pattern comparable to that of individuals with right superior temporal lobe lesions that include primary auditory cortex (Heschl’s gyrus). Right primary auditory cortex dysfunction may protect against hallucinations by influencing laterality of functioning.
Keywords: schizophrenia, sensory processing, spatial localization, Heschyl’s gyrus, hallucinations, cognitive functioning
Introduction
Schizophrenia is a severe neuropsychiatric illness associated with widespread deficits in neurocognition. Although deficits have been studied primarily in relationship to higher cognitive function, increasing evidence implicates dysfunction within primary sensory cortex. In the auditory system, deficits in functioning of primary sensory cortex are supported by the observation that patients show significant impairment in ability to match tones following brief delay (Hunter 2004; Hunter et al. 2003; Javitt et al. 2000; Rabinowicz et al. 2000; Strous et al. 1995) with no increased susceptibility to within-modal (Rabinowicz et al. 2000) or cross-modal (Javitt et al. 1997) distraction.
Although less well studied, sound localization also appears to be impaired in schizophrenia. Balogh et al (1979) found that individuals with schizophrenia were less accurate at determining the location of sound relative to the midline. Behavioral and electrophysiological findings from a recent event-related potential (ERP) study using manipulation of intra-aural cues to simulate location differences also provide evidence for impairments (Matthews et al. 2007). Individuals with schizophrenia were less accurate at detecting location deviants than controls and mismatch negativity (MMN) amplitude was attenuated in the schizophrenia group, suggesting that deficits in early auditory processing may account for performance impairments (Matthews et al. 2007).
In humans, auditory regions are located within superior temporal gyrus (STG), with primary auditory cortex located on the transverse gyri of Heschl (HG) (Morosan et al. 2001). Recently, effects of temporal lobe lesions on auditory localization have been detailed (Zatorre and Penhune 2001). In both cats and monkeys, both hemispheres participate primarily in contralateral spatial localization and primary cortical lesions impair localization within but not across midlines (Heffner and Heffner 1990). Humans, however, show a different pattern in which right hemisphere lesions affect localization and discrimination in both hemifields (Karnath 2001; Weeks et al. 1999; Zatorre and Penhune 2001). Furthermore, lesions affecting the primary auditory cortex are distinguished from other lesions based upon increased variability of localization, rather than just decreased accuracy. The present study assessed localization and discrimination in schizophrenia using free-field stimuli in order to compare patterns of dysfunction in schizophrenia to published patterns in individuals with known auditory cortical lesions. We hypothesized that patients should show patterns consistent with bilateral primary auditory cortical dysfunction, including decreased accuracy and increased variability.
A secondary aim of the study was to evaluate the relationship between performance in schizophrenia and symptom severity. Left STG abnormalities are implicated in cognitive symptoms such as thought disorder based upon both structural (Rajarethinam et al. 2000), (Sun et al. 2009) and functional (Ford et al. 2009) investigations. In addition, hallucinations may represent “mislocalization” of thoughts generated within the head to external locations (Hunter 2004; Hunter et al. 2003), and have also been associated with auditory cortical dysfunction based upon electrophysiological (Fisher et al. 2008; Youn et al. 2003), functional (Barta et al. 1990; Flaum et al. 1995; Ford et al. 2009; O’Daly et al. 2007) and structural (Gaser et al. 2004; Rajarethinam et al. 2000) brain imaging. To date no studies have evaluated symptom severity relative to localization and discrimination abilities in schizophrenia. An exploratory analysis in the present study thus assessed the relationship between impaired spatial localization ability particularly within right hemifield and severity of thought disorder, hallucinations, and other potential symptom correlates.
Experimental/Materials and Methods
1.1 Participants
Participants included 21 patients with schizophrenia diagnosed based upon the Structured Clinical Interview for DSM-IV (Spitzer et al. 1992) and 20 healthy controls. Patients were recruited from an inpatient unit and outpatient facilities associated with Nathan Kline Institute. Controls who met diagnostic criteria for a psychiatric disorder or reported a history of substance abuse during the initial screening were excluded. Individuals with hearing problems were also excluded.
Socioeconomic status was calculated based on the Hollingshead scale (Hollingshead and Redlich 1954). In patients, symptom severity was assessed using the Positive and Negative Syndrome Scale (Kay et al. 1988). Participants provided informed consent prior to participation and the protocol was approved by the appropriate institutional review boards.
1.2 Sound Localization and Spatial Discrimination Paradigms
Stimuli were projected from seven speakers concavely arranged with 30 degrees separation to enable assessments across 180° in the horizontal plane with positions as illustrated in Figure 1. Two paradigms were presented in two blocks each in counterbalanced order. In the sound localization paradigm, 20 tones were generated randomly from each of the seven speakers (140 total). To reduce the likelihood of recording biases, participants pointed to the speaker they thought the sound came from and experimenter recorded the reported location. Because subjects were permitted to view the speakers, positions other than legitimate speaker locations were rarely reported. However, if it was unclear which speaker the participant was pointing to, they were asked to clarify. In the spatial discrimination paradigm, 16 trials per speaker pair (700 trials total) were presented in random order. In each trial, two tones were sequentially presented 150 ms apart from either the same or different speakers. The participant was asked to report whether the tones came from the same or different speakers, ignoring relative position of the tones.
Figure 1. Speaker Arrangement.
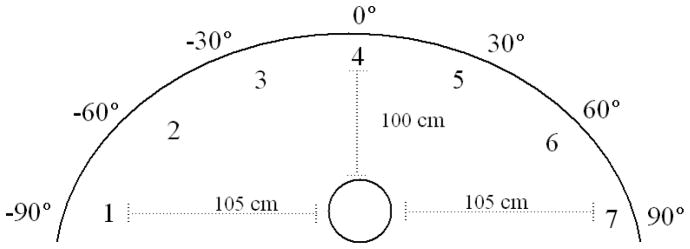
Seven speakers were concavely arranged with 30° spatial separation enabling assessments across 180° in the horizontal plane. Subjects were seated 100cm from the front and sides of the structure and the speakers were adjusted to be approximately at the level of the subject’s pinnae.
For both paradigms, stimuli consisted of 300ms 10ms rise/fall, 1000Hz tones presented at 60dB. Prior to each stimulus presentation, participants were instructed to fixate on a light presented at the midline. Subsequent stimuli were presented only after a response was provided and the subject redirected their focus to the midline. Participants completed approximately 35 practice trials for the localization task and 70 for the discrimination task. Feedback was not provided for either task.
1.3 Statistical Analysis
Mixed Model Analysis of Variance (RM ANOVA) using SPSS version 17.0 were performed to compare performance between patients and controls for both tasks. Comparable analyses were used to examine performance differences between patients and controls in the monaural cue conditions.
1.4 Sound Localization Paradigm
In the sound localization paradigm, accuracy and precision were measured on three indices: percent correct, error difference and response variability. Approximately 20 trials were completed for each speaker location for each subject. Percent correct was calculated as the number of correct responses at each location. Responses were considered to be correct when the reported location was the same as the actual location. To calculate error differences, the differences between reported and actual location were averaged across trials for each location. Response variability was calculated by taking the standard deviation of the error difference across trials for each individual at each location. In order to examine the effect of speaker location on performance, 2 (group status: patients vs controls) × 7 (speaker location: −90°, −60°, −30°, 0°, 30°, 60°, 90°) RM ANOVAs were completed for each accuracy assessment with group status as the between subjects factor and speaker location as the repeated factor. Preplanned follow-up analyses assessed performance within each hemifield using separate 2 (group status: patients vs. controls) × 3 (speaker location: 30°, 60°, 90° relative to the midline) RM ANOVAs for each hemifield.
1.5 Spatial Discrimination Paradigm
For the spatial discrimination paradigm, performance was assessed by calculating the d prime (d′) for each speaker combination (Macmillan and Creelman 2005). Because d′ represents the deviance between the two distributions, larger d′ values are indicative of greater accuracy.
For within hemifield comparisons, a 2 (group status: patients vs. controls) × 12 (speaker combination position: −90°/−60°, −60°/−30°, −90°/−30°, −90°/−0°, −60°/−0°, −30°/0°, 90°/60°, 60°/30°, 90°/30°, 90°/0°, 60°/0°, and 30°/0°) RM ANOVA was conducted with group status as the between subjects factor and position as the repeated factor. For across midline analyses, a 2 (group status) × 3 (degree of separation between speakers: 60 degrees, 120 degrees and 180 degrees) RM ANOVA was performed. For within versus across hemifield comparisons, a 2 (group status) × 3 (hemifield: within left hemifield, within right hemifield or across the midline) RM ANOVA was performed with group status as the between subject factor and hemifield as the repeated measure. To control for degree of separation, the analysis was restricted to combinations with 60 degrees of separation (i.e., −30°/−90° and 30°/90° for within hemifield differences and −30°/30° for across midline differences).
1.6 Performance and Symptom Severity
Symptom severity was assessed for total, positive, negative, and cognitive symptoms on the PANSS (Levine and Rabinowitz 2007). Severity of hallucinations was assessed using the single item from the PANSS. Stepwise multiple regression analyses were performed to assess potential associations, with symptom severity as the dependent variable and location specific performance as the independent variables. For the sound localization task, individual performance was averaged within each hemifield and at the midline. For the spatial discrimination task, individual performance was averaged for eccentric and central combinations in each hemifield and across the midline. Data in text represent mean±sem.
Results
2.1 Sample Characteristics
Table 1 presents relevant sample characteristics. No significant between group differences were observed for any of the demographic characteristics.
Table 1.
Sample Characteristics
| Patients n (%) | Controls n (%) | X2 | p-value | |
|---|---|---|---|---|
| Gender | 1.78 | 0.238 | ||
| Male | 19 (90.5) | 15 (75.0) | ||
| Female | 2 (9.5) | 5 (25.0) | ||
| Ethnicity | 2.99 | 0.489 | ||
| White/Caucasian | 11 (52.4) | 13 (65.0) | ||
| Black | 9 (42.9) | 4 (20.0) | ||
| Hispanic/Latino | 1 (4.8) | 1 (5.0) | ||
| Asian/Other | 0 (0.0) | 2 (10.0) |
| Mean (SD) | Mean (SD) | p-value | |
|---|---|---|---|
| Age at Interview (years) | 39.2 (10.1) | 38.1 (13.0) | 0.783 |
| Parental Socioeconomic Statusa | 40 (14.9) | 44 (15.2) | 0.465 |
N missing: cases = 5, controls = 2
2.2 Sound Localization Analysis
Sound localization analyses focused on three measures: overall percent correct at each location, degree of difference between actual and indicated position at each location and the degree of response variability at each location. For each measure, primary analysis consisted of a group × location RM ANOVA. Separate analyses were conducted across and within hemifields.
2.2.1 Across Hemifield Comparisons
For the percent correct performance analysis, there was a highly significant main effect of group [F(1, 39) = 9.65, p =0.004)] reflecting significantly lower performance in patients (56.3±3.4%) compared to controls (71.3±3.5%). Both groups were more accurate at central compared to peripheral locations as shown by a significant main effect of location [F(6, 34) = 3.55, p =0.008]. However, the groups did not differ significantly in performance distribution across locations, as shown by non-significant group × location interaction [F(6, 34) = 1.52, p = 0.200].
In the absolute error difference comparison, a significant main effect of group was also observed [F(1, 39) = 5.21, p =0.028], reflecting greater error difference for patients (19.2±2.4°) than controls (11.3±2.5°). Both the main effect of location [F(6, 34) = 2.11, p = 0.077] and the group × location interaction [F(6,34) = 2.24, p = 0.068] tended toward significance.
With respect to response variability, a significant main effect for group [F(1, 39) = 4.94 p= 0.028] and location [F(6, 34) = 2.74, p = 0.028] was again observed with no significant group × location interaction [F(6, 34) = 0.96, p = 0.469].
2.2.2 Within Hemifield Comparisons
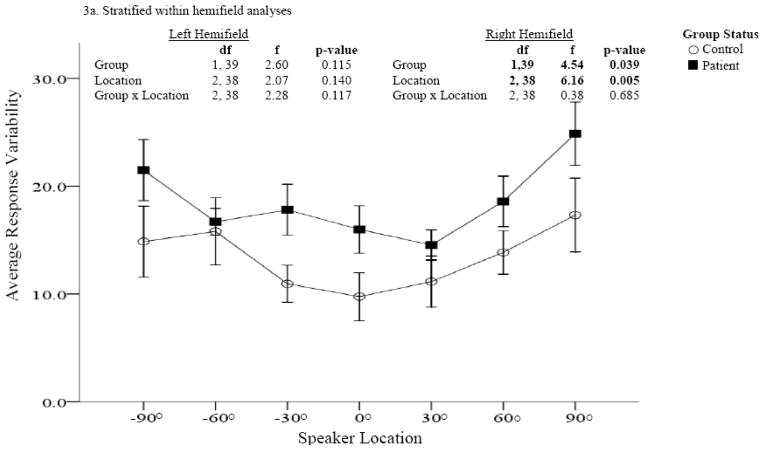
Performance comparisons for absolute error difference and response variability are provided in Figures 2 and 3. In both assessments, significant main effects were found for group and location in the right hemifield only. In the right hemifield, patients were less accurate and more variable in their responses than controls and both groups were more accurate at locations closer to the midline than eccentric locations. The greatest differences in accuracy between patients and controls were found for more eccentric locations (i.e., 60° and 90).
Figure 2. Error difference between patients and controls (Sound Localization task).
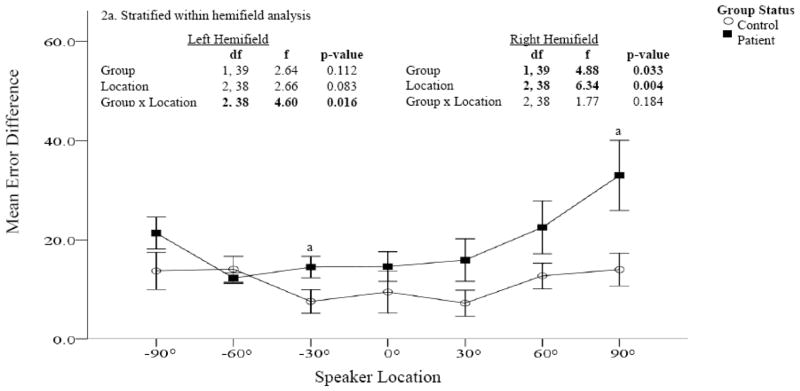
Accuracy was measured at each speaker location using error difference (the absolute difference between the actual and reported speaker location); greater error differences are indicative of poorer accuracy. Patients are represented by the dark squares and controls by the open circles. Significantly greater error differences were found in patients compared to controls, particularly in the right hemifield. Within hemifield analysis confirm significant differences in the right but not left hemifield (Figure 2a).
Figure 3. Response variability comparisons between patients and controls (Sound Localization task).
Performance was measured at each speaker location using response variability (the standard deviation of the error difference). Patients are represented by dark squares and controls by open circles. Across locations, response variability was significantly greater among patients compared to controls. Within hemifield analysis revealed significant group differences in the right but not left hemifield (Figure 3a).
2.3 Spatial Discrimination Analysis
For the spatial discrimination analysis, performance was measured using d′. Separate RM ANOVAs were performed for comparisons across the midline, within hemifield and within hemifield vs. across the midline.
2.3.1 Across Midline Comparisons
Overall accuracy was significantly worse in patients than controls for across midline comparisons [F(1, 39) = 6.16, p = 0.018]. Both groups were more accurate at combinations with 120 degrees separation (i.e, −60°/60°) than either 60 (i.e., −30°/30°) or 180 (i.e, −90°/90°) degrees separation. The group × location interaction was not significant [F(2, 38) = 1.06, p = 0.356]. (Figure 4a)
Figure 4.
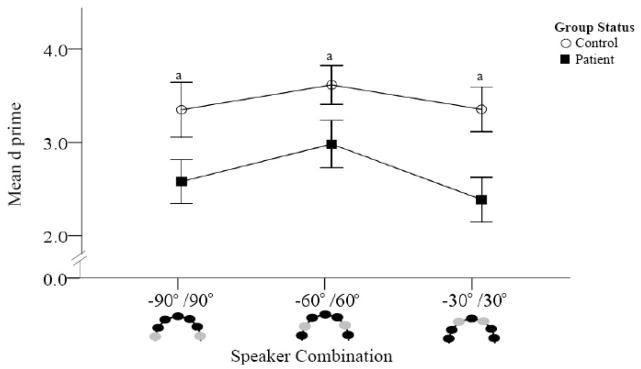
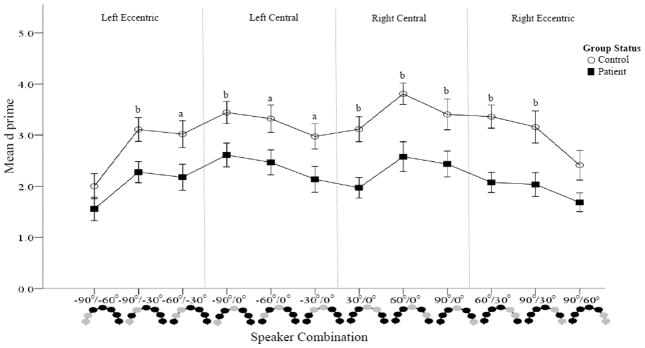
Figure 4a. Accuracy comparisons between patients and controls (Spatial Discrimination task).
D prime was calculated for each speaker combination. Higher d′ values are indicative of better accuracy. Combinations crossing the midline are presented. Schematic representations of the speakers are provided at the bottom of the figure with light colored circles representing the sound locations. Patients are represented by dark squares and controls by open circles. Poorer accuracy was found in patients compared to controls at all three speaker combinations.
Figure 4b. Accuracy comparisons between patients and controls (Spatial Discrimination task). D prime was calculated for each speaker combination. Higher d′ values are indicative of better accuracy. Within hemifield speaker combinations are presented. Schematic representations of the speakers are provided at the bottom of the figure with the light colored circles representing the sound locations. Patients are represented by dark squares and controls by open circles. With the exception of the most eccentric combinations, poorer accuracy was found in patients compared to controls at all speaker combinations.
2.3.2 Within Hemifield Comparisons
Within hemifield comparisons yielded significant main effects for group [F(1, 39) = 10.41, p = 0.003], and location [F(11, 29) = 7.13, p < 0.001] and a significant group × location interaction [F(11, 29) = 2.51, p < 0.023]. Across speaker combinations, average d′ prime was significantly lower in patients (3.09±2.0) than controls (2.17±.20). With the exception of the most eccentric combinations in both hemifields (−90°/−60° and 90°/60°), patients were less accurate than controls for all speaker combinations (Figure 4b)
2.3.3 Within Hemifield vs. Across Midline Comparison
For within hemifield versus across midline comparisons, overall accuracy was significantly worse in patients than controls [F(1, 39) = 10.20, p = 0.003]. No performance differences were found for across vs. within hemifield comparisons [F(2, 38) = 2.44, p =0.101] and the group × hemifield interaction failed to reach significance [F(2,38) = 0.39, p= 0.681].
2.4 Symptom Correlations
For cognitive symptoms, poorer performance in the right hemifield predicted greater severity of cognitive symptoms in both the sound localization (β = −0.51, p = 0.043) and spatial discrimination (β = −0.59, p = 0.013) tasks. At all three speaker combinations, severity of cognitive symptoms was inversely correlated with performance (Table 2). Significant correlations were also observed for disorganization and difficulty in abstract thinking symptoms considered independently (Table 2). No other cognitive symptoms significantly correlated with performance.
Table 2.
Correlations between performance and symptom severity
| Localization | 30° | 60° | 90° |
|---|---|---|---|
| Cognitive Symptoms | −0.33 | −0.38 | −0.50a |
| Conceptual disorganization | −0.40 | −0.58b | −0.07 |
| Poor attention | −0.20 | −0.14 | −0.21 |
| Mannerisms and posturing | 0.18 | 0.22 | −0.38 |
| Difficulty in abstract thinking | −0.22 | −0.31 | −0.60b |
| −30° | −60° | −90° | |
| Hallucinations | −0.37 | −0.13 | −0.46a |
| Discrimination | 0°/30° | 0°/60° | 0°/90° |
|---|---|---|---|
| Cognitive Symptoms | −0.53a | −0.53a | −0.58b |
| Conceptual disorganization | −0.34 | −0.26 | −0.37 |
| Poor attention | −0.22 | −0.13 | −0.17 |
| Mannerisms and posturing | −0.07 | −0.10 | −0.17 |
| Difficulty in abstract thinking | −0.51a | −0.72b | −0.67b |
p<0.05;
p < 0.01
Greater accuracy (i.e., lower average absolute difference) in the left hemifield was associated with greater severity of hallucinations (β = −0.54, 95% CI: −0.03 to −0.02, p =0.026). Post hoc exploratory correlation analyses were performed to identify whether specific locations accounted for the significant associations (Table 2). Significant correlations were found for the most eccentric location for both symptom types. Correlations at other locations were moderate, but failed to reach significance.
Discussion
Deficits in auditory processing are now well documented in schizophrenia, primarily using measures such as pitch or duration discrimination that localize to primary auditory cortex (Heschl’s gyrus, HG) (Javitt et al. 2000; Javitt et al. 1997; Rabinowicz et al. 2000; Strous et al. 1995). Spatial localization, an additional HG-dependent process, has been studied in schizophrenia to only a limited degree. This is the first study to evaluate localization using free-field sound within and across hemifields and to assess not only correct performance, but also absolute and relative localization disparities, which are known to be specifically sensitive to HG dysfunction.
The primary finding of the present study is that schizophrenia patients, as predicted, showed reduced accuracy and increased variability of localization relative to controls. Although reduced localization accuracy is seen following lesions of either primary or secondary auditory cortex, increased variability is reported as a hallmark of primary auditory cortical dysfunction. Patients had the greatest difficulty with the spatial discrimination task in which reduced accuracy was found at nearly every location. In contrast, impairments on the sound localization task were seen throughout the right hemifield, but only at specific locations (−30 & −90) on the left. Increased variability of sound localization was also observed in the right-, but not left-, hemifield.
In imaging studies, spatial discrimination is associated with bilateral activation of posterior auditory association regions (Alain et al. 2001; Arnott et al. 2004). Lesion studies, however, provide evidence that hemispheric involvement depends on the hemisphere and region of damage (Bellmann et al. 2001; Clarke et al. 2000; Spierer et al. 2009). For example, in one study of post-stroke individuals, sound localization ability in both hemifields was affected primarily by lesions in the right hemisphere, whereas left hemisphere lesions affected primarily spatial discrimination abilities. Furthermore, lesions that affected HG were distinguished from those that spared HG based upon pattern of deficit. Discrimination abilities were impaired in individuals with HG lesions in either hemisphere; whereas localization abilities were only affected if the lesion was in the right hemisphere(Zatorre and Penhune 2001).
Similar findings were obtained in other studies of post-stroke individuals (Spierer et al. 2009; Yamada et al. 1997). These studies thus provide a neuroanatomical basis for interpretation of deficit patterns in schizophrenia. In the present study, deficits were seen in patients across both left and right hemifields, with greater within- hemifield discrimination impairments found in the right hemifield compared to the left hemifield and across the midline. This pattern closely resembles the pattern observed for patients with brain lesions affecting right temporal lobe including primary auditory cortex (HG) (Zatorre and Penhune 2001) but differs from patterns seen following HG sparing (Zatorre and Penhune 2001). Subtle findings in the present study, however, support a bilateral, rather than purely right unilateral lesion. First, patients showed preserved function at the left mid-hemifield location (−60°). At this site, left temporal lesions lead to somewhat improved accuracy relative to controls (Zatorre and Penhune 2001), suggesting that lack of deficit in patients may reflect combined effects of left and right auditory dysfunction.
Second, the greater deficit in right vs. left hemifield may reflect additive effects of left and right hemispheric dysfunction. However, isolated bilateral brain damage is rare, so there is relatively little information regarding effects of bilateral vs. unilateral auditory lesions. As a result, it is difficult to determine whether present findings reflect solely right temporal dysfunction or combined left and right dysfunction. Results from one MMN study do, however, suggest that impairments are likely due to disruptions in hemispheric communication. Patients have attenuated MMN response to deviants created by interaural time but not loudness cues, suggesting that temporal synchrony abnormalities may cause impairments(Matthews et al. 2007). A second MMN of location deviants created using inteaural time differences failed to find significant differences in MMN response (Fisher et al., 2008). Inconsistencies may be due to differences in spatial separation between standards and deviants. MMN has yet to be evaluated for sounds presented free-field as in the present study, which would provide a greater understanding of these inconsistencies. Further, the MMN component is generated by detecting a deviance relative to frequent stimuli. As such, comparisons are useful for identifying early processing deficits associated with discriminating between spatial locations, but not necessarily detecting impairments associated with localization. The present findings suggest that early auditory processing involved in both relative discrimination and absolute localization are impaired in schizophrenia.
3.1 Symptom Severity and Spatial Localization
In the present study, we also observed significant correlations of cognitive symptoms and hallucinations, but not other symptoms, with spatial localization ability. Within the sound localization paradigm, significant associations were only found for left hemifield performance, which is indicative of right hemisphere involvement. Paradoxically, however, better performance on the task (greater accuracy) was associated with greater hallucination severity. Given the inverse correlation, results might best be understood in terms of interhemispheric balance, with bilateral dysfunction producing the types of auditory deficits typically seen in schizophrenia, but relatively preserved right vs. left hemisphere dysfunction leading to overstimulation of external localization percepts. This theory is supported by electrophysiological evidence suggesting that schizophrenia is characterized by altered functional hemispheric asymmetry of the STG (Youn et al. 2003).
In contrast to the left hemifield correlations with hallucinations, cognitive symptoms correlated most strongly with right hemifield dysfunction, suggesting preferential association with left hemisphere dysfunction. In our study, performance was associated with conceptual disorganization and abstract thinking difficulties, cognitive symptoms which are heavily dependent on language function. As language dysfunction is known to be localized to left hemisphere and correlated with left STG dysfunction (Sun et al. 2009), right hemifield sound localization deficits may provide a selective index of left auditory cortical dysfunction in schizophrenia.
3. 2 Conclusion
Overall, sensory deficits have been increasingly documented in schizophrenia, along with histopathological changes in primary sensory cortices. The present study demonstrates impaired spatial localization ability in schizophrenia in a pattern consistent with right or bilateral primary cortex dysfunction. These findings support generalized models of cognitive dysfunction in schizophrenia, and suggest need for further studies investigating the relationship of sensory cortical dysfunction to symptoms and deficits of schizophrenia.
Acknowledgments
Role of Funding Source
Patient recruitment, data collection and manuscript preparation were supported by USPHS grants R37 MH49334 and the P50 MH086385 (NYU Conte Center for Schizophrenia Research) to DCJ.
We wish to thank Jonathan Lehrfeld for his assistance with data collection.
Footnotes
Contributors
Ms. Perrin designed the study, collected the data, managed the literature searches, performed the statistical analysis and wrote the first draft of the paper. Dr. Butler and Ms. Silipo supervised recruitment and clinical interviewing of study participants and reviewed the final draft of the manuscript. Ms. DiConstanzo and Ms. Forchelli recruited participants and conducted clinical interviews. Dr. Javitt supervised the study design, hypothesis formulation and data collection. Dr. Javitt also critically reviewed all analyses and manuscript preparation.
Conflicts of Interest:
Ms. Perrin has no conflict of interest to disclose.
Dr. Butler has no conflict of interest to disclose.
Ms. DiConstanzo has no conflict of interest to disclose.
Ms. Forchelli has no conflict of interest to disclose.
Ms. Silipo has no conflict of interest to disclose.
Dr. Javitt has no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. What” and “where” in the human auditory system. Proc Natl Acad Sci U S A. 2001;98(21):12301–12306. doi: 10.1073/pnas.211209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott SR, Binns MA, Grady CL, Alain C. Assessing the auditory dual-pathway model in humans. Neuroimage. 2004;22(1):401–408. doi: 10.1016/j.neuroimage.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147(11):1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bellmann A, Meuli R, Clarke S. Two types of auditory neglect. Brain. 2001;124(Pt 4):676–687. doi: 10.1093/brain/124.4.676. [DOI] [PubMed] [Google Scholar]
- Clarke S, Bellmann A, Meuli RA, Assal G, Steck AJ. Auditory agnosia and auditory spatial deficits following left hemispheric lesions: evidence for distinct processing pathways. Neuropsychologia. 2000;38(6):797–807. doi: 10.1016/s0028-3932(99)00141-4. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119(4):909–921. doi: 10.1016/j.clinph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Flaum M, O’Leary DS, Swayze VW, 2nd, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res. 1995;29(4):261–276. doi: 10.1016/0022-3956(94)00046-t. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Jorgensen KW, Turner JA, Brown GG, Notestine R, Bischoff-Grethe A, Greve D, Wible C, Lauriello J, Belger A, Mueller BA, Calhoun V, Preda A, Keator D, O’Leary DS, Lim KO, Glover G, Potkin SG, Mathalon DH. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophr Bull. 2009;35(1):58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Volz HP, Buchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14(1):91–96. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64(3):915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich F. Schizophrenia and social structure. American Journal of Psychiatry. 1954;110(9):695–701. doi: 10.1176/ajp.110.9.695. [DOI] [PubMed] [Google Scholar]
- Hunter MD. Locating voices in space: a perceptual model for auditory hallucinations? Cogn Neuropsychiatry. 2004;9(1–2):93–105. doi: 10.1080/13546800344000174. [DOI] [PubMed] [Google Scholar]
- Hunter MD, Griffiths TD, Farrow TF, Zheng Y, Wilkinson ID, Hegde N, Woods W, Spence SA, Woodruff PW. A neural basis for the perception of voices in external auditory space. Brain. 2003;126(Pt 1):161–169. doi: 10.1093/brain/awg015. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111(10):1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106(2):315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Karnath HO. New insights into the functions of the superior temporal cortex. Nat Rev Neurosci. 2001;2(8):568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23(1):99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Levine SZ, Rabinowitz J. Revisiting the 5 dimensions of the Positive and Negative Syndrome Scale. J Clin Psychopharmacol. 2007;27(5):431–436. doi: 10.1097/jcp/.0b013e31814cfabd. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Matthews N, Todd J, Budd TW, Cooper G, Michie PT. Auditory lateralization in schizophrenia--mismatch negativity and behavioral evidence of a selective impairment in encoding interaural time cues. Clin Neurophysiol. 2007;118(4):833–844. doi: 10.1016/j.clinph.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13(4):684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Frangou S, Chitnis X, Shergill SS. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. 2007;156(1):15–21. doi: 10.1016/j.pscychresns.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57(12):1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41(2):303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Spierer L, Bellmann-Thiran A, Maeder P, Murray MM, Clarke S. Hemispheric competence for auditory spatial representation. Brain. 2009;132(Pt 7):1953–1966. doi: 10.1093/brain/awp127. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I. History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152(10):1517–1519. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61(1):14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Aziz-Sultan A, Bushara KO, Tian B, Wessinger CM, Dang N, Rauschecker JP, Hallett M. A PET study of human auditory spatial processing. Neurosci Lett. 1999;262(3):155–158. doi: 10.1016/s0304-3940(99)00062-2. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kaga K, Uno A, Shindo M. Comparison of interaural time and intensity difference discrimination in patients with temporal lobe lesions. Acta Otolaryngol Suppl. 1997;532:135–137. doi: 10.3109/00016489709126163. [DOI] [PubMed] [Google Scholar]
- Youn T, Park HJ, Kim JJ, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophr Res. 2003;59(2–3):253–260. doi: 10.1016/s0920-9964(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Penhune VB. Spatial localization after excision of human auditory cortex. J Neurosci. 2001;21(16):6321–6328. doi: 10.1523/JNEUROSCI.21-16-06321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]