Abstract
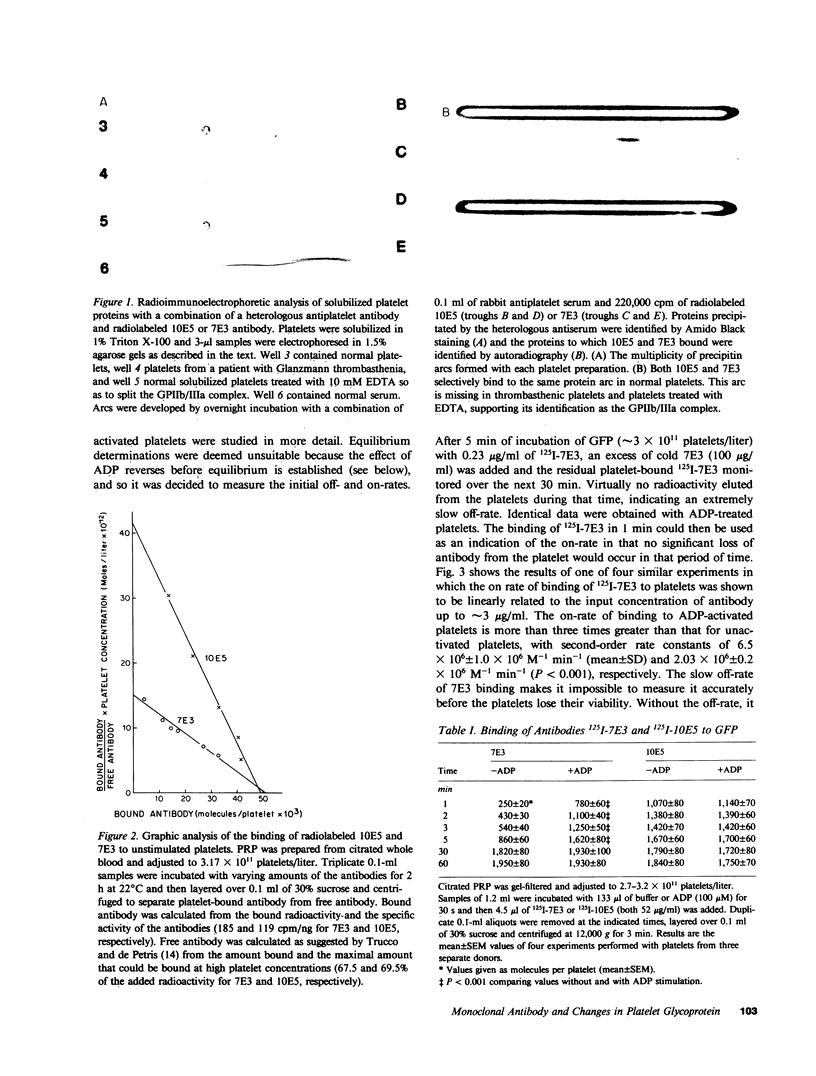
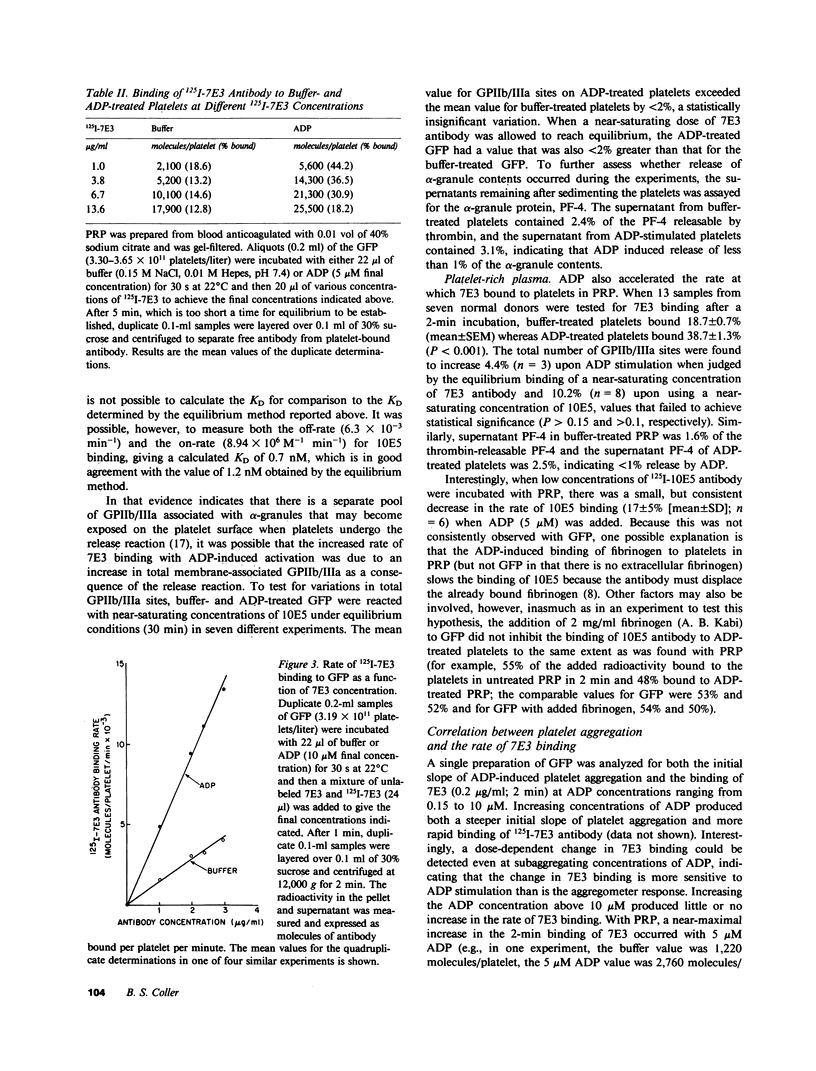
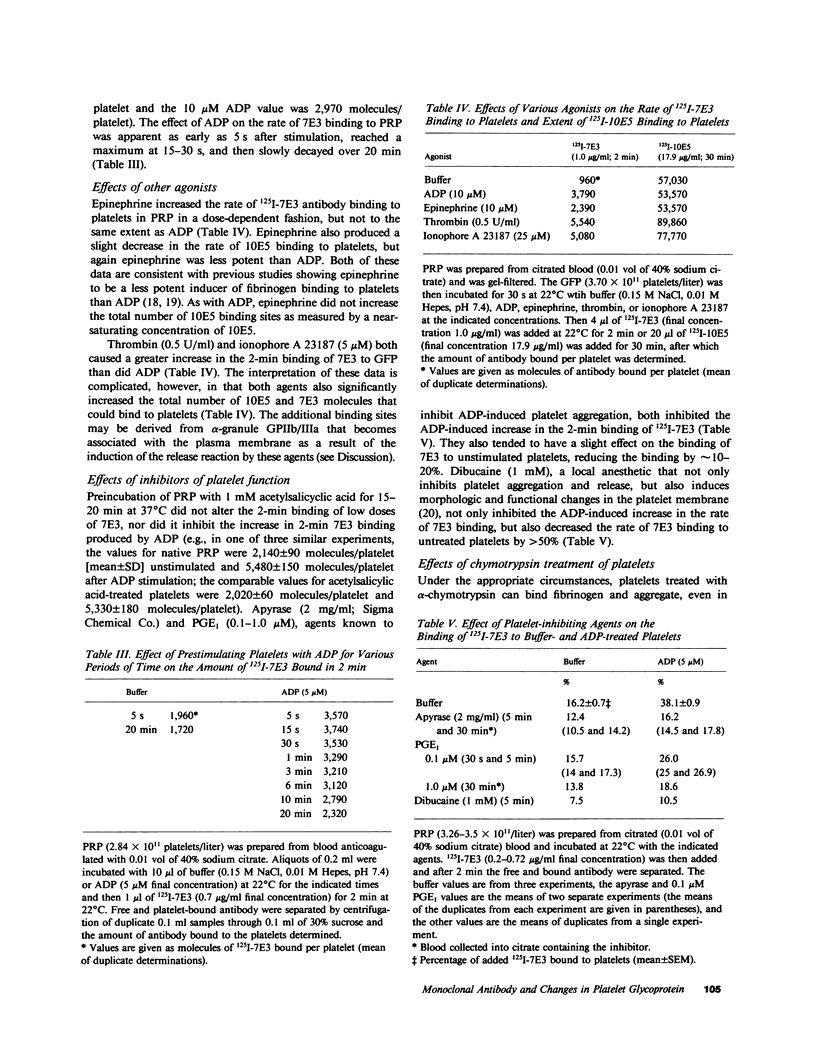
Considerable evidence indicates that the glycoprotein (GP) IIb/IIIa complex on human platelets functions as a receptor for fibrinogen, but little is known about the mechanism of receptor "exposure." To investigate this mechanism, our previously described murine monoclonal antibody (10E5) and a new monoclonal antibody (7E3), both of which block the binding of fibrinogen to platelets and bind to GPIIb and/or GPIIIa, were radiolabeled and their rates of binding to native and ADP-activated platelets were studied. At low concentrations, 125I-10E5 bound nearly equally rapidly to both native and activated platelets, whereas 125I-7E3 bound slowly to native platelets and much more rapidly to activated platelets. This increased rate of 7E3 binding is unlikely to be due to an increase in the number of GPIIb/IIIa sites on the surface of activated platelets because: (a) the rate of 10E5 binding was unchanged; (b) the total number of surface GPIIb/IIIa sites increased by only 2-10% with activation as judged by equilibrium binding of near-saturating concentrations of 10E5 and 7E3, and (c) there was less than 1% release of platelet factor 4 with activation, indicating minimal fusion of alpha-granule membranes (a potential source of GPIIb/IIIa) with the plasma membrane. Other activators (epinephrine, thrombin, and ionophore A 23187) also increased the rate of 7E3 binding, as did digestion of platelets with chymotrypsin. Aspirin did not affect the rate of binding of 7E3, whereas apyrase, prostaglandin E1, and dibucaine all inhibited the enhancement of the 7E3-binding rate produced by ADP. These data provide evidence for an activation-dependent change in the conformation and/or microenvironment of the GPIIb/IIIa complex, and offer a method of studying the receptor exposure mechanism that does not rely on the binding of fibrinogen itself.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S. Effects of tertiary amine local anesthetics on von Willebrand factor-dependent platelet function: alteration of membrane reactivity and degradation of GPIb by a calcium-dependent protease(s). Blood. 1982 Sep;60(3):731–743. [PubMed] [Google Scholar]
- Coller B. S., Franza B. R., Jr, Gralnick H. R. The pH dependence of quantitative ristocetin-induced platelet aggregation: theoretical and practical implications-a new device for maintenance of platelet-rich plasma pH. Blood. 1976 May;47(5):841–854. [PubMed] [Google Scholar]
- Coller B. S. Interaction of normal, thrombasthenic, and Bernard-Soulier platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia. Blood. 1980 Feb;55(2):169–178. [PubMed] [Google Scholar]
- Coller B. S., Kalomiris E., Steinberg M., Scudder L. E. Evidence that glycocalicin circulates in normal plasma. J Clin Invest. 1984 Mar;73(3):794–799. doi: 10.1172/JCI111273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller H. A., Coller B. S. Statistical analysis of repetitive subcloning by the limiting dilution technique with a view toward ensuring hybridoma monoclonality. Hybridoma. 1983;2(1):91–96. doi: 10.1089/hyb.1983.2.91. [DOI] [PubMed] [Google Scholar]
- Gogstad G. O., Hagen I., Korsmo R., Solum N. O. Characterization of the proteins of isolated human platelet alpha-granules. Evidence for a separate alpha-granule-pool of the glycoproteins IIb and IIIa. Biochim Biophys Acta. 1981 Sep 29;670(2):150–162. doi: 10.1016/0005-2795(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Coller B. S. Fibrinogen competes with von Willebrand factor for binding to the glycoprotein IIb/IIIa complex when platelets are stimulated with thrombin. Blood. 1984 Oct;64(4):797–800. [PubMed] [Google Scholar]
- Gugler E., Lüscher E. F. Platelet function in congenital afibrinogenemia. Thromb Diath Haemorrh. 1965 Nov 15;14(3-4):361–373. [PubMed] [Google Scholar]
- Inceman S., Caen J., Bernard J. Aggregation, adhesion, and viscous metamorphosis of platelets in congenital fibrinogen deficiencies. J Lab Clin Med. 1966 Jul;68(1):21–32. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Mustard J. F., Kinlough-Rathbone R. L., Packham M. A., Perry D. W., Harfenist E. J., Pai K. R. Comparison of fibrinogen association with normal and thrombasthenic platelets on exposure to ADP or chymotrypsin. Blood. 1979 Nov;54(5):987–993. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L., Perry D. W., Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978 Aug;52(2):453–466. [PubMed] [Google Scholar]
- Niewiarowski S., Budzynski A. Z., Morinelli T. A., Brudzynski T. M., Stewart G. J. Exposure of fibrinogen receptor on human platelets by proteolytic enzymes. J Biol Chem. 1981 Jan 25;256(2):917–925. [PubMed] [Google Scholar]
- Peerschke E. I., Coller B. S. A murine monoclonal antibody that blocks fibrinogen binding to normal platelets also inhibits fibrinogen interactions with chymotrypsin-treated platelets. Blood. 1984 Jul;64(1):59–63. [PubMed] [Google Scholar]
- Peerschke E. I. Effect of epinephrine on fibrinogen receptor exposure by aspirin-treated platelets and platelets from concentrates in response to ADP and thrombin. Am J Hematol. 1984 May;16(4):335–345. doi: 10.1002/ajh.2830160404. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Induction of human platelet fibrinogen receptors by epinephrine in the absence of released ADP. Blood. 1982 Jul;60(1):71–77. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B. Fibrinogen receptor exposure and aggregation of human blood platelets produced by ADP and chilling. Blood. 1981 Apr;57(4):663–670. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A. Induction of the fibrinogen receptor on human platelets by epinephrine and the combination of epinephrine and ADP. J Biol Chem. 1980 Nov 25;255(22):10971–10977. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A. Participation of ADP in the binding of fibrinogen to thrombin-stimulated platelets. Blood. 1980 Sep;56(3):553–555. [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligsohn U., Rososhansky S. A Glanzmann's thrombasthenia cluster among Iraqi Jews in Israel. Thromb Haemost. 1984 Dec 29;52(3):230–231. [PubMed] [Google Scholar]
- Weiss H. J., Rogers J. Fibrinogen and platelets in the primary arrest of bleeding. Studies in two patients with congenital afibrinogenemia. N Engl J Med. 1971 Aug 12;285(7):369–374. doi: 10.1056/NEJM197108122850703. [DOI] [PubMed] [Google Scholar]




