Abstract
It is well known that the process of plant cell differentiation depends on the symplasmic isolation of cells. Before starting the differentiation programme, the individual cell or group of cells should restrict symplasmic communication with neighbouring cells. We tested the symplasmic communication between epidermal cells in the different root zones of parental barley plants Hordeum vulgare L., cv. ‘Karat’ with normal root hair development, and two root hairless mutants (rhl1.a and rhl1.b). The results clearly show that symplasmic communication was limited during root hair differentiation in the parental variety, whereas in both root hairless mutants epidermal cells were still symplasmically connected in the corresponding root zone. This paper is the first report on the role of symplasmic isolation in barley root cell differentiation, and additionally shows that a disturbance in the restriction of symplasmic communication is present in root hairless mutants.
Keywords: 8-Hydroxypyrene-1,3,6-trisulphonic acid, trisodium salt; cell differentiation; fluorescein diacetate; Hordeum vulgare; root hair cells; symplasmic communication
INTRODUCTION
Barley (Hordeum vulgare L.) is one of the major cereals cultivated worldwide. In a time where there is a rapid increase in human population and the absence of new areas under cultivation, improvement of cereals and other crops is a major task of plant research. Root systems play a critical role in plant growth and development, since they are responsible for water and nutrient uptake from the soil (Cailloux 1972; Itoh & Barber 1983; Föhse et al. 1991). A larger surface interface between the roots and the soil allows more efficient nutrient uptake by plants (Bates & Lynch 2000). One morphological strategy to increase root–soil (rhizosphere soil) contact is to develop root hairs, the tubular outgrowths of specialised root epidermal cells, called trichoblasts (Grabov & Bottger 1994; Lauter et al. 1996). It has been proved that barley cultivars with longer root hairs absorb more phosphorus from rhizosphere soil than cultivars with shorter root hair (Gahoonia et al. 2001). Moreover, an analysis of the root hairless mutant brb (bald root barley) and its parental variety ‘Pallas’ revealed almost twofold depletion of inorganic and organic phosphorus fractions compared to mutants without root hairs (Gahoonia et al. 2001).
Although root hairs play an important role in the uptake of nutrients and the production of biomass by crops, their development in monocotyledons is still not well understood. However, a few genes involved in root hair differentiation in monocotyledons have been described recently, e.g. β-expansin in barley (HvEXPB1; Kwaśniewski & Szarejko 2006), apyrase (OsAPY1; Yuo et al. 2009) and cellulose synthase-like D1 (OsCSLD1; Kim et al. 2007) in the root hairs of rice. However, in comparison to Arabidopsis thaliana, where a dozen or so genes have been recognised for each stage of root hair development (for review see Horn et al. 2009), our knowledge on the genetic control of root hair development in crop plants is limited. Lately identified root hair mutants of crop plants (Wen & Schnable 1994; Engvild & Rasmussen 2004; Szarejko et al. 2005; Rebouillat et al. 2009) are very useful for gene identification and recognition of molecular mechanisms underlying the process of differentiation of root epidermal cells in monocots.
Many studies have demonstrated that the process of plant cell differentiation depends on symplasmic isolation of these cells (Rinne & van der Schoot 1998; Ruan et al. 2004; Kim & Zambryski 2005; Kurczyńska et al. 2007). The process of symplasmic communication depends on the presence of plasmodesmata – the cytoplasmic channels within cell walls of neighbouring cells (Lucas et al. 1993; Roberts & Oparka 2003). Plasmodesmata are structures that regulate the passage of molecules between plant cells (Crawford & Zambryski 2001; Maule 2008) that constitute the route between neighbouring cells for proteins and RNAs (Oparka & Cruz 2000; Lucas et al. 2001; Jorgensen 2002; Ruiz-Medrano et al. 2004). It has been postulated that only when plasmodesmata are closed or absent can cells realise their own individual genetic programme. Symplasmic isolation is involved in development of the embryo sac in Torenia fournieri (Han et al. 2000; Dresselhaus 2006), establishment of the apical–basal axis and tissue differentiation during embryogenesis in Arabidopsis (Kim & Zambryski 2005; Kim et al. 2005; Stadler et al. 2005), androgenesis in barley (Wrobel et al. 2011) and the differentiation of root epidermal cells in Arabidopsis (Duckett et al. 1994). Duckett et al. (1994) showed that in the meristematic zone of the Arabidopsis root all epidermal cells were symplasmically connected, whereas in the differentiation zone, both types of epidermal cell, trichoblasts and atrichoblasts, were symplasmically isolated from neighbouring cells. These results confirmed the key role of symplasmic isolation in plant cell differentiation, especially in initially homogeneous cell systems. The explanation of why symplasmic isolation is so important for the differentiation of root epidermal cells may be the fact that some proteins involved in root hair development in Arabidopsis may be transported via plasmodesmata. The CPC (CAPRICE) gene, a positive regulator of root hair formation, is transcribed only in atrichoblasts, but CPC protein is present in all root epidermal cells (Wada et al. 2002; Kurara et al. 2005). On the other hand, negative regulators of root hair formation, such as GL3 (GLABRA3) and EGL3 (ENHANCER OF GLABRA3), may be transported through plasmodesmata from trichoblasts to atrichoblasts (Bernhardt et al. 2005). CPL3 (CAPRICE-LIKE MYB3) is another protein that is also involved in root hair development and is transported via plasmodesmata (Tominaga et al. 2008). On the basis of results obtained to date, new models depicting the differentiation of root epidermal cells in Arabidopsis, which critically depend on the movement of proteins between neighbouring cells via plasmodesmata, have been proposed (Benítez et al. 2008; Petricka & Benfey 2008; Savage et al. 2008). Furthermore, a similar dependence between cell differentiation and the movement of proteins through plasmodesmata was observed in the leaf epidermis of Arabidopsis (Digiuni et al. 2008; Zhao et al. 2008). These results show that genetic control of cell fate and patterning may be supported by the restriction of symplasmic communication.
The main aim of the presented studies was to describe symplasmic communication between different cells of the barley root epidermis during cell differentiation in the parental variety and two root hairless mutants, rhl1.a and rhl1.b, in order to understand whether there is any difference in the transport via plasmodesmata between the analysed genotypes. This would suggest the importance of symplasmic isolation during the differentiation of barley root hairs.
MATERIAL AND METHODS
Plant material
The mutants analysed were obtained after treatment with N-methyl-N-nitrosourea (MNU) and sodium azide (NaH3) of spring barley (Hordeum vulgare L., cv. ‘Karat’) at the Department of Genetics, University of Silesia. Both mutants (rhl1.a and rhl1.b) are completely hairless and their root hair phenotypes are controlled via a single recessive gene (Szarejko et al. 2005).
The seeds of barley mutants were surface sterilised with a 20% bleach solution (v/v) and germinated in hydroponic conditions using glass tubes sealed with Parafilm. All of the plants investigated were grown in a growth chamber under a 16-/8-h photoperiod at 20 °C, 180 μmol·m−2·s−1 light intensity. All experiments were carried out on 5-day-old seedlings.
Light microscopy
For histological examination, combined conventional and microwave-assisted fixation, substitution and embedding of 2-mm root segments were performed using a PELCO BioWave 34700-230 (TedPella, Redding, CA, USA) according to the procedure described in Thiel et al. (2012). The semi-thin sections (ca. 2-μm thick) were cut from the embedded samples, mounted on slides and stained for 2 min with 1% (w/v) methylene blue/1% (w/v) Azur II in 1% (w/v) aqueous borax at 60 °C prior to light microscopic examination with a Zeiss Axiovert 135 microscope.
Transverse and longitudinal sections of at least 20 roots from different individuals of ‘Karat’ and both mutants were studied at each root developmental zone: meristematic, elongation, differentiation and mature root hair zone (3 cm from the tip).
Fluorescence microscopy analysis of symplasmic transport tracers in roots
Fluorescein diacetate (FDA)
FDA (Sigma-Aldrich, Cat. No. F7378, Poznan, Poland) stock solution prepared in acetone (5 mg·ml−1) was dissolved in demineralised water (0.5 ml per 24.5 ml) to obtain FDA working solution. Roots, still attached to the whole plant, were treated with the FDA working solution in darkness for 15 min. The roots were then washed in demineralised water, placed on microscopic slides and covered with a cover glass.
Fluorescence recovery after photobleaching (FRAP) was used to monitor movement of the FDA within the epidermis of the barley root. For fluorescence analyses, a 20 × PlanFluor objective lenses (NA 0.5) and a scan format of 512 × 512 pixels was used in an Olympus FV1000 confocal system (Olympus, Warszawa, Poland). The pre- and post-bleaching events were imaged using 0.5% power of a 488-nm line from an argon-ion laser (Melles Griot BV, the Netherlands); emission was detected using a 505–530 nm band pass filter. For photobleaching of the fluorescence in a single cell, 90% power of the laser line was used for 10 s. This procedure was repeated at least ten times for each root zone of all genotypes.
8-Hydroxypyrene-1,3,6-trisulphonic acid, trisodium salt (HPTS)
In order to monitor the movement of the HPTS (5 mg·ml−1 in demineralised water; Sigma-Aldrich, Cat. No. H1529) in barley root cells, the tracer was injected into cortical cells of the meristematic zone using a tissue cell pressure probe (Steudle 1993) provided with a microcapillary preceded by treatment with a 0.1 mM demineralised water solution of DDG (2-deoxy-d-glucose; Sigma-Aldrich, Cat. No. D4601) for 30 min to inhibit deposition of callose during injection. Observations of roots after the injection were carried out under an Olympus BX40 epifluorescence microscope equipped with an Olympus 3040 CCD camera (Olympus). Tracer was excited with 480-nm light produced by a UV lamp, and a 500–530 nm band pass filter was used for excitation of HPTS (Mercury BX-FLA fluorescence illuminator, Olympus, Warszawa, Poland). Injection analyses were carried out on ten roots from each genotype.
Electron microscopy analysis
Ultrastructure analysis
For ultrastructure examination, combined conventional and microwave-assisted fixation, substitution and embedding of 2-mm root segments was performed using a PELCO BioWave 34700-230 (TedPella) according to the procedure described in Thiel et al. (2012). For electron microscopy analysis with a Tecnai Sphera G2 (FEI Co., Eindhoven, the Netherlands) transmission electron microscope at 120 kV, ultrathin sections ca. 70-nm thick were cut with a diamond knife and contrasted with a saturated methanolic solution of uranyl acetate and lead citrate before examination. For each genotype, sections from at least ten roots were analysed.
Immunogold analysis
Roots used for immunogold labelling of callose were cut into 2-mm segments, vacuum infiltrated for a short period with 50 mM cacodylate buffer (pH 7.2) containing 0.5% (v/v) glutaraldehyde and 2.0% (v/v) formaldehyde, and kept for 4 h at room temperature in the same medium. Samples were washed with the same buffer for 15 min, followed by two washing steps for 15 min with distilled water. Dehydration of samples was done step-wise, increasing the concentration of ethanol. The steps were performed as follows: 30% (v/v), 40% (v/v), 50% (v/v), 60% (v/v), 70% (v/v), 90% (v/v) and two times 100% (v/v) ethanol for 45 min each. After dehydration the samples were infiltrated subsequently with Lowycryl HM20 resin (Plano, Wetzlar, Germany): 25% (v/v) HM20 resin in ethanol overnight, 50% (v/v) and 75% (v/v) for 4 h each and then 100% HM20 overnight. Samples were transferred into gelatin capsules and an automated freeze substitution unit (Leica Microsystems, Bensheim, Germany), kept there for 3 h in fresh resin at −35 °C and polymerised at −35 °C for 72 h in UV light. Immunogold labelling of 70-nm ultrathin sections was carried out as described previously (Teige et al. 1998). Anti-mouse monoclonal (1,3)-b-D-glucan antibody (Biosupplies, Parkville, Vic., Australia) 1:100 diluted was used as a primary antibody against callose, and goat anti-mouse conjugated to 10-nm gold particles was used as a secondary antibody. The ultrathin sections were contrasted in LEICA EM stain (Leica Microsystems) with uranyl acetate (Polyscience Inc., Eppelheim, Germany) for 30 min and subjected to transmission electron microscope (Tecnai Sphera G2) at 120 kV. Sections from at least seven roots from each genotype were analysed.
RESULTS
Considering their morphology and histology, the phenotype of ‘Karat’ roots was similar to those of barley described earlier (Clowes 2000). Three zones were detected along the root longitudinal axis: meristematic, elongation and differentiation zones. Histological analysis of roots of both the parental variety and the mutants showed the presence of: epidermis, parenchyma, endodermis, pericycle and central root stele (Fig.1). For comparison of the root anatomy of the parental variety and the two mutants, sections from 20 different roots of 20 different plants for each genotype were analysed. There were no differences between parental plants and mutants in the case of cell size, number of epidermal cells in traverse sections, number of parenchyma cell layers and number of metaxylem vessels (Fig.1).
Figure 1.
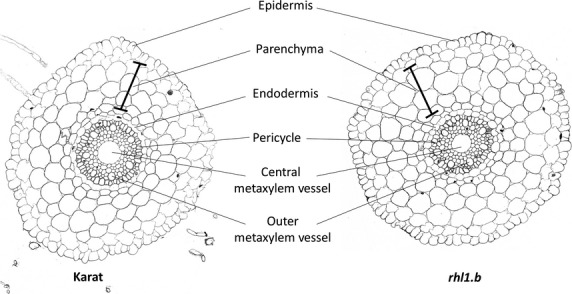
Histology of roots of the mutant rhl1.b and parental variety ‘Karat’ of Hordeum vulgare L. Cross-sections through the root differentiation zone.
Symplasmic communication between epidermal root cells was monitored using a confocal laser scanning microscope and FRAP (Fig.2). In the meristematic zone of all three genotypes (‘Karat’, rhl1.a and rhl1.b), recovery time of fluorescence was very short: 0.25 min in rhl1.a and 0.5 min in other genotypes analysed (Table1). In the apical elongation zone, the fluorescence recovery time was 3 min, regardless of root genotype. The most visible differences were observed in the basal part of the elongation and differentiation zones. In the basal part of the elongation zone, the shortest observed time was in the rhl1.a mutant, which was more than three times shorter in comparison to ‘Karat’ and the rhl1.b mutant. No recovery of fluorescence was observed in the differentiation zone of ‘Karat’, regardless of which cell (producing or not producing root hairs) was photobleached (Fig.3, Table1), while in both mutants the time needed for recovery was about 10 min within the differentiation zone (Fig.4, Table1). The obtained results suggest that in both mutants all epidermal cells in the differentiation zone were symplasmically connected, whereas in the parental variety, root hair cells and non-root hair cells were symplasmically isolated from neighbouring cells (Table1). These results were also confirmed using caged fluorescein (data not shown).
Figure 2.
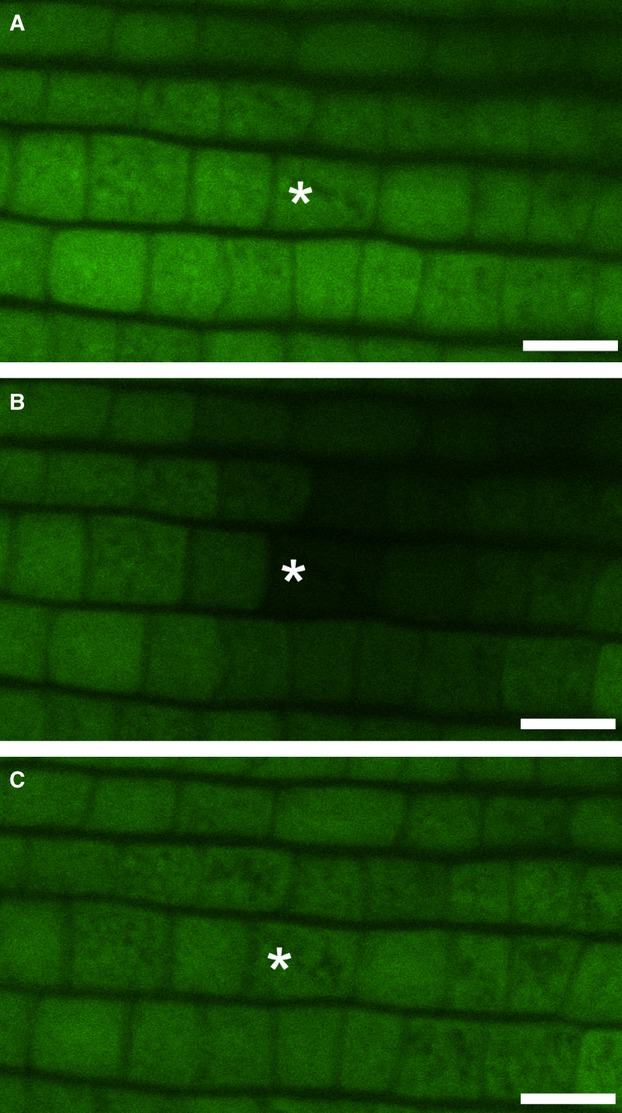
Fluorescence analysis of the symplasmic tracer FDA in epidermal root cells during the FRAP experiment. The meristematic zone of ‘Karat’ before photobleaching (A): immediately after photobleaching (B): and 30 min after photobleaching (C): Bar = 20 μm.
Table 1.
Transport of fluorescent probes between root epidermal cells. Quantification of fluorochrome movement after photobleaching of Hordeum vulgare L., cv. ‘Karat’ root epidermal cells at different stages of development
| ‘Karat’ | |||||
|---|---|---|---|---|---|
| mz | aez | bez | dz | ||
| hc | nhc | ||||
| % bleached cells that recovered fluorescence (n) | 100 (15) | 100 (10) | 53.8 (13) | 8.3 (12) | 14.3 (14) |
| 0.5 min | 3.0 min | 10.0 min | nfr | ||
| Average time of fluorescence recovery after photobleaching | 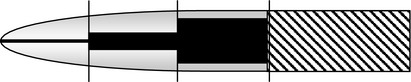 |
||||
| rhl1.a | |||||
|---|---|---|---|---|---|
| mz | aez | bez | dz | ||
| % bleached cells that recovered fluorescence (n) | 100 (10) | 100 (12) | 92.9 (14) | 78.6 (14) | |
| Average time of fluorescence recovery after photobleaching | 0.25 min | 3.00 min | 3.00 min | 10.00 min | |
 | |||||
| rhl1.b | |||||
|---|---|---|---|---|---|
| mz | aez | bez | dz | ||
| % bleached cells that recovered fluorescence (n) | 100 (10) | 100 (14) | 84.6 (13) | 84.6 (13) | |
| Average time of fluorescence recovery after photobleaching | 0.5 min | 3.0 min | 10.0 min | 10.0 min | |
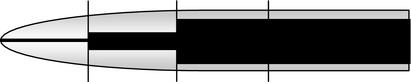 | |||||
The width of the black rectangle schematically represents the time of fluorescence recovery, the striped rectangle represents no fluorescence recovery; aez, apical elongation zone; bez, basal elongation zone; dz, differentiation zone; hc, hair cell; mz, meristematic zone; n, total number of analysed cells; nhc, non-hair cell; nfr, no fluorescence recovery.
Figure 3.
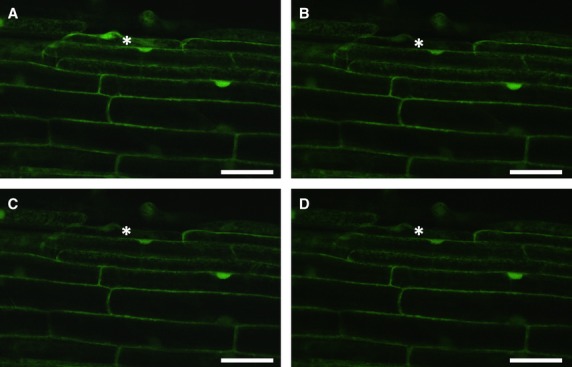
Lack of symplasmic communication between root epidermal cells of ‘Karat’. The differentiation zone of ‘Karat’ before photobleaching (A): immediately after photobleaching (B): 10 min after photobleaching (C): and 30 min after photobleaching (D): Bar = 50 μm.
Figure 4.
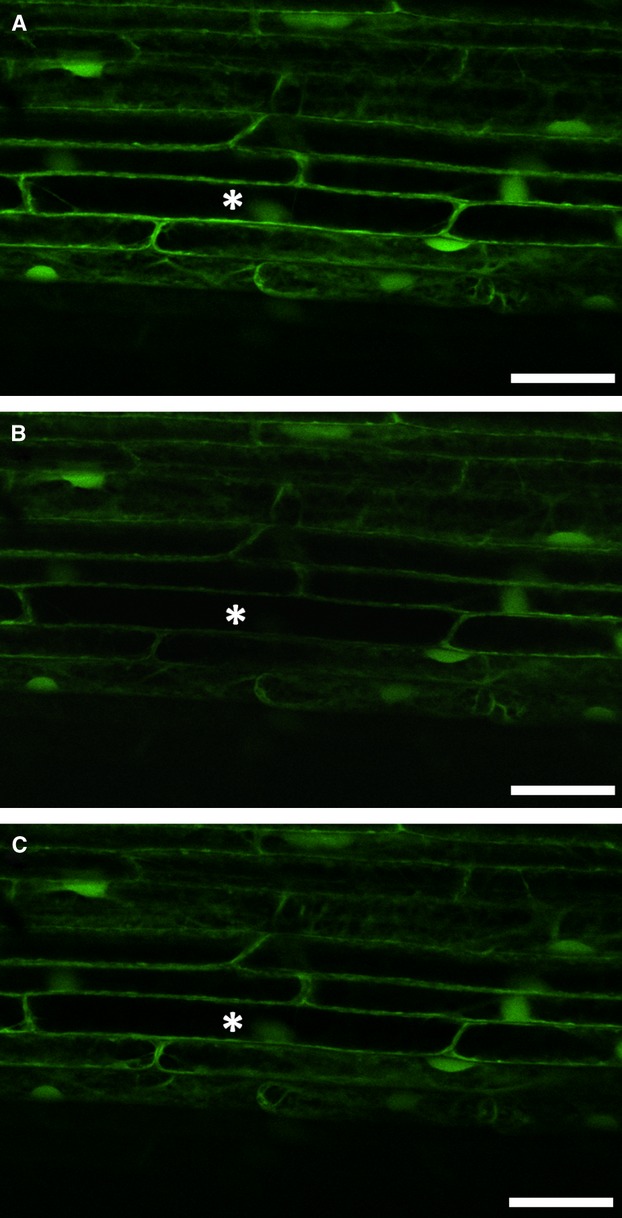
Symplasmic communication between root epidermal cells of rhl1.b. The differentiation zone of the mutant before photobleaching (A): immediately after photobleaching (B): and 10 min after photobleaching (C): Bar = 50 μm.
The next question was whether epidermal cells are symplasmically connected to other root tissues in different zones of barley root. In order to investigate this, HPTS was inserted with a cell pressure probe. After local insertion into cortical cells, it was clearly visible that the fluorochrome did not pass to epidermal cells but freely shifted to apical and basal part of the root within the other cortical cells. These results were obtained for ‘Karat’ and both allelic root hairless mutants (Fig.5).
Figure 5.
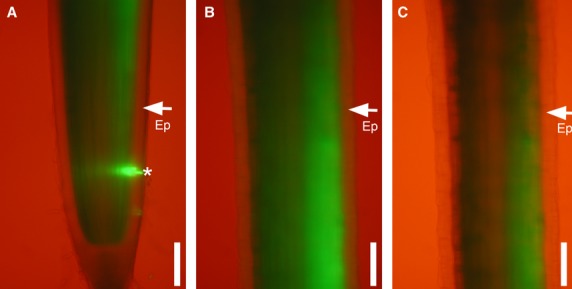
Fluorescence analysis of the HTPS symplasmic tracer in roots of rhl1.a after injection into the cortex of the meristematic zone. Meristematic zone (A): elongation zone (B): and differentiation zone (C): A star indicates the position of fluorochrome injection; arrows indicate the epidermis (Ep). Bar = 100 μm.
To understand symplasmic isolation during differentiation of barley epidermal cells, the ultrastructure of plasmodesmata and callose deposition in the parental variety and the rhl1.b mutant was analysed. No differences in plasmodesmata ultrastructure between plasmodesmata connecting the neighbouring epidermal cells in the meristematic zone, zone of cell elongation and zone of cell differentiation in ‘Karat’ were observed. In the case of the wild type, only simple plasmodesmata were observed in the root epidermis and not a single branched plasmodesmata was found in any of analysed zones of the root (Fig.6A,B). Similar results were obtained for rhl1.b; there were no differences between plasmodesmata localised in different zones of roots (Fig.6C,D), nor between the parental variety and mutant (Fig.6).
Figure 6.
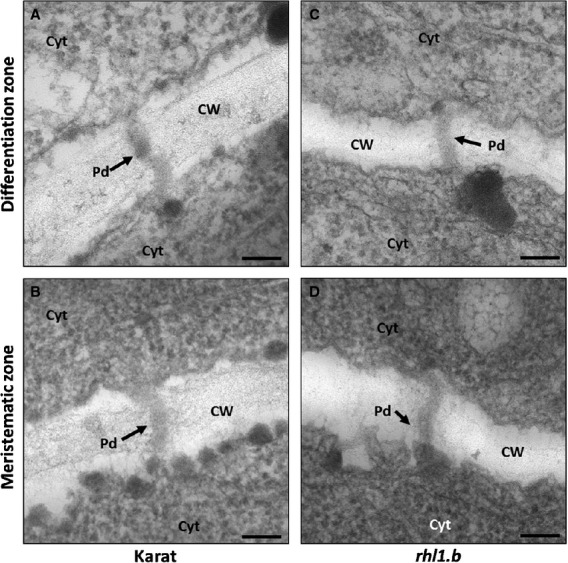
The ultrastructure of plasmodesmata. No differences were observed in the structure of plasmodesmata localised between root epidermal cells of the ‘Karat’ differentiation zone (A): and meristematic zone (B): Plasmodesmata in differentiation zone (C): and meristematic zone (D): of rhl1.b showed no differences. Pd, Plasmodesmata, Cyt, cytoplasm, CW, cell wall, bar = 100 μm.
Immunogold localisation of callose in ‘Karat’ revealed increasing deposition of this polysaccharide inside plasmodesmata between epidermal cells during their development. In the meristematic zone of roots, single molecules of callose had gold-labelled antibodies (Fig.7A,B); whereas in the zone where root epidermal cells started elongation and differentiation, a higher number of callose molecules was observed (Fig.7C,D). In contrast, in the case of the rhl1.b mutant, comparable amount of callose was deposited in plasmodesmata between root epidermal cells, regardless of the zone of the root (Fig.7E–H).
Figure 7.
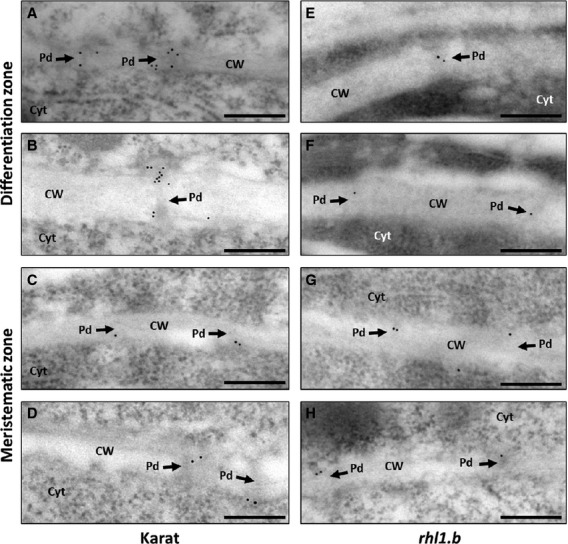
Immunolocalisation of callose in plasmodesmata. Callose deposition in plasmodesmata labelled with 10-nm gold particles in the periclinal cell wall of two epidermal cells of ‘Karat’ roots at the differentiation zone (A, B): and meristematic zone (C, D): and of the rhl1.b mutant root differentiation zone (E, F): and meristematic zone (G, H): Pd, Plasmodesmata, Cyt, cytoplasm, CW, cell wall, bar = 200 μm.
DISCUSSION
It is well known that cell-to-cell communication via plasmodesmata is important in determining the direction of cell differentiation (Rinne & van der Schoot 1998; Ruan et al. 2004; Kim & Zambryski 2005; Kurczyńska et al. 2007). The presented results show that in the ‘Karat’ variety during normal development of root epidermal cells into trichoblasts and atrichoblasts, symplasmic communication between epidermal cells decreased during cell differentiation. Communication via plasmodesmata between epidermal cells was shown in the meristematic zone of all three genotypes analysed; similar results were obtained for the same root part in A. thaliana (Duckett et al. 1994). Symplasmic transport between all epidermal cells indicates that at this stage of development the epidermis is a single symplasmic domain connected by open plasmodesmata; as also found for undifferentiated group of cells such as young embryo sacs of Torenia fournieri before fertilisation (Han et al. 2000) or young embryos of Arabidopsis (Kim & Zambryski 2008). In all of these cases, neighbouring epidermal cells were connected via open plasmodesmata. The results presented in this paper show that reduced symplasmic communication between trichoblasts and atrichoblasts occurs in the elongation zone of roots of the parent variety. Additionally, complete symplasmic isolation was detected in the mature root hair zone of the ‘Karat’ variety, what indicates that the root epidermis is no longer a single symplasmic domain but that symplasmic subdomains are generated within this tissue, including domains built by individual atrichoblasts and individual trichoblasts. Moreover, in both of the analysed root hairless mutants the restriction of symplasmic communication between root epidermal cells during cell elongation and differentiation was lower. It is well documented that plasmodesmata are the route for molecules such as RNAs and proteins, which play a key role during plant cell differentiation (Zambryski 2012). This is why symplasmic isolation of plant cells is necessary in order to start the individual developmental programme of a single or a group of cells. This could be why increased symplasmic permeability in barley root epidermal cells correlates with defects in root hair development. In addition, the obtained results suggest that symplasmic isolation or restriction of symplasmic communication is essential for the correct development of root hairs in barley, as in the case of Arabidopsis (Duckett et al. 1994). It is well documented that the number of different symplasmic domains present in a plant organ is positively correlated with the stage of cell development – in the mature stage of plant development there are a higher number of symplasmic domains present (Stadler et al. 2005), as found here for the mature root hair zone of barley.
In the presented paper HPTS and FDA were used as tracers with a molecular diameter around 0.9 nm (Heinlein & Epel 2004). In this case, the term ‘symplasmic isolation’ means that restriction of the plasmodesmata pathway concerns molecules with a diameter of 0.9 nm and more, suggesting that transport of water (0.96-nm molecular diameter) and nutrients via symplasm may occur without hindrance.
The presented results show that symplasmic communication between root epidermal cells in the two analysed root hairless mutants differed from the parent variety. In both mutants, movement of symplasmic tracers between epidermal cells was detected in all investigated root zones, i.e. the differentiation of cells is correlated with the degree of symplasmic communication between them. This is a first report indicating that in root hairless mutants, root epidermal cells are still symplasmically connected in all root zones, whereas symplasmic communication is restricted in wild-type plants. Moreover, this is the first time that a disturbance in regulation of symplasmic communication could be correlated with the root hairless phenotype in plants. Symplasmic isolation may take place in two different ways: by decreasing the number of plasmodesmata between cells, as in the case of guard cells (Wille & Lucas 1984), or by blocking the plasmodesmata with callose, as in Chara vulgaris, L. during spermatogenesis (Kwiatkowska & Maszewski 1985). Immunogold localisation of callose indicates that higher deposition of this polysaccharide plays a crucial role in the restriction of symplasmic communication between barley root epidermal cells during their differentiation. Moreover, the lack of increasing deposition of callose inside the plasmodesmata in the mutant with a root hairless phenotype confirms our hypothesis on the importance of symplasmic isolation in the process of cell differentiation. Although, also in both analysed mutants, there was a longer fluorescence recovery time in the elongation and differentiation zones in comparison to the meristematic zone of the root. These results may be explained by the lower number of plasmodesmata between cells localised above the meristematic zone; however this hypothesis remains to be confirmed during further experiments. The future work will focus on analysis of the number and distribution of plasmodesmata in barley epidermal cells of the root epidermis during differentiation, and also on analysis of expression profiles of genes encoding barley callose synthase proteins in roots of the parent varieties and different mutants of barley.
CONCLUSION
Our studies show that symplasmic communication between trichoblasts and atrichoblasts decreases gradually during the differentiation of these two types of cell in the parental variety of barley. This means that the mechanism of specialisation of epidermal root cell in monocots is similar to that of dicots. In root hairless mutants such a decrease in symplasmic communication was not observed, and this is the first report describing disorders in the regulation of symplasmic communication in root hairless mutants. To identify any correlations between the phenotype and the regulation of symplasmic communication in roots, additional studies are required. Root hairless mutants provide a good subject for future research, not only in terms of root hair development but also for symplasmic communication between cells.
Acknowledgments
We would like to thank M. Wiesner and K. Hoffie from the Structural Cell Biology Group, Leibniz Institute of Plant Genetics and Crop Plant Research, for the technical assistance. This research was supported by grants from the German Academic Exchange Service (50755272) and Polish National Science Center (2011/01/M/NZ2/02979). M.M. thanks the UPGOW project, co-financed by the European Social Fund, for a scholarship.
REFERENCES
- Bates TR, Lynch JP. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany. 2000;87:964–970. [PubMed] [Google Scholar]
- Benítez M, Espinosa-Soto C, Padilla-Longoria P, Alvarez-Buylla ER. Interlinked nonlinear subnetworks underlie the formation of robust cellular patterns in Arabidopsis epidermis: a dynamic spatial model. BMC Systems Biology. 2008;2:98. doi: 10.1186/1752-0509-2-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development. 2005;132:291–298. doi: 10.1242/dev.01565. [DOI] [PubMed] [Google Scholar]
- Cailloux M. Metabolism and the absorption of water by root hairs. Canadian Journal of Botany. 1972;50:557–573. [Google Scholar]
- Clowes FAL. Pattern in root meristem development in angiosperms. New Phytologist. 2000;146:83–94. [Google Scholar]
- Crawford KM, Zambryski PC. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiology. 2001;125:1802–1812. doi: 10.1104/pp.125.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiuni S, Schellmann S, Geier F, Greese B, Pesch M, Wester K, Dartan B, Mach V, Srinivas BP, Timmer J, Fleck C, Hulskamp M. A competitive complex formation mechanism underlies trichome patterning on Arabidopsis leaves. Molecular Systems Biology. 2008;4:217. doi: 10.1038/msb.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T. Cell–cell communication during double fertilization. Current Opinion in Plant Biology. 2006;9:41–47. doi: 10.1016/j.pbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Duckett CM, Oparka KJ, Prior DAM, Dolan L, Roberts K. Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development. 1994;120:3247–3255. [Google Scholar]
- Engvild KC, Rasmussen SK. Root hair mutants of barley. Barley Genetics Newsletter. 2004;34:13–15. [Google Scholar]
- Föhse D, Claassen N, Jungk A. Phosphorus efficiency in plants. Plant and Soil. 1991;132:261–272. [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. A root hairless barley mutant for elucidating genetics of root hairs and phosphorus uptake. Plant and Soil. 2001;237:211–219. [Google Scholar]
- Grabov A, Bottger M. Are redox reactions involved in regulation of K+ channels in the plasma membrane of Limnobium stoloniferum root hairs? Plant Physiology. 1994;105:927–935. doi: 10.1104/pp.105.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YZ, Huang BQ, Zee SY, Yuan M. Symplastic communication between the central cell and the egg apparatus cells in the embryo sac of Torenia fournieri Lind. before and during fertilization. Planta. 2000;211:158–162. doi: 10.1007/s004250000289. [DOI] [PubMed] [Google Scholar]
- Heinlein M, Epel BL. Macromolecular transport and signaling through plasmodesmata. International Review of Cytology. 2004;235:93–164. doi: 10.1016/S0074-7696(04)35003-5. [DOI] [PubMed] [Google Scholar]
- Horn R, Yi K, Menand B, Pernas-Ochoa M, Takeda S, Walker T, Colan L. Root epidermal development in Arabidopsis. Annual Review of Plant Biology. 2009;37:64–82. [Google Scholar]
- Itoh S, Barber SA. Phosphorus uptake by six plant species as related to root hairs. Agronomy Journal. 1983;75:457–461. [Google Scholar]
- Jorgensen RA. RNA traffics information systemically in plants. Proceedings of the National Academy of Sciences USA. 2002;99:11561–11563. doi: 10.1073/pnas.192449099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Zambryski PC. Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Current Opinion in Plant Biology. 2005;8:593–599. doi: 10.1016/j.pbi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Kim I, Zambryski PC. Intercellular trafficking of macromolecules during embryogenesis. Methods in molecular biology. 2008;427:145–155. doi: 10.1007/978-1-59745-273-1_12. [DOI] [PubMed] [Google Scholar]
- Kim I, Kobayashi K, Cho E, Zambryski PC. Subdomains for transport via plasmodesmata corresponding to the apical–basal axis established during Arabidopsis embryogenesis. Proceedings of the National Academy of Sciences USA. 2005;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CM, Park SH, Je BI, Park SH, Park SJ, Piao HL, Eun MY, Dolan L, Han C. OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiology. 2007;143:1220–1230. doi: 10.1104/pp.106.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurara T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, Sano R, Nagasaka R, Tominaga R, Koshino-Kimura Y, Kato T, Sato S, Tabata S, Okada K, Wada T. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development. 2005;132:5387–5398. doi: 10.1242/dev.02139. [DOI] [PubMed] [Google Scholar]
- Kurczyńska EU, Gaj MD, Ujczak A, Mazur E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta. 2007;226:619–628. doi: 10.1007/s00425-007-0510-6. [DOI] [PubMed] [Google Scholar]
- Kwaśniewski M, Szarejko I. Molecular cloning and characterization of β-expansin gene related to root hair formation in barley. Plant Physiology. 2006;141:1149–1158. doi: 10.1104/pp.106.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska M, Maszewski J. Changes in ultrastructure of plasmodesmata during spermatogenesis in Chara vulgaris L. Planta. 1985;166:46–50. doi: 10.1007/BF00397384. [DOI] [PubMed] [Google Scholar]
- Lauter FR, Ninnemann O, Bucher M, Riesmeier JW, Frommer WB. Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato. Proceedings of the National Academy of Sciences USA. 1996;93:8139–8144. doi: 10.1073/pnas.93.15.8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Ding B, van der Schoot C. Plasmodesmata and the supracellular nature of plants. The Journal of Physiology. 1993;125:435–476. doi: 10.1111/j.1469-8137.1993.tb03897.x. [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Yoo BC, Kragler F. RNA as a long-distance information macromolecule in plants. Nature Reviews, Molecular Cell Biology. 2001;2:849–857. doi: 10.1038/35099096. [DOI] [PubMed] [Google Scholar]
- Maule AJ. Plasmodesmata: structure, function and biogenesis. Current Opinion in Plant Biology. 2008;11:680–686. doi: 10.1016/j.pbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Cruz SS. The great escape: phloem transport and unloading of macromolecules. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:323–347. doi: 10.1146/annurev.arplant.51.1.323. [DOI] [PubMed] [Google Scholar]
- Petricka JJ, Benfey PN. Root layers: complex regulation of developmental patterning. Current Opinion in Genetics & Development. 2008;18:354–361. doi: 10.1016/j.gde.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouillat J, Dievart A, Verdeil JL, Escoute J, Giese G, Breitler JC, Gantet P, Espeout S, Guiderdoni E, Périn C. Molecular genetics of rice root development. Rice. 2009;2:15–34. [Google Scholar]
- Rinne PLH, van der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development. 1998;125:1477–1485. doi: 10.1242/dev.125.8.1477. [DOI] [PubMed] [Google Scholar]
- Roberts AG, Oparka KJ. Plasmodesmata and the control of symplastic transport. Plant, Cell & Environment. 2003;23:103–124. [Google Scholar]
- Ruan YL, Xu SM, White R, Furbank RT. Genotypic and developmental evidence for the role of plasmodesmatal regulation in cotton fiber elongation mediated by callose turnover. Plant Physiology. 2004;136:4104–4113. doi: 10.1104/pp.104.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Current Opinion in Plant Biology. 2004;7:641–650. doi: 10.1016/j.pbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Savage NS, Walker T, Wieckowski Y, Schiefelbein J, Dolan L, Monk NAM. A mutual support mechanism through intercellular movement of CAPRICE and GLABRA3 can pattern the Arabidopsis root epidermis. PLoS Biology. 2008;6:1899–1909. doi: 10.1371/journal.pbio.0060235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Lauterbach C, Sauer N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiology. 2005;139:701–712. doi: 10.1104/pp.105.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In: Smith JAC, Griffiths H, editors. Water deficits: plant responses from cell to community. Oxford, UK: Bios Scientific; 1993. pp. 5–36. [Google Scholar]
- Szarejko I, Janiak A, Chmielewska B, Nawrot M. Description of several root hair mutants of barley. Barley Genetics Newsletter. 2005;35:36–38. [Google Scholar]
- Teige M, Melzer M, Süss K-H. Purification, properties and in situ localization of the amphibolic enzymes D-ribulose-5-phosphate 3-epimerase and transketolase from spinach chloroplasts. European Journal of Biochemistry. 1998;252:237–244. doi: 10.1046/j.1432-1327.1998.2520237.x. [DOI] [PubMed] [Google Scholar]
- Thiel J, Riewe D, Rutten T, Melzer M, Friedel S, Bollenbeck F, Weschke W, Weber H. Differentiation of endosperm transfer cells of barley – a comprehensive analysis at the micro-scale. The Plant Journal. 2012;71:639–655. doi: 10.1111/j.1365-313X.2012.05018.x. [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. (Arabidopsis CAPRICE-LIKE MYB 3 CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development. 2008;135:1335–1345. doi: 10.1242/dev.017947. [DOI] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development. 2002;129:5409–5419. doi: 10.1242/dev.00111. [DOI] [PubMed] [Google Scholar]
- Wen TJ, Schnable PS. Analysis of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggests that root hairs are dispensable. American Journal of Botany. 1994;81:833–842. [Google Scholar]
- Wille AC, Lucas WJ. Ultrastructural and histochemical studies on guard cells. Planta. 1984;160:129–142. doi: 10.1007/BF00392861. [DOI] [PubMed] [Google Scholar]
- Wrobel J, Barlow PW, Gorka K, Nabialkowska D, Kurczynska EU. Histology and symplasmic tracer distribution during development of barley androgenic embryos. Planta. 2011;233:873–881. doi: 10.1007/s00425-010-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuo T, Toyota M, Ichii M, Taketa S. Molecular cloning of root hairless gene rth1 in rice. Breeding Science. 2009;59:13–20. [Google Scholar]
- Zambryski P. Plasmodesmata paradigm shift: regulation from without versus within. Annual Review of Plant Biology. 2012;63:239–260. doi: 10.1146/annurev-arplant-042811-105453. [DOI] [PubMed] [Google Scholar]
- Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development. 2008;135:1991–1999. doi: 10.1242/dev.016873. [DOI] [PubMed] [Google Scholar]


