Abstract
Aims
To characterize the pathological features of pulmonary cysts, and to elucidate the possible mechanism of cyst formation in the lungs of patients with Birt–Hogg–Dubé syndrome (BHDS), a tumour suppressor gene syndrome, using histological and morphometric analyses.
Methods and results
We evaluated 229 lung cysts from 50 patients with BHDS and 117 from 34 patients with primary spontaneous pneumothorax (PSP) for their number, size, location and absence or presence of inflammation. The BHDS cysts abutted on interlobular septa (88.2%) and had intracystic septa (13.6%) or protruding venules (39.5%) without cell proliferation or inflammation. The frequencies of these histological characteristics differed significantly from those seen in the lungs of patients with PSP (P < 0.05). Although the intrapulmonary BHDS cysts were smaller than the subpleural BHDS cysts (P < 0.001), there was no difference in size between them when there was no inflammation. The number of cysts diminished logarithmically and the proportion of cysts with inflammation increased as their individual sizes became greater (P < 0.05).
Conclusions
These results imply that the BHDS cysts are likely to develop in the periacinar region, an anatomically weak site in a primary lobule, where alveoli attach to connective tissue septa. We hypothesize that the BHDS cysts possibly expand in size as the alveolar walls disappear at the alveolar-septal junction, and grow even larger when several cysts fuse.
Keywords: alveolar-septal junction, cell–matrix interaction, folliculin, mechanical stresses, TGF-β
Introduction
Birt–Hogg–Dubé syndrome (BHDS) is an autosomal dominant disorder characterized by hamartomas of the hair follicle, renal tumours, and multiple lung cysts accompanying spontaneous recurrent pneumothorax.1 The FLCN gene responsible for BHDS was cloned in 2002,2 but the function of folliculin, the protein encoded by FLCN, is not completely clear. Several studies have shown that the function of folliculin-binding proteins (FNIP1 and FNIP2) involves 5′-AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR) pathway, and that a complete loss of folliculin function leads to BHDS-associated tumorigenesis through dysregulation of AMPK and the mTOR pathway.3–8
Clinically, approximately 85% of BHDS patients have fibrofolliculoma diagnosed by histological testing of skin or lung cysts detected by CT imaging of the chest; additionally, 29–34% of these patients have renal tumours visible by CT imaging.1,9 On molecular analysis, BHDS-associated renal tumours have a somatic mutation of a second copy of FLCN,10 whereas fibrofolliculomas of BHDS patients do not necessarily have FLCN loss of heterozygosity (LOH), indicating that haploinsufficiency of FLCN leads to tumour-like lesions of the hair follicle.11 In contrast to the kidney and skin lesions, neither tumour formation nor proliferation of abnormal cells has ever been reported as a feature of the pulmonary manifestations, for which multiple cysts constitute the sole abnormality in both radiological and pathological studies. In addition, as for fibrofolliculomas, the high penetrance of lung cysts11,12 may indicate that the latter occur through haploinsufficiency of FLCN, and that LOH analysis of cysts is not as useful as it is for renal tumours and fibrofolliculomas.
The mechanism of cyst formation in BHDS is not well understood. Therefore, we believe that it is necessary to define the histopathological findings for these cysts and underlying parenchyma from a large number of BHDS patients. Previously, in lung specimens from such patients, bullae or blebs were found with underlying emphysematous changes,13–15 thin-walled cysts were surrounded by normal parenchyma,16–18 or the cysts showed a predominance of type II pneumocyte-like cuboidal cells.4 However, the number of BHDS patients examined in these studies was small, and the focus might have been on pleural or subpleural cysts, the pathological findings for which would be significantly influenced by pneumothorax and pneumothorax-associated inflammation.
Here, we report the histological and morphometric characteristics of 229 lung cysts from 50 patients with BHDS, the largest cohort ever included in an investigation of the lung pathology of this disorder.
Materials and methods
Lung specimens were obtained from 50 Asian patients (49 Japanese and one Chinese) with BHDS from the archives or consultation files in the Pneumothorax Centre, Tamagawa Hospital, and Division of Respiratory Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine (Table1). BHDS was diagnosed by the use of FLCN genetic tests, as described previously.19,20 The age (median) at operation in the 50 patients was 38.5 years, ranging from 24 to 66 years (38 years, ranging from 27 to 50 years, in 19 men; 41 years, ranging from 24 to 66 years, in 31 women) (Table1). Thirteen patients were smokers, four were ex-smokers, 30 had never smoked, and three lacked any documented smoking history. A total of 229 lung cysts (79 in men; 150 in women) were identified in the 350 tissue sections that we examined.
Table 1.
Summary of Birt–Hogg–Dubé syndrome cases
| No | Age (years) | Sex | Smoking history | Location | FLCN mutation | No. of tissue sections | No. of cysts | Other findings |
|---|---|---|---|---|---|---|---|---|
| 1 | 38 | M | S | Exon 4 | c.119delG | 7 | 2 | |
| 2 | 38 | F | S | Exon 5 | c.328C>T | 1 | 1 | |
| 3 | 38 | F | S | Intron 5 | c.396 + 1G>A | 3 | 3 | |
| 4 | 29 | M | N | Intron 5 | c.397-2A>C | 8 | 1 | |
| 5 | 36 | F | U | Exon 6 | c.397-13_397-4delGGCCCTCCAG | 1 | 3 | |
| 6 | 37 | F | U | Exon 6 | c.402delC | 2 | 3 | |
| 7 | 39 | F | S | Exon 7 | c.769_771delTCC | 7 | 8 | |
| 8 | 40 | F | N | Exon 7 | c.769_771delTCC | 9 | 8 | |
| 9 | 47 | F | N | Exon 8 | c.853C>T | 7 | 1 | Fibrosis |
| 10 | 38 | F | N | Exon 9 | c.889_890delGA | 7 | 5 | |
| 12 | 48 | F | N | Exon 9 | c.932_933delCT | 6 | 3 | |
| 11 | 44 | F | N | Exon 9 | c.991_992dupTC | 10 | 11 | |
| 13 | 48 | M | N | Exon 9 | c.997_998delTC | 8 | 4 | |
| 14 | 34 | M | S | Intron 9 | c.1063-2A>G | 8 | 8 | Emphysema |
| 15 | 53 | F | N | Exon 10 | c.1063-10_1065delTCTTGTTTAGGTC | 5 | 6 | |
| 16 | 24 | F | S | Exon 11 | c.1285dupC | 6 | 1 | |
| 17 | 29 | M | S | Exon 11 | c.1285dupC | 4 | 2 | |
| 18 | 33 | F | N | Exon 11 | c.1285dupC | 7 | 1 | Granuloma |
| 19 | 35 | F | S | Exon 11 | c.1285dupC | 7 | 13 | |
| 20 | 35 | M | N | Exon 11 | c.1285dupC | 3 | 1 | |
| 21 | 38 | M | S | Exon 11 | c.1285dupC | 5 | 3 | |
| 22 | 39 | M | S | Exon 11 | c.1285dupC | 12 | 6 | |
| 23 | 41 | F | N | Exon 11 | c.1285dupC | 20 | 9 | |
| 24 | 43 | F | N | Exon 11 | c.1285dupC | 4 | 8 | |
| 25 | 47 | F | N | Exon 11 | c.1285dupC | 6 | 1 | |
| 26 | 50 | M | N | Exon 11 | c.1285dupC | 10 | 6 | |
| 27 | 62 | F | N | Exon 11 | c.1285dupC | 4 | 1 | |
| 28 | 64 | F | S | Exon 11 | c.1285dupC | 24 | 15 | Granuloma |
| 29 | 31 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 5 | 4 | |
| 30 | 32 | M | U | Exon 12 | c.1347_1353dupCCACCCT | 3 | 7 | |
| 31 | 38 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 15 | 5 | |
| 32 | 42 | F | S | Exon 12 | c.1347_1353dupCCACCCT | 7 | 8 | |
| 33 | 43 | F | S | Exon 12 | c.1347_1353dupCCACCCT | 7 | 5 | |
| 34 | 43 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 5 | 6 | |
| 35 | 45 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 8 | 4 | |
| 36 | 48 | M | S | Exon 12 | c.1347_1353dupCCACCCT | 6 | 4 | |
| 37 | 57 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 5 | 4 | |
| 38 | 66 | F | N | Exon 12 | c.1347_1353dupCCACCCT | 4 | 2 | Emphysema |
| 39 | 46 | M | N | Exon 12 | c.1429C>T | 5 | 2 | |
| 40 | 32 | F | N | Intron 12 | c.1433-1G>T | 8 | 7 | |
| 46 | 43 | M | N | Exon 13 | c.1489_1490delGT | 4 | 1 | |
| 41 | 26 | F | S | Exon 13 | c.1533_1536delGATG | 1 | 1 | |
| 42 | 27 | M | S | Exon 13 | c.1533_1536delGATG | 5 | 1 | |
| 43 | 34 | M | N | Exon 13 | c.1533_1536delGATA | 15 | 6 | |
| 44 | 35 | M | S | Exon 13 | c.1533_1536delGATG | 21 | 8 | |
| 45 | 38 | M | N | Exon 13 | c.1533_1536delGATG | 4 | 3 | |
| 47 | 29 | M | N | Exon 14 | c.1539-?_c.1740 + ?del | 8 | 8 | |
| 48 | 46 | M | N | Exon 14 | c.1539-?_c.1740 + ?del | 6 | 6 | |
| 49 | 31 | F | N | Exons 9–14 | c.872-?_c.1740 + ?del | 6 | 2 | |
| 50 | 58 | F | N | Exons 9–14 | c.872-?_c.1740 + ?del | 1 | 1 |
N, never smoker; S, current smoker; U, unknown.
Lung tissues were obtained using video-assisted thoracic surgery (VATS), undertaken for the treatment of pneumothorax or for the diagnosis of cystic lung diseases, and were then appropriately inflated, and fixed with 10% buffered formaldehyde. After routine preparation, the formalin-fixed paraffin-embedded tissues were sectioned and stained with haematoxylin and eosin and Elastica–Masson trichrome (EM) or Elastica–Van Gieson (EVG) stains. We evaluated chronic inflammation in each cyst on low-power magnification (×4 objective lens attached to a BX51 microscope; Olympus, Tokyo, Japan): the presence of cellular inflammation was defined as the accumulation of lymphocytes or plasma cells; the presence of fibrous inflammation was defined by the presence of dense (sub)pleural scars and/or fibrotic lung tissue with replacement of architecture.21 We measured the maximum diameter of each cyst on the sections stained with EM or EVG by using the ocular micrometer on a microscope (U-OCM10/100; Olympus) or micrometer callipers on a glass slide (Shinwa, Nagoya, Japan).
As a control for the analysis of pulmonary cysts, lung tissues were used from 34 Japanese patients presenting with primary spontaneous pneumothorax (PSP) to the Japanese Red Cross Medical Centre. The median age of these 34 patients was 24 years, ranging from 18 to 30 years (33 men, and one woman aged 25 years). All of the 117 cysts associated with PSP were diagnosed as bullae and/or blebs.
Statistical analysis was performed using the Mann–Whitney U-test and χ2-test (statmate III for Windows; ATMS, Tokyo, Japan), or the Kruskal–Wallis test (ibm spss statistics; IBM Japan, Tokyo, Japan). A P-value of <0.05 was considered to be statistically significant. This study was approved by the ethical committee in Juntendo University School of Medicine (No. 17053) and by the ethical committee for clinical studies in the Japanese Red Cross Medical Centre (No. 429).
Results
Histological Characteristics
The lung tissues obtained from 45 of 50 patients with BHDS had normal parenchyma, whereas those from the other five had centrilobular emphysema (two patients), granulomas (two patients) or fibrosis (one patient) in the parenchyma (Table1, and data not shown). Macroscopic findings demonstrated that the lung cysts, which occasionally contained intracystic septa, had very thin and translucent walls, and were surrounded by normal lung parenchyma in all patients (Figure1A and B). The intracystic septa seen in 13.6% of BHDS cysts were composed of interlobular septa, and venules protruding into the cyst (observed in 39.5% of BHDS cysts) sometimes showed regression of surrounding connective tissue (Figure1C). The anatomical and histological findings were characterized by the following features (Table2). Half of the lung cysts were located in the subpleural area (Figure1D), and the remainder in the intrapulmonary area (Figure1E); the cysts abutted on interlobular septa but rarely on bronchioles. The BHDS cysts, especially those located in lung parenchyma, were not at all or little affected by inflammation, including fibrosis (Figure1F), although approximately one-third of subpleural cysts showed mild inflammation, including fibroblast proliferation (Figure1G) and lymphocyte infiltration (Figure1H). The pulmonary cysts of BHDS patients showed far more clearly defined pathological features than the pulmonary cysts (bullae or blebs) of PSP patients, and differences were statistically significant (Table2). In BHDS patients: (i) cysts were present in both subpleural and intrapulmonary areas; (ii) cysts frequently abutted on interlobular septa, often had venules protruding into the cyst, and occasionally accompanied intracystic septa, suggesting the periacinar development of cysts in a primary lobule; and (iii) cysts usually had no sign of inflammation, especially those in intrapulmonary areas.
Figure 1.

Representative pathological findings of pulmonary cysts from patients with Birt–Hogg–Dubé syndrome (BHDS). A, A cyst in the subpleural area has, macroscopically, a very thin, translucent wall with an intracystic septum indicated by a white arrow (scale bar: 5 mm). B, The cyst shown in A was located in the area adjacent to an interlobular septum including pulmonary veins, and has a very thin intracystic septum (indicated by the black arrow) (Elastica–Masson trichrome stain). C, Vessels in the interlobular septa frequently protrude into the cyst. Note that the connective tissue surrounding one of the of the vessels is decreased (indicated by a small black arrow) (Elastica–Masson trichrome stain). D, Two subpleural cysts abut on an interlobular septum (small arrowheads), and the opposite side of each cyst wall is composed of thin pleural wall (CL indicates a centrilobular area). E, An intrapulmonary cyst abuts on an interlobular septum (large arrowheads), and the other side of the cyst wall is composed of thin alveolar wall (CL indicates a centrilobular area.). F, Approximately half of all cysts that we examined in this study were composed of normal alveolar walls with neither cell proliferation nor inflammatory cell infiltrates (* indicates intracystic area). However, some cysts from BHDS have inflammation, and representative photomicrographs of subpleural cysts are presented in G and H. In G, the basal side of a subpleural cyst abuts on an interlobular septum without inflammation, whereas its pleural side shows thickened visceral pleura with fibroblast proliferation. In H, the very thin wall of a subpleural cyst shows but lymphocyte infiltration but no fibrous thickening.
Table 2.
Comparison of the numbers of cysts in lung specimens from patients with Birt–Hogg–Dubé syndrome (BHDS) and primary spontaneous pneumothorax (PSP) [no. (%)]
| Histological findings | Cysts from BHDS patients (n = 229) | Cysts from PSP patients (n = 117) | χ2 -test |
|---|---|---|---|
| Cysts located in | |||
| Subpleural area | 116 (50.7) | 115 (98.3) | P < 0.001 |
| Intrapulmonary area | 113 (49.3) | 2 (1.7) | |
| Cysts abutting on | |||
| Interlobular septa | 202 (88.2) | 16 (13.7) | P < 0.001 |
| Bronchiole | 11 (4.8) | 42 (35.9) | P < 0.001 |
| Intracystic septa | 31 (13.6) | 0 (0) | P < 0.001 |
| Venules protruding into the cyst | 90 (39.5) | 2 (1.7) | P < 0.001 |
| Cysts without inflammation | |||
| Total | 125/229 (54.6) | 2/117 (1.7) | P < 0.001 |
| Subpleural area | 37/116 (31.9) | 2/115 (1.7) | P < 0.001 |
| Intrapulmonary area | 88/113 (77.9)* | 0/2 (0) | NS (P = 0.177) |
P < 0.001 for comparison of the numbers of cysts without inflammation between the subpleural and intrapulmonary areas.
NS, not significant.
BHDS and PSP patients were then compared for the pathological features (inflammatory site and type) of subpleural cysts with inflammation (Figure2). Most cysts from PSP patients were located in subpleural areas and had inflammatory infiltrates. Subpleural BHDS cysts that were inflamed were less likely to have such inflammation (especially of the fibrous type) at a basal site (i.e. proximal part of a subpleural cyst) (Table3). The most prominent feature of subpleural BHDS cysts that distinguished them from PSP cysts was the former's almost complete absence of fibrous inflammation in the basal area, with 94.8% sensitivity and 92.2% specificity.
Figure 2.
Representative photomicrographs showing cyst-associated cellular and/or fibrous inflammation: A, no inflammation (from an intrapulmonary cyst in Birt–Hogg–Dubé syndrome, BHDS: note that the cyst abuts on an interlobular septum in the upper area); B, cellular inflammation (from a subpleural cyst in BHDS); C, fibrous inflammation (from bullae in primary spontaneous pneumothorax, PSP); and D, cellular and fibrous inflammation (from bullae in PSP).
Table 3.
Comparison of the numbers of subpleural cysts with inflammation in Birt–Hogg–Dubé syndrome (BHDS) and primary spontaneous pneumothorax (PSP). Inflammation was examined with regard to the location of the cyst (pleural or basal site) and type of inflammation (cellular or fibrous)
| No. of subpleural cysts examined | No. of cysts with inflammation (%) | Inflammation at pleural site, no. (%) | Inflammation at basal site, no. (%) | |||
|---|---|---|---|---|---|---|
| Cellular | Fibrous | Cellular | Fibrous | |||
| BHDS | 116 | 79 (68.1) | 75 (64.7) | 55 (47.4) | 19 (16.4) | 6 (5.2) |
| PSP | 115 | 115 (100) | 73 (63.5) | 115 (100) | 56 (48.7) | 106 (92.2) |
| χ2- test | P < 0.001 | P = 0.852 | P < 0.001 | P < 0.001 | P < 0.001 | |
Morphometric Analysis
We examined the histological features of cysts in terms of size and location in the lung parenchyma. The maximum diameter of cysts associated with BHDS ranged from 1.0 to 15.7 mm (median: 3.8 mm), and two-thirds of them had diameters of ≤5 mm. A histogram depicting our analysis of size shows that the number of the cysts logarithmically diminished as the maximum cyst size increased [correlation coefficient for the fitted curve, y = −23.3 ln(x) + 63.0, R2 = 0.925] (Figure3). In addition, the proportion of cysts with inflammation increased as the maximum cyst size increased. However, no significant difference was noted in maximal cyst size between men and women [median 4.0 mm (range 1–15.7 mm) versus 3.5 mm (range 1.0–13.2 mm), P = 0.6908] or between patients with or without a history of smoking [median 4.0 mm (range 1.0–12.6 mm) versus 3.4 mm (range 1.0–15.7), P = 0.1508]. Statistical significance was evident for the larger size of subpleural cysts than of intrapulmonary cysts [median 5.0 mm (range 1.0–15.7 mm) versus 3.0 mm (range 1.0–9.8 mm), P < 0.0001] and for the larger size of cysts with inflammation than of those without inflammation [median 4.7 mm (range 1.1–15.7 mm) versus 3.3 mm (range 1.0–9.8 mm), P < 0.0001]. When we evaluated the influence of location or inflammation on maximum cyst size, the results demonstrated that subpleural cysts with inflammation were significantly larger than those without inflammation. However, the size of intrapulmonary cysts was not affected by the presence or absence of inflammation, and the size of subpleural cysts without inflammation resembled that of non-inflamed intrapulmonary cysts (Figure4).
Figure 3.
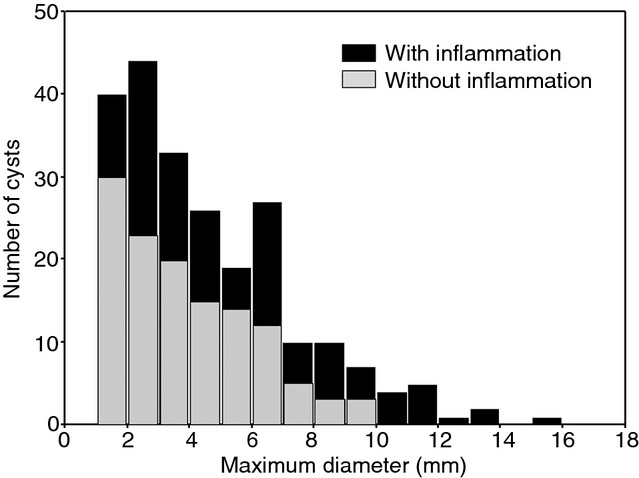
Distribution of the maximum diameter of pulmonary cysts in patients with Birt–Hogg–Dubé syndrome. Black and grey columns indicate the numbers of pulmonary cysts with and without inflammation, respectively.
Figure 4.

Comparison of the maximum diameters of intrapulmonary and subpleural cysts in patients with BHDS.
Discussion
We have demonstrated the unique histological characteristics of pulmonary cysts from 50 unrelated patients with BHDS, the largest cohort ever included in a study of lung pathology focusing on BDHS. Our results show that pulmonary BHDS cysts are: (i) surrounded by normal alveolar walls; (ii) abut on interlobular septa; and (ii) may have intracystic septa and/or protrusion of venules into the cystic space, indicating disappearance of the surrounding alveolar wall and/or regression of connective tissue of interlobular septa. These histological characteristics can differentiate BHDS from other cystic lung diseases. For example, tuberous sclerosis complex (TSC)-associated lymphangioleiomyomatosis (LAM) always shows LAM cell proliferation in the cyst walls. In other hereditary cystic lung diseases, such as cystic fibrosis, Ehlers–Danlos syndrome, and Marfan syndrome, patients have non-specific cystic lesions with cellular or fibrous inflammation.22–24 In the non-hereditary lung cystic diseases, including Langerhans cell histiocytosis, amyloidosis, Sjögren syndrome, and lymphocytic interstitial pneumonia, infiltration of inflammatory cells and/or matrix deposition always occurs.25
The present study clearly establishes that neither inflammation nor cell proliferation contributes to cyst formation in patients with BHDS, because most of their cysts, especially intrapulmonary BHDS cysts that do not suffer from the secondary effects of pneumothorax, show neither inflammation nor abnormal cell proliferation. As the majority of BHDS cysts are located far from bronchioles, the mechanism for cyst formation in BHDS is less likely to be associated with a check-valve mechanism, which is supposedly operative on cyst formation in PSP, smoking-related diseases, Sjögren syndrome, and other non-hereditary cystic lung diseases. We have demonstrated in the present study that most of the intrapulmonary BHDS cysts (88/113, 77.9%) lack inflammation, whereas only approximately one-third of the subpleural cysts (37/116, 31.9%) have no inflammation (Table2). Accordingly, we think that most, if not all, of the inflammatory changes observed were associated with pneumothorax. It has already been well described that pneumothorax causes pleural inflammation at the pleural side of the cyst, but not at the basal side.26 In addition, an animal experiment clearly showed that repeated injection of air into the pleural space caused inflammation and the formation of ‘neomembranes’ composed of fibroblasts and collagen that was variably covered by proliferation of mesothelial cells.27 In addition, the finding that the BHDS cysts without inflammation had no significant difference in size, irrespective of whether they were subpleural or intrapulmonary (Figure4), suggests that inflammation secondary to pneumothorax is likely to contribute to the subsequent growth of subpleural cysts in BHDS. Interestingly, we have demonstrated a logarithmic decline in the number of cysts as the size of individual cysts increases. Possibly, the fusion of small cysts resulted in the enlargement that we noted. This process may also explain how intracystic septa develop in BHDS cysts, as abutting cysts could fuse with intervening interlobular septa. In this context, the protrusion of venules into ∼40% of the BHDS cysts may have been caused not only by the disappearance of alveolar walls adjoining interlobular septa, but also by regression of their surrounding connective tissue in the septa.
The mechanism for development of pulmonary cysts in BHDS has been discussed in several reports. Graham et al.14 speculated that a genetic abnormality was responsible for postnatal alveolar proliferation of the peripheral lung, on the basis of pathological examination of three BHDS non-smokers, as they found cysts predominantly in the subpleural area. Warren et al.28 found that FLCN was expressed in type I pneumocytes and stromal cells, including fibroblasts and macrophages in the lungs, and hence proposed a possible role for functional abnormalities of these folliculin-expressing cells in cyst formation. Recently, Furuya et al.4 reported that dysregulation of the mTOR pathway resulting from haploinsufficiency of FLCN may induce cyst formation through proliferation of type II pneumocytes. However, the above mechanisms were deduced from an examination of limited numbers of lung specimens, and are therefore unlikely to fit the histopathological features of BHDS cysts and lungs defined after detailed analysis of the much larger specimen sample presented here. For example, cysts are present not only in subpleural areas but also in parenchyma, and most cysts have neither cellular proliferation nor inflammation, especially cysts in the parenchymal area, where secondary changes resulting from pneumothorax would not affect the pathological findings, in contrast to cysts at subpleural sites. In addition, when bullae/blebs are affected by pneumothorax, they will usually have reactive proliferation of type II pneumocytes. If proliferation of type II pneumocytes were actively involved in cyst formation, those cysts would show no predilection for the location and distribution recorded here; instead, they should be detectable not only in the area surrounded by interlobular septa, but also in the centrilobular area. Furthermore, one would expect cyst formation to proceed by proteolysis, as in smoke-related inflammatory diseases, collagen diseases, and neoplastic diseases such as LAM.29 Otherwise, the proliferation of type II pneumocytes might form a lung tumour, like multifocal micronodular pneumocyte hyperplasia occurring via dysregulation of the mTOR pathway in patients with TSC.18 However, in the present study, lung specimens from BHDS patients showed no destruction of lung architecture by either proliferating type II pneumocytes or inflammation, indicating that BHDS cysts may not develop through proliferation of type II pneumocytes or proteolysis mediated by proliferating type II pneumocytes.
Presumably, considering the unique histological characteristics of BHDS cysts defined by the present study, almost all cysts abutting on interlobular septa without significant inflammation should ensue naturally from the inherent mechanism of cyst formation. In this context, we postulate that FLCN mutation results in abnormalities at the alveolar-septal junction. Several reports have described folliculin, the protein encoded by FLCN, as a regulator of TGF-β signalling, especially TGF-β2,30 or cell–cell adhesion through the interaction with adherence junction protein.31 TGF-β2 is involved in epithelial–mesenchymal interactions, cell growth, the production of extracellular matrix proteins, and tissue remodelling during development or normal subepithelial matrix homeostasis.32,33 Warren et al.28 demonstrated that FLCN mRNA was strongly expressed in stromal cells within the connective tissue and weakly in type I pneumocytes in the lung. This topographic expression pattern indicates that folliculin may participate in maintaining the homeostasis of alveolar walls through effects on stromal cells and/or type I pneumocytes. We recently found that lung fibroblasts isolated from patients with BHDS showed diminished migration as well as decreased expression of TGF-β and extracellular matrix proteins (manuscript in preparation). These findings imply that the alveolar-septal junction may become vulnerable to mechanical forces throughout the entire alveolar wall meshwork during respiratory cycles of inflation and deflation, leading to cyst formation resulting from the disruption of alveolar homeostasis. Supporting this notion is the fact that the periacinar regions where alveoli attach to connective tissue septa are weak from an anatomical as well as a physiological viewpoint. Capillaries in alveolar walls abutting on connective tissue septa or visceral pleura are less numerous than elsewhere, and the periacinar regions then have less vascularity and greater compliance.34 Furthermore, our hypothesis based on histopathological study seems to be well formulated to explain the results of our previous radiological study, showing that BHDS cysts are irregularly shaped rather than oval, and are predominantly located in the lower medial lung zone:35 (i) a cyst's periacinar development would contribute to its irregular shape, caused by imbalanced elastic recoil forces of the remaining alveolar tissues within a unit structure of a secondary lobule;36 and (ii) greater mechanical force would be loaded onto the lower lobes than onto the upper lobes during respiratory cycles. However, further studies are needed to confirm this hypothesis.
In conclusion, we have defined the unique histological features of pulmonary cysts in patients with BHDS, almost all cysts abutting on interlobular septa without significant inflammation. The 229 BHDS cysts derived from lung specimens of 50 unrelated patients surveyed here constitute the most extensive of such studies.
Acknowledgments
The authors greatly thank Dr Hitoshi Niino, Division of Clinical Laboratory, National Hospital Organization Yokohama Medical Centre, Kanagawa, Japan, for providing the macroscopic figures of lung tissue, and Dr Noriyo Yanagawa, Department of Radiology, Tsukuba Memorial Hospital, Tochigi, Japan, for fruitful discussion. We also thank Ms Phyllis Minick for her excellent proofreading of our English.
References
- Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt–Hogg–Dube syndrome. Am. J. Hum. Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt–Hogg–Dube syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl Acad. Sci. USA. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Tanaka R, Koga S, et al. Pulmonary cysts of Birt–Hogg–Dube syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am. J. Surg. Pathol. 2012;36:589–600. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- Hartman TR, Nicolas E, Klein-Szanto A, et al. The role of the Birt–Hogg–Dube protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi H, Baba M, Hong SB, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM, Bratslavsky G, Pinto PA, et al. Molecular diagnosis and therapy of kidney cancer. Annu. Rev. Med. 2010;61:329–343. doi: 10.1146/annurev.med.042808.171650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Kobayashi T, Shiono M, et al. Interaction of folliculin (Birt–Hogg–Dube gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27:5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt–Hogg–Dube syndrome: a new series of 50 families and a review of published reports. J. Med. Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt–Hogg–Dube-associated renal tumors. J. Natl Cancer Inst. 2005;97:931–935. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- van Steensel MA, Verstraeten VL, Frank J, et al. Novel mutations in the BHD gene and absence of loss of heterozygosity in fibrofolliculomas of Birt–Hogg–Dube patients. J. Invest. Dermatol. 2007;127:588–593. doi: 10.1038/sj.jid.5700592. [DOI] [PubMed] [Google Scholar]
- Furuya M, Nakatani Y. Birt–Hogg–Dube syndrome: clinicopathological features of the lung. J. Clin. Pathol. 2013;66:178–186. doi: 10.1136/jclinpath-2012-201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayo DS, Aughenbaugh GL, Yi ES, Hand JL, Ryu JH. Cystic lung disease in Birt–Hogg–Dube syndrome. Chest. 2007;132:679–684. doi: 10.1378/chest.07-0042. [DOI] [PubMed] [Google Scholar]
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am. J. Respir. Crit. Care Med. 2005;172:39–44. doi: 10.1164/rccm.200501-143OC. [DOI] [PubMed] [Google Scholar]
- Ishii H, Oka H, Amemiya Y, et al. A Japanese family with multiple lung cysts and recurrent pneumothorax: a possibility of Birt–Hogg–Dube syndrome. Intern. Med. 2009;48:1413–1417. doi: 10.2169/internalmedicine.48.2144. [DOI] [PubMed] [Google Scholar]
- Koga S, Furuya M, Takahashi Y, et al. Lung cysts in Birt–Hogg–Dube syndrome: histopathological characteristics and aberrant sequence repeats. Pathol. Int. 2009;59:720–728. doi: 10.1111/j.1440-1827.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- Butnor KJ, Guinee DG., Jr Pleuropulmonary pathology of Birt–Hogg–Dube syndrome. Am. J. Surg. Pathol. 2006;30:395–399. doi: 10.1097/01.pas.0000183571.17011.06. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Takayanagi N, Ishiguro T, Sugita Y, Kawabata Y, Fukuda Y. Birt–Hogg–Dube syndrome with multiple cysts and recurrent pneumothorax: pathological findings. Intern. Med. 2010;49:2137–2142. doi: 10.2169/internalmedicine.49.3670. [DOI] [PubMed] [Google Scholar]
- Gunji Y, Akiyoshi T, Sato T, et al. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J. Med. Genet. 2007;44:588–593. doi: 10.1136/jmg.2007.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunogi M, Kurihara M, Ikegami TS, et al. Clinical and genetic spectrum of Birt–Hogg–Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J. Med. Genet. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar SP. Pneumothorax. In: Tomashefski JF Jr, Cagle PT, Farver C, Fraire AE, editors. Dail and Hammar's pulmonary pathology, Vol. 1: Nonneoplastic lung disease. 3rd ed. New York: Springer Science/Business Media, LLC; 2008. pp. 1167–1168. [Google Scholar]
- Tomashefski JF, Jr, Bruce M, Stern RC, Dearborn DG, Dahms B. Pulmonary air cysts in cystic fibrosis: relation of pathologic features to radiologic findings and history of pneumothorax. Hum. Pathol. 1985;16:253–261. doi: 10.1016/s0046-8177(85)80011-3. [DOI] [PubMed] [Google Scholar]
- Kawabata Y, Watanabe A, Yamaguchi S, et al. Pleuropulmonary pathology of vascular Ehlers-Danlos syndrome: spontaneous laceration, haematoma and fibrous nodules. Histopathology. 2010;56:944–950. doi: 10.1111/j.1365-2559.2010.03574.x. [DOI] [PubMed] [Google Scholar]
- Dyhdalo K, Farver C. Pulmonary histologic changes in Marfan syndrome: a case series and literature review. Am. J. Clin. Pathol. 2011;136:857–863. doi: 10.1309/AJCP79SNDHGKQFIN. [DOI] [PubMed] [Google Scholar]
- Seaman DM, Meyer CA, Gilman MD, McCormack FX. Diffuse cystic lung disease at high-resolution CT. AJR Am. J. Roentgenol. 2011;196:1305–1311. doi: 10.2214/AJR.10.4420. [DOI] [PubMed] [Google Scholar]
- Lichter I, Gwynne JF. Spontaneous pneumothorax in young subjects. A clinical and pathological study. Thorax. 1971;26:409–417. doi: 10.1136/thx.26.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg AM. Pneumothorax. In: Thurlbeck WM, Churg AM, editors. Pathology of the lung. 2nd ed. New York, NY: Thieme Medical Publishers; 1995. pp. 1078–1080. [Google Scholar]
- Warren MB, Torres-Cabala CA, Turner ML, et al. Expression of Birt–Hogg–Dube gene mRNA in normal and neoplastic human tissues. Mod. Pathol. 2004;17:998–1011. doi: 10.1038/modpathol.3800152. [DOI] [PubMed] [Google Scholar]
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur. Respir. J. 2008;32:1399–1403. doi: 10.1183/09031936.00132007. [DOI] [PubMed] [Google Scholar]
- Hong SB, Oh H, Valera VA, et al. Tumor suppressor FLCN inhibits tumorigenesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol. Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvetz DA, Khabibullin D, Hariharan V, et al. Folliculin, the product of the Birt–Hogg–Dube tumor suppressor gene, interacts with the adherens junction protein p0071 to regulate cell–cell adhesion. PLoS ONE. 2012;7:e47842. doi: 10.1371/journal.pone.0047842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HG, Mih JD, Krasieva TB, Tromberg BJ, George SC. Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1277–L1285. doi: 10.1152/ajplung.00057.2006. [DOI] [PubMed] [Google Scholar]
- Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaise LR, Senior RM. Bullous disease of the lung. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, editors. Fishman's pulmonary diseases and disorders. 4th ed. New York, NY: McGraw-Hill; 2008. pp. 913–929. [Google Scholar]
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt–Hogg–Dube syndrome: thin-section CT findings of the chest in 12 patients. Eur. J. Radiol. 2011;77:403–409. doi: 10.1016/j.ejrad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Morgan MD, Edwards CW, Morris J, Matthews HR. Origin and behaviour of emphysematous bullae. Thorax. 1989;44:533–538. doi: 10.1136/thx.44.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]



