Abstract
Introduction
Pharmacogenetics attempts to identify inter-individual genetic differences that are predictive of variable drug response and propensity to side effects, with the prospect of assisting physicians to select the most appropriate drug and dosage for treatment. However, many concerns regarding genetic tests exist. We sought to test the opinions of undergraduate science and medical students in southern Ontario universities toward pharmacogenetic testing.
Methods and Results
Questionnaires were completed by 910 undergraduate medicine and science students from 2005 to 2007. Despite students' concerns that the results of genetic tests may be used for other purposes without consent (71%) or lead to discrimination (78%), an overwhelming number of students were in favor of pharmacogenetic testing (90%).
Discussion
To our knowledge, this study is the first to survey a large sample for their attitude toward pharmacogenetic testing for psychotropic medications. Our results indicate that, although concerns remain and scientific advancements are required, respondents were in support of pharmacogenetic testing for medications used to treat schizophrenia. © 2014 The Authors. Human Psychopharmacology: Clinical and Experimental published by John Wiley & Sons, Ltd.
Keywords: pharmacogenetics, psychopharmacology, bioethics, genetics, antipsychotics
INTRODUCTION
Twenty to 25% of the general population suffers from a diagnosable mental illness, ranging from the most common major depressive disorder and anxiety disorders to psychotic and cognitive disorders (National Institute of Mental Health; Kessler et al., 2005). Mental health issues are the leading cause of health-related disability in children and adolescent with long-lasting effects throughout their lives (Kieling et al., 2011). According to the World Health Report 2001, four of the 10 leading causes of disability worldwide are neuropsychiatric disorders, which account for over 30% of total disability and more than 12% of the total burden of disease. Moreover, the World Health Organization reported that depression is the leading cause of years lost due to disability and mental disorders account for approximately 160 million lost years of healthy life, with at least 30%, which can be averted with existing treatment (WHO, 2009). Psychotropic medications are generally the first-line treatment of severe psychiatric disorders, and among them include antidepressants, antipsychotics, mood stabilizers, and anxiolytic medications, and so on. (APA, 2013). Antidepressants are the top therapeutic class by prescriptions in the United States since 2011 (IMS, 2012). Thus, medications are an important part of the management of psychiatric disorders, and both clinicians and patients should be aware of their benefits and risks.
Pharmacogenetics is the study of the genetic variation that contributes to inter-individual differences in drug response and propensity to side effects (Roses, 2000). Pharmacogenetics holds the potential to assist in the care of psychiatric conditions, perhaps more than in other medical disorders, due to the nature of psychotropic medications. Variable response to psychotropic medication remains a critical issue in psychiatric care, as a large proportion of patients continues to experience significant symptoms despite treatment (MacGillivray et al., 2003; Corey-Lisle et al., 2004). Selecting the appropriate dose is often difficult, requiring long periods of patient assessment and drug titration (Reynolds, 2012). Psychotropic medications utilize a variety of drug targets and methods of action, creating a range of prescription options to select between, often with little evidence (Shuchman et al., 2008). Furthermore, a significant number of patients develop drug-induced side effects and adverse events; many of which are potentially avoidable if the appropriate medication and dosage are prescribed (Reynolds, 2012).
Subsequent to the completion of the human genome project and with the advancement of high-throughput sequencing and genotyping technologies, an understanding of how genomes vary from person to person is growing at an exponential rate (The International HapMap Consortium, 2010). Initial studies into the pharmacogenetics of psychotropic drugs have provided optimism for the potential of tests regarding both safety and efficacy (Reynolds, 2012). Genetic variation in genes producing neurotransmitter receptors, transporters, neurotrophic factors, and drug metabolizing enzymes has all been associated with antidepressant response (Fabbri et al., 2013; Kato and Serretti, 2008). With respect to side effects, efforts have been made toward identifying patients with increased propensity for antipsychotic-induced weight gain (Lett et al., 2012; Malhotra et al., 2012). A genetic test that investigates genetic variants in the drug metabolizing enzymes CYP2D6 and CYP2C19 (Vetti et al., 2010), which are two major mechanisms impacting on inter-individual variability in response and side effects is commercially available with Food and Drug Administration approval (AmpliChip® CYP450 Test; Table 1). Brandl et al. have reported that this Roche AmpliChip® CYP450 test was useful in providing significant prediction of outcome in antidepressant treatment of obsessive-compulsive disorder (Brandl et al., 2012). In general, genes associated with drug response and safety require further validation and verification, including in vitro and in vivo functional studies, animal studies, and large randomized controlled trials (Reynolds, 2012). A proposal has been made to establish criteria to ensure the quality of pharmacogenetic studies (Serretti et al., 2008).
Concerns with regard to genetic testing in general, and pharmacogenetics specifically, do exist. Within the literature, the most commonly cited concerns include fears of further use of the test result without consent, potential discrimination of individuals seen to be at “genetic disadvantage”, exacerbation of concerns regarding orphan drugs (medications that have been developed to treat a rare medical condition), and additional inequities in the delivery of health care (Breckenridge and Walley, 2008). The hereditary nature of genetic information means that the ramifications of test results on family members must also be considered (Haga and Burke, 2008). Concerns with regard to performing tests on vulnerable individuals or those who are unable to provide informed consent add to the complexity of the issue (Morley and Hall, 2004). Additionally, one must consider ancillary information that can be inferred by the results of many pharmacogenetic test results, as variants associated with drug response are often also associated with disease risk (Haga et al., 2012).
Different factors influence opinion in genetic testing, including general knowledge, level of education, culture, ethnicity, religion, prior exposure and experience, policy and law, and so on. A recent study from the United States implicated that majority of participants indicated willingness to test for curable disorders; however, participants with religion background tend to have an indirect negative effect on attitude toward genetic testing (Botoseneanu et al., 2011). In another study, a small percentage of participants prefer genetic testing as the basis for prescription of medications if issues of cost, discrimination, and privacy have been addressed (Bevan et al., 2003). Regarding attitudes in genetic testing between the healthcare professionals and patients, gaps were identified because of no clear model focusing on clinical practice, high expectations from patients, and current understanding of genetic testing (Fargher et al., 2007). Furthermore, there appeared to be a lack of consensus regarding safeguards and risks of genetic testing with the early use of genetic testing in psychiatry (Hoop et al., 2010). In view of the differences, including methodological, ancestral and cultural, across populations, caution should be taken when comparing results from multiple studies.
With pharmacogenetic tests already being marketed directly to consumer (Couzin, 2008; Table S1,), it is vital that we open a discussion to the public regarding interest and concerns toward pharmacogenetic tests. To our knowledge, this is the first study evaluating opinions toward pharmacogenetics of psychotropic medications.
METHODS
A questionnaire was developed with a specific focus on genetic and pharmacogenetic testing of complex psychiatric traits (Supporting Information). Demographic data including age, sex, religion, importance of religion, and race was collected. A draft of the questionnaire was piloted on 30 students, and comments were collected to ensure the clarity, timing, and relevance of the questionnaire. However, no formal verification of reliability was performed. The study received approval from the Ethics Review Boards of the Centre for Addiction and Mental Health, the University of Waterloo, and the University of Western Ontario.
Invited participants were undergraduate science and medical students at the University of Waterloo, the University of Western Ontario, and the University of Toronto. Data was collected over 3 years from 2005 to 2007, collated in 2007, and analyzed in 2009 to 2010. With the permission of professors and department heads, one of the investigators would enter the lecture hall at the end of a lecture and deliver a standardized recruitment announcement. Questionnaires were completed anonymously, and students were informed that they were under no obligation to participate in the survey and could leave at any time. The students were instructed that any questions that they were uncomfortable about or unsure could be checked “Don't Know” or left blank. Completely blank questionnaires were not included in the analysis. In partially completed questionnaires, blank questions were coded as “data missing”. We did observe an increase in blank question responses toward the end of the questionnaire, but of the 910 participants who started the survey, 889 completed the survey in its entirety (97.7%). Religion was included in the questionnaire because of the differences in the opinion of genetic testing due to religious beliefs. Simple statistical analyses including descriptive comparison, non-parametric analyses of categorical variables were performed. All statistics were performed in sas v9.1 (SAS Institute, Cary, NC, USA).
RESULTS
In total, the questionnaire was completed by undergraduate science students (total n = 910) at the University of Waterloo (n = 542) and undergraduate medical students at the University of Western Ontario (n = 268) and the University of Toronto (n = 100). The response rate, given the number of surveys distributed, and the class size are estimated to be ∼85%. The demographic data of the participants are presented in Table 1. A majority of students reported exposure to some information regarding genetic testing (86%) and mental illness (81%) within the classroom and thus are likely to be more informed with regard to the issues than the general public (67% in the public; Wilde et al., 2010). To determine opinions toward pharmacogenetics, the following was asked: “Schizophrenia drugs can have severe side effects. Assume that a genetic test could determine which drug will give the best results with the least side effects for each individual patient. If schizophrenia has been diagnosed, should genetic testing be used to determine which drug to prescribe?” Of respondents, 90% (816 out of 908) selected “Yes” (Figure 1). Within the sampled university students, the response to the query was not significantly modified by the addition of age, sex, race, religion, or importance of religion.
Table 1.
Demographic data
| Characteristic | Responses | # (%) of participants |
|---|---|---|
| Sex | Male | 331 (36.6) |
| Female | 571 (63.2) | |
| Data missing | 8 (0.9) | |
| Age | 16–19 years | 92 (10.2) |
| 20–23 years | 597 (66.1) | |
| 24–27 years | 181 (20.0) | |
| >27 years | 33 (3.7) | |
| Data missing | 7 (0.8) | |
| Children | Yes | 10 (1.1) |
| No | 885 (98.8) | |
| Expecting | 1 (0.1) | |
| Data missing | 14 (1.5) | |
| Do you plan on having children in the future? | Yes | 741 (81.4) |
| No | 18 (2.0) | |
| Do not know | 117 (12.9) | |
| Data missing | 34 (3.7) | |
| Race | Caucasian | 525 (57.7) |
| Asian | 288 (31.7) | |
| Native Canadian | 6 (0.7) | |
| Hispanic | 4 (0.4) | |
| Black | 4 (0.4) | |
| Other | 72 (7.9) | |
| Data missing | 11 (1.2) | |
| Religion | Catholic | 215 (23.6) |
| Protestant | 154 (16.9) | |
| Jewish | 42 (4.6) | |
| Muslim | 46 (5.0) | |
| Hindu | 33 (2.8) | |
| Buddhist | 25 (3.6) | |
| None | 242 (26.6) | |
| Other | 129 (12.3) | |
| Data missing | 24 (2.6) | |
| Importance of Religion in my life | Extremely important | 116 (12.8) |
| Very important | 152 (16.7) | |
| Moderately important | 283 (31.1) | |
| Hardly important | 170 (18.7) | |
| Not important at all | 171 (18.8) | |
| Data missing | 18 (2.0) | |
| Students | Undergraduate | 542 (59.6) |
| Medical | 368 (40.4) |
Figure 1.
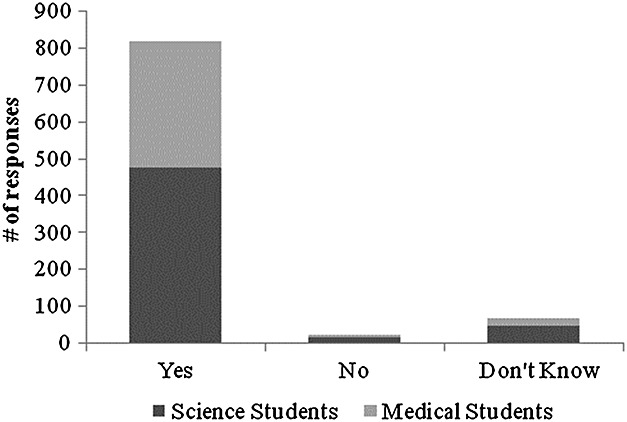
Result of the question “Schizophrenia drugs can have severe side effects. Assume that a genetic test could determine which drug will give the best results with the least side effects for each individual patient. If schizophrenia has been diagnosed, should genetic testing be used to determine which drug to prescribe?”
We did not find significant differences regarding students' opinion on genetic testing between the different age, sex, race, and religion groups.
Questions were formed to query possible concerns for respondents. Seventy-one percent (638 of 898) of respondents were concerned that genetic tests could lead toward discrimination, and seventy-eight percent (703 of 897) was concerned that the results of a genetic test might later be used without consent.
DISCUSSION
The current study aimed to investigate opinions of undergraduate science and medical students toward the use of pharmacogenetics for psychotropic medications. We found 90% support for using genetic information to assist in the selection of pharmacological treatments for schizophrenia when a diagnosis has already been made, efficacy can be predicted, and side effects can be reduced. This is an overwhelming positive response for pharmacogenetic testing; however, it is likely that an important component of the question was that efficacy can be predicted and side effects can be reduced. Although there are examples of advancements of psychiatric pharmacogenetics (Reynolds, 2012), it is vital that carefully designed studies are carried out so that the promise of pharmacogenetics for psychotropic medications can be fulfilled (Serretti et al., 2008). However, this sample might not be generalizable across the general population given the level of education (post-secondary) and the field of study (science or medicine) with more exposure and understanding toward medical/mental illnesses and genetic testing.
A substantial amount of psychoeducation and/or counseling is required before a patient can come to an informed decision regarding a genetic test for risk for a disease. Terms such as absolute and relative risk, sensitivity and specificity, and positive and negative predictive values are unfamiliar and difficult to interpret, yet many of these terms are common place in the practice of evidence-based medicine and regularly need to be dealt with (Breckenridge et al., 2004). As proposed by ethicists, respondents were concerned that genetic test results could lead to discrimination, and that the results might later be used without consent. Issues of confidentiality are common in modern medicine and will hopefully improve with the implementation of electronic health records (Masys et al., 2002). In the end, the question is “are the risks connected with the pharmacogenetic test smaller than the benefits that such knowledge may provide?” Counseling is not usually necessary unless the genetic testing is related to the risk for a disease, as opposed to pharmacogenetic testing, and this may, in part, be because the prescribing physician uses the genetic information only as a guide for choice and a dose of medication. As pharmacogenetic tests, and the evidence supporting them, improve, the balance will continue to shift in favor of pharmacogenetic testing.
An important limitation of the current study was that participants were a convenience sample of medicine and science students and thus may be biased toward the acceptance of new technologies. However, because of their education, the students should be more informed with respect to the pros and cons of genetic testing including bioethical concerns especially in medicine, than the general public, and as future physicians and scientists, they also represent important decision makers of the future. Furthermore, it should be noted that the scenarios in the survey generally described the benefits of genetic testing, as opposed to the limitations and potential risks—this bias may have contributed to the observed very high endorsement of pharmacogenetic testing.
It appears that there is a growing marketplace for genetic and pharmacogenetic testing, particularly available through the internet, often without the necessary counseling or full understanding of test results. Private companies are proceeding with the commercialization of many pharmacogenetic and genetic tests for psychiatric conditions. In regard to direct-to-consumer pharmacogenetic testing, a good deal of caution should be taken by the consumer, because, without the knowledge and judgment of a trained clinician, harmful misinterpretations may occur. The results of the current study indicate that a large majority of participants believe that pharmacogenetic testing should be used for the selection of the appropriate psychotropic medication. A good understanding of society's needs, desires, and opinions toward psychiatric pharmacogenetic testing will help guide future research and be an asset when instituting policies regarding the acquisition and use of genetic information.
CONFLICT OF INTEREST
Dr JL Kennedy is Scientific Advisory Board member of AssureRx (unpaid), speaker honorarium of Eli Lilly and Novartis and consultant of Roche. Other authors have no financial disclosure.
Acknowledgments
Dr. Lanktree is supported by the Canadian Institute of Health Research (CIHR) MD/PhD Studentship Award. Dr. Zai is currently in the Clinician-Scientist Program at the Department of Psychiatry, Clinical Investigator Program at the Faculty of Medicine, University of Toronto. This study is supported by the Ontario Ministry of Research and Innovation (JLK). The authors are grateful for the assistance of all study participants. The study was performed with no additional funding.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site.
Supporting info item
Supporting info item
REFERENCES
- American Psychiatric Association (APA) 2013. Clinical practice guidelines http://www.psychiatry.org/practice/clinical-practice-guidelines (Accessed on 15 October 2013)
- Bevan JL, Lynch JA, Dubriwny TN, et al. Informed lay preferences for delivery of racially varied pharmacogenomics. Genet Med. 2003;5(5):393–399. doi: 10.1097/01.gim.0000087989.12317.3f. [DOI] [PubMed] [Google Scholar]
- Botoseneanu A, Alexander JA, Banaszak-Holl J. To test or not to test? The fole of attitudes, knowledge, and religious involvement among U.S. adults on intent-to-obtain adult genetic testing. Health Educ Behav. 2011;38(6):617–628. doi: 10.1177/1090198110389711. [DOI] [PubMed] [Google Scholar]
- Brandl EJ, Müller DJ, Hwang R, et al. The AmpliChip(®) CYP450 test and response to treatment in schizophrenia and obsessive compulsive disorder: a pilot study and focus on cases with abnormal CYP2D6 drug metabolism. Genet Test Mol Biomarkers. 2012;16(8):897–903. doi: 10.1089/gtmb.2011.0327. [DOI] [PubMed] [Google Scholar]
- Breckenridge A, Lindpaintner K, Lipton P, McLeod H, Rothstein M, Wallace H. Pharmacogenetics: ethical problems and solutions. Nat Rev Genet. 2004;5(9):676–680. doi: 10.1038/nrg1431. [DOI] [PubMed] [Google Scholar]
- Breckenridge A, Walley T. Early access to new medicines. Clin Pharmacol Ther. 2008;84(1):23–25. doi: 10.1038/clpt.2008.19. [DOI] [PubMed] [Google Scholar]
- Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Arch Intern Med. 2004;164(11):1197–1204. doi: 10.1001/archinte.164.11.1197. [DOI] [PubMed] [Google Scholar]
- Couzin J. Science and commerce. Gene tests for psychiatric risk polarize researchers. Science. 2008;319(5861):274–277. doi: 10.1126/science.319.5861.274. [DOI] [PubMed] [Google Scholar]
- Fabbri C, Di Girolamo G, Serretti A. Pharmacogenetics of antidepressant drugs: an update after almost 20 years of research. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(6):487–520. doi: 10.1002/ajmg.b.32184. [DOI] [PubMed] [Google Scholar]
- Fargher EA, Eddy C, Newman W, et al. Patients' and healthcare professionals' views on pharmacogenetic testing and its future delivery in the NHS. Pharmacogenomics. 2007;8(11):1511–1519. doi: 10.2217/14622416.8.11.1511. [DOI] [PubMed] [Google Scholar]
- Haga SB, Burke W. Pharmacogenetic testing: not as simple as it seems. Genet Med. 2008;10(6):391–395. doi: 10.1097/GIM.0b013e31817701d4. [DOI] [PubMed] [Google Scholar]
- Haga SB, Tindall G, O'Daniel JM. Public perspectives about pharmacogenetic testing and managing ancillary findings. Genet Test Mol Biomarkers. 2012;16(3):193–197. doi: 10.1089/gtmb.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoop JG, Lapid MI, Paulson RM, Roberts LW. Clinical and ethical considerations in pharmacogenetic testing: views of physicians in 3 " early adopting" departments of psychiatry. J Clin Psychiatry. 2010;71(6):745–753. doi: 10.4088/JCP.08m04695whi. [DOI] [PubMed] [Google Scholar]
- IMS Institute for Healthcare Informatics. 2012. The use of medicines in the United States: review of 2011 http://www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdf (Accessed on 15 October 2013)
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2008;15(5):473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieling C, Baker-Henningham H, Belfer M, et al. Child and adolescent mental health worldwide: evidence for action. Lancet. 2011;378(9801):1515–1525. doi: 10.1016/S0140-6736(11)60827-1. [DOI] [PubMed] [Google Scholar]
- Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Müller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry. 2012;17(3):242–266. doi: 10.1038/mp.2011.109. [DOI] [PubMed] [Google Scholar]
- MacGillivray S, Arroll B, Hatcher S, et al. Efficacy and tolerability of selective serotonin reuptake inhibitors compared with tricyclic antidepressants in depression treated in primary care: systematic review and meta-analysis. BMJ. 2003;326(7397):1014. doi: 10.1136/bmj.326.7397.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Correll CU, Chowdhury NI, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch Gen Psychiatry. 2012;69(9):904–912. doi: 10.1001/archgenpsychiatry.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masys D, Baker D, Butros A, Cowles KE. Giving patients access to their medical records via the internet: the PCASSO experience. J Am Med Inform Assoc. 2002;9(2):181–191. doi: 10.1197/jamia.M1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley KI, Hall WD. Using pharmacogenetics and pharmacogenomics in the treatment of psychiatric disorders: some ethical and economic considerations. J Mol Med. 2004;82(1):21–30. doi: 10.1007/s00109-003-0496-x. [DOI] [PubMed] [Google Scholar]
- Reynolds GP. The pharmacogenetics of symptom response to antipsychotic drugs. Psychiatry Investig. 2012;9(1):1–7. doi: 10.4306/pi.2012.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Pharmacogenetics and the practice of medicine. Nature. 2000;405(6788):857–865. doi: 10.1038/35015728. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, Kennedy JL. Pharmacogenetic studies in depression: a proposal for methodologic guidelines. Pharmacogenomics J. 2008;8(2):90–100. doi: 10.1038/sj.tpj.6500477. [DOI] [PubMed] [Google Scholar]
- Shuchman M, Hebert PC, Kale R, Sibbald B, Flegel K, MacDonald N. Bringing a research base to psychiatry. CMAJ. 2008;178(10):1257–1260. doi: 10.1503/cmaj.080530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International HapMap Consortium. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetti HH, Molven A, Eliassen AK, et al. Is pharmacogenetic CYP2D6 testing useful? Tidsskr Nor Laegeforen. 2010;130:2224–2228. doi: 10.4045/tidsskr.09.1445. [DOI] [PubMed] [Google Scholar]
- Wilde A, Meiser B, Mitchell PB, Schofield PR. Public interest in predictive genetic testing, including direct-to-consumer testing, for susceptibility to major depression: preliminary findings. Eur J Hum Genet. 2010;18(1):47–51. doi: 10.1038/ejhg.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2009. Global health risks: mortality and burden of disease attributable to selected major risks. WHO Press, Geneva, Switzerland. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (Accessed on 15 October 2013)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item


