Abstract
Therapeutic drug concentrations measured in plasma are of limited value as reference intervals for interpretation in post-mortem (PM) toxicology. In this study, drug concentration distributions were studied in PM femoral venous blood from 57 903 Finnish autopsy cases representing all causes of death during an 11-year period. Cause-of-death information was obtained from death certificates issued by forensic pathologists. Median, mean, and upper percentile (90th, 95th, 97.5th) concentrations were calculated for 129 drugs. To illustrate how PM median concentrations relate to established therapeutic ranges in plasma, a PM blood/plasma relationship was calculated for each drug. Males represented 75% of the subjects and showed a lower median age (55 yrs) than females (59 yrs). In 43% of these cases, blood alcohol concentration was higher than 0.2‰, and the median was 1.8‰. Sixty-one (47%) of the 129 drugs showed a PM blood/plasma relationship of 1. For 22 drugs (17%), the relationship was <1, and for 46 drugs (35%), the relationship was >1. No marked correlation was found between the PM blood/plasma relationship and the volume of distribution (Vd). For 36 drugs, more than 10% of cases were fatal poisonings attributed to this drug as the main finding. These drug concentration distributions based on a large database provide a helpful reference not only to forensic toxicologists and pathologists but also to clinical pharmacologists in charge of interpreting drug concentrations in PM cases. © 2013 The Authors. Drug Testing and Analysis published by John Wiley & Sons, Ltd.
Keywords: post-mortem toxicology, post-mortem drug redistribution, cause of death, drug concentration, blood
Introduction
Post-mortem (PM) toxicology aims to detect xenobiotics in autopsy specimens, determine the concentrations of the relevant compounds, and contribute to the interpretation of the findings for cause of death investigations. In today’s society, medicines and drugs of abuse account for a majority of the compounds causing fatal poisonings. Drug concentrations measured in PM blood play a key role in determining the cause and manner of death in suspected overdose cases.
In a clinical context, therapeutic and toxic drug concentrations in plasma have been thoroughly investigated, and they are readily available from extensive compilations.1 These data serve as appropriate references in therapeutic drug monitoring, clinical toxicology, and monitoring of compliance.2 Unfortunately reference plasma concentrations are applied rather carelessly even to PM blood, as many compilations do not clearly state whether the particular data are from blood or plasma. Yet, an early compilation by Osselton distinguished between plasma and PM blood values, and indicated drug distribution in blood as a percentage in plasma for a number of compounds.3 In the most recent editions of the handbook by Baselt, the blood/plasma ratio is included where available,4 indicating more than two-fold differences between blood and plasma concentrations with certain drugs.
Little was known about PM drug redistribution (PMR) until the papers by Pounder and Jones5 and Prouty and Anderson6 appeared in 1990. It was realized that PM drug concentrations are not necessarily the same as those at the time of death, as drug levels may vary according to the sampling site and the interval between death and specimen collection (PM interval). This finding gave rise to extensive research, including the investigation of cardiac blood to peripheral blood concentration ratios and the use of experimental animals.7–11 The underlying mechanisms include passive drug release from drug reservoirs such as the gastrointestinal tract, liver, lungs, and myocardium immediately after death and, later, cell autolysis and the putrefactive process.12 Today, it is a general conclusion that PM blood from a femoral vein, exhibiting less PMR than central blood, should be used for quantitative determinations on a routine basis.13,14
Numerous case notes and small case series have reported drug concentrations related to fatal poisonings.4 Although useful, these data are very heterogeneous in terms of the origin of blood and the quality of methods, resulting in very broad concentration ranges with little statistical value. There is a risk that published cases represent the higher end of fatal concentrations, because a high concentration found for a novel drug may trigger the publication of a case note. Ferner stated that concentrations measured after death cannot generally be interpreted to yield concentrations present before death and the definition of ‘lethal concentrations’ is extremely difficult.15 Furthermore, Ferner pointed out that PM concentrations have been over-interpreted in the past, and good evidence should be required before ‘lethal concentrations’ are defined in the future.15 Interestingly, far fewer studies have dealt with ‘normal’ PM concentrations, although these data would be even more important for the interpretation of the concentrations measured in casework.
In some countries, a high autopsy rate and a high PM toxicology rate make it possible to generate PM drug concentration data that is less biased by fatal poisonings. Especially valuable data can be obtained from laboratories that analyze a broad range of drugs using quality-controlled methods on a routine basis. A straightforward approach to establishing normal concentrations was taken by Jones and Holmgren, utilizing Swedish material under well-defined conditions.16 Using a database gathered from 24 876 PM femoral blood samples, they presented concentration distributions of the 25 drugs most frequently identified representing all causes of death, using 50th, 90th, 95th, and 97.5th percentiles. The advantage of this approach is that representative data can be extracted relatively simply from a comprehensive laboratory database without including cause-of-death information.
Our objectives were to exploit an even larger database in order to establish concentration distributions in PM blood for 129 drugs representing all causes of death and to evaluate the general applicability of the concentration data by comparison with the Jones and Holmgren study.16 We present the proportion of fatal poisonings attributed to each drug to facilitate interpretation. In addition, we compare the PM concentrations found in blood with the average therapeutic ranges in plasma, as exemplified recently by Linnet,17 in order to get a general idea of the prevalence and magnitude of the differences.
Materials and methods
Forensic toxicology in Finland
In Finland, a sudden or unexpected death leads to an inquest conducted by the police. If considered necessary, the police launch a medico-legal investigation by a forensic pathologist. An investigation is always conducted when a death is not known to be caused by a disease, or is known to be related to an accident, crime, suicide, poisoning, occupational illness, medical procedure, or war. This also includes deaths that do not occur under medical care or are otherwise sudden or unclear. Even reasonable doubt about the causes mentioned above is enough for an investigation and no permission from the next-of-kin is needed. Approximately 50 000 Finns die each year and a medico-legal autopsy is performed in about 11 000 cases. Toxicological samples are collected after the decision by the forensic pathologist. All toxicological analyses are performed in one laboratory at the Department of Forensic Medicine of Hjelt Institute, University of Helsinki. This leads to toxicological analysis of over 6500 cases a year, which represents over 13% of all deaths nationwide. The laboratory has been accredited since 1997 by the Finnish Centre for Metrology and Accreditation (FINAS).
The samples undergo screening and quantification analysis for legal and illicit drugs and poisonous substances, and the analysis results are then sent to the forensic pathologist and stored in the laboratory’s toxicology database. Later, a death certificate, including the cause and manner of death, is issued by the forensic pathologist and the appropriate data are integrated in the toxicology database.
Toxicological analysis
The PM interval from death to autopsy was on average five days, and on arrival at morgue the cadavers were put in cold storage (+3–5 °C) pending autopsy. The concentration data were acquired from femoral venous blood taken at autopsy. The samples, containing 1% NaF to prevent microbial processes, were stored at +4 °C until and during the laboratory investigation, which in most cases was completed within 12 days of arrival of the sample.
A multi-technique approach was used for the comprehensive toxicological analysis of blood and urine samples. Urine samples were analysed using the laboratory’s routine qualitative drug screening methods involving immunoassay for drugs of abuse and liquid chromatography/time-of-flight mass spectrometry (LC-TOF-MS) for a broad range of therapeutic and abused illicit drugs.18,19 Simultaneously, blood samples were quantitatively monitored for about 200 drugs using the following three methods: For acidic and neutral drugs, dual-column gas chromatography with nitrogen phosphorus detection (GC-NPD)20 was used until replaced in February 2007 by a method based on GC coupled with mass spectrometry (GC-MS).21 For benzodiazepines, a GC method with electron capture detection (GC-ECD)22 was used until March 2010, after which a method based on GC and negative-ion chemical ionization MS (GC-NCIMS)23 was adopted for routine use. A dual-column GC-NPD method was used for basic drug screening.24 Confirmation and additional determinations were carried out by GC-MS and LC coupled to triple quadrupole MS/MS for drugs not covered by the quantitative monitoring methods. The analytical procedure covered the majority of psychotropic drugs available on the legal and illicit markets in Finland, with special emphasis on abused substances. The validation data are summarized in an online supplementary file.
Data refining
During the 11-year study period (1 January 2000 – 31 December 2010), drug findings in PM femoral venous blood from 57 903 autopsy cases were entered in the toxicology database (Table 1). From this material, the drugs that had been detected at least 50 times in PM blood were selected. These data also included findings below the limit of quantification (LOQ) reported as ‘positive’.
Table 1.
Characteristics of autopsy cases with comprehensive post-mortem drug analysis completed
| Autopsies | Age, years | Blood alcohol ≥ 0.2‰a | Blood alcohol concentration ‰ | |
|---|---|---|---|---|
| Gender | N | Mean ± SD (median) highest | N | Mean ± SD (median) highest |
| Male | 43,458 | 53.4 ± 16.5 (55) 102 | 20,193 | 1.85 ± 1.11 (1.80) 7.60 |
| Female | 14,428 | 58.3 ± 18.5 (59) 99 | 4685 | 1.79 ± 1.12 (1.70) 8.50 |
| All | 57,903b | 54.6 ± 17.1 (56) 102 | 24,879 | 1.84 ± 1.16 (1.80) 8.50 |
Limit of quantification for blood ethanol in post-mortem blood.
No age data was available for 15 cadavers that remained unidentified.
Due to changes in the quantitative analytical methods, there had been some significant changes in the LOQ for some drugs in the course of time. For uniformity, the benzodiazepine and nifedipine results included were obtained solely from the 53 095 cases analysed by GC-ECD.22 Similarly, the results included for the acidic/neutral drugs phenobarbital, phenytoin, ibuprofen, indometacin, ketoprofen, levetiracetam, meprobamate, naproxen, oxcarbazepine, theophylline, and warfarin were obtained solely from the 23 385 cases analysed by GC-MS.21 If there had been only a slight change in LOQ, all results were included and both LOQ values indicated.
Median, mean and upper percentile (90th, 95th, 97.5th) concentrations (mg/L) were calculated from findings with a numerical value for the drug concentration (Table 2). The proportion of fatal drug poisonings caused by each of the drugs, as the most important finding, was obtained from death certificate data. The forensic pathologist had provided the cause of death according to the 10th revision of the International Classification of Diseases (ICD-10, WHO) and the ATC code (Anatomical Therapeutic Chemical classification, WHO) of the particular drug as an external cause of death.
Table 2.
Concentration distribution in post-mortem (PM) femoral blood and proportion of fatal poisonings for 129 drugs
| Drug | Cases | LOQ | PM mean | PM median | PM upper percentiles mg/L | Therapeutic range in plasmaa | Proportion of fatal poisonings | PM blood/therapeutic plasma concentration relationshipb | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | mg/L | mg/L | mg/L | 90th | 95th | 97.5th | mg/L | % | |||
| 1 | Acebutolol | 85 | 0.25 | 11 | 0.84 | 25 | 86 | 100 | 0.2-2 | 20 | 1 |
| 2 | Alprazolam | 940 | 0.02 | 0.09 | 0.05 | 0.20 | 0.30 | 0.40 | 0.005-0.05 | 13 | 1 |
| 3 | Aminophenazone, 4-methyl | 330 | 2/3c | 25 | 15 | 58 | 82 | 103 | 10d | 1 | 1 |
| 4 | Amiodarone | 125 | 0.4 | 3.4 | 1.8 | 7.7 | 11 | 15 | 1-2 | 1 | 1 |
| 5 | Amitriptyline | 1589 | 0.1 | 1.5 | 0.40 | 2.9 | 5.5 | 8.8 | 0.05-0.3 | 27 | 1.3 |
| 6 | Amlodipine | 313 | 0.006 | 0.10 | 0.07 | 0.20 | 0.26 | 0.34 | 0.003-0.015 | 3 | 4.5 |
| 7 | Amphetamine | 565 | 0.04 | 0.91 | 0.28 | 2.1 | 3.7 | 6.2 | 0.02-0.1 | 12 | 2.8 |
| 8 | Atenolol | 415 | 0.2 | 1.3 | 0.64 | 2.6 | 4.6 | 6.9 | 0.1-1 | 6 | 1 |
| 9 | Betaxolol | 124 | 0.003 | 0.22 | 0.10 | 0.31 | 0.41 | 0.43 | 0.005-0.05 | 2 | 1.9 |
| 10 | Biperiden | 30 | 0.1 | 0.20 | 0.10 | 0.30 | 0.30 | 0.66 | 0.05-0.1 | 0 | 1 |
| 11 | Bisoprolol | 3633 | 0.01 | 0.12 | 0.05 | 0.17 | 0.26 | 0.43 | 0.01-0.1 | 1 | 1 |
| 12 | Bupivacaine | 49 | 0.2 | 1.2 | 0.40 | 1.6 | 3.0 | 4.3 | 0.5-1.5 | 0 | 0.80 |
| 13 | Buprenorphine | 671 | 0.0002 | 0.0033 | 0.0012 | 0.0058 | 0.0093 | 0.022 | 0.0005-0.005 | 45 | 1 |
| 14 | Caffeine | 22125 | 1/3c | 4.0 | 3.0 | 7.5 | 10 | 13 | 4-10 | 0 | 0.75 |
| 15 | Carbamazepine | 1482 | 1/0.3c | 7.9 | 6.2 | 13 | 19 | 27 | 2-8 | 2 | 1 |
| 16 | Carvedilol | 134 | 0.003 | 0.05 | 0.02 | 0.08 | 0.13 | 0.43 | appr. 0.02-0.15 | 0 | 1 |
| 17 | Celiprolol | 105 | 0.03 | 2.1 | 0.28 | 2.7 | 5.2 | 6.9 | 0.05-0.5 | 6 | 1 |
| 18 | Cetirizine | 99 | 0.005 | 0.41 | 0.10 | 0.92 | 1.9 | 2.9 | appr. 0.02-0.3 | 0 | 1 |
| 19 | Chlordiazepoxide | 1714 | 0.2 | 1.4 | 0.70 | 3.5 | 5.2 | 7.6 | 0.4-3 | 1 | 1 |
| 20 | Chloroquine | 47 | 0.2 | 12 | 1.3 | 43 | 62 | 69 | 0.02-0.5 | 15 | 2.6 |
| 21 | Chlorpromazine | 246 | 0.05 | 1.0 | 0.30 | 2.2 | 3.9 | 6.5 | 0.03-0.1 | 11 | 3.0 |
| 22 | Chlorprothixene | 519 | 0.1 | 1.7 | 0.30 | 3.0 | 5.2 | 9.0 | 0.02-0.3 | 18 | 1 |
| 23 | Citalopram | 3542 | 0.1 | 0.97 | 0.40 | 1.4 | 2.4 | 5.3 | 0.05- 0.11 | 4 | 3.6 |
| 24 | Clonazepam | 77 | 0.01 | 0.06 | 0.03 | 0.10 | 0.20 | 0.31 | 0.02-0.08 | 5 | 1 |
| 25 | Clozapine | 445 | 0.1 | 2.5 | 1.1 | 4.4 | 8.9 | 17 | 0.35-0.6 | 13 | 1.8 |
| 26 | Codeine | 1903 | 0.02 | 0.72 | 0.16 | 1.8 | 3.2 | 5.1 | 0.03-0.25 | 23 | 1 |
| 27 | Cyclizine | 48 | 0.005 | 1.1 | 0.30 | 1.2 | 2.1 | 6.0 | 0.1-0.25 | 2 | 1.2 |
| 28 | Desmethyldiazepam | 9459 | 0.02 | 0.21 | 0.10 | 0.50 | 0.70 | 1.0 | 0.2 -0.8 | 0 | 0.13 |
| 29 | Dextromethorphan | 54 | 0.1 | 0.69 | 0.40 | 1.6 | 2.2 | 2.8 | 0.01-0.04 | 15 | 10 |
| 30 | Dextropropoxyphene | 249 | 0.1 | 6.5 | 2.6 | 12 | 17 | 38 | 0.05-0.3 | 59 | 8.7 |
| 31 | Diazepam | 7404 | 0.02 | 0.17 | 0.09 | 0.4 | 0.6 | 0.8 | 0.1-2 | 0 | 0.90 |
| 32 | Diclofenac | 20 | 0.5 | 2.5 | 1.3 | 6.6 | 7.1 | 8.5 | 0.5-3 | 0 | 1 |
| 33 | Diltiazem | 315 | 0.1 | 2.5 | 0.30 | 1.8 | 7.9 | 22 | 0.03-0.13 | 9 | 2.3 |
| 34 | Diphenhydramine | 57 | 0.005 | 0.35 | 0.20 | 0.80 | 0.90 | 0.96 | 0.05-0.1 | 0 | 2.0 |
| 35 | Dixyrazine | 52 | 0.003 | 0.70 | 0.10 | 1.8 | 3.9 | 6.4 | appr. 0.3 | 42 | 0.33 |
| 36 | Donepezil | 102 | 0.003 | 0.23 | 0.20 | 0.41 | 0.49 | 0.60 | appr. 0.03-0.075 | 0 | 2.7 |
| 37 | Doxepin | 678 | 0.05 | 2.4 | 0.60 | 6.7 | 12 | 15 | 0.01-0.2 | 42 | 3.0 |
| 38 | Duloxetine | 109 | 0.01 | 0.37 | 0.14 | 0.64 | 1.1 | 2.4 | 0.03-0.12 | 8 | 1.2 |
| 39 | Ephedrine | 156 | 0.04 | 0.66 | 0.21 | 1.1 | 1.6 | 3.1 | 0.02-0.2 | 2 | 1.1 |
| 40 | Ethylmorphine | 71 | 0.02 | 0.44 | 0.10 | 1.3 | 2.6 | 2.8 | 0.3-0.6 | 6 | 0.33 |
| 41 | Etoricoxib | 55 | 0.5 | 3.5 | 1.2 | 4.2 | 5.5 | 6.8 | 1.3-3.6 | 0 | 0.92 |
| 42 | Fentanyl | 419 | 0.0001 | 0.012 | 0.0058 | 0.023 | 0.040 | 0.061 | 0.001-0.01e | 12 | 1 |
| 43 | Flecainide | 101 | 0.2 | 4.7 | 1.9 | 9.8 | 19 | 23 | 0.4-0.8 | 18 | 2.4 |
| 44 | Fluconazole | 275 | 0.1 | 6.3 | 3.8 | 15 | 23 | 28 | appr. 1-5 | 0 | 1 |
| 45 | Fluoxetine | 649 | 0.2 | 0.80 | 0.50 | 1.6 | 2.5 | 3.6 | 0.12-0.5 | 3 | 1 |
| 46 | Fluvoxamine | 79 | 0.03 | 2.6 | 0.70 | 7.7 | 11 | 21 | 0.06-0.23 | 13 | 3.0 |
| 47 | Furosemide | 294 | 0.1 | 2.6 | 0.90 | 5.5 | 9.5 | 16.7 | 2-5 | 0 | 0.45 |
| 48 | Gabapentine | 135 | 1 | 29 | 11 | 60 | 103 | 157 | appr. 0.5-6 | 8 | 1.8 |
| 49 | Galantamine | 44 | 0.01 | 0.21 | 0.11 | 0.38 | 0.46 | 1.1 | appr. 0.03-0.06 | 0 | 1.8 |
| 50 | Glimepiride | 177 | 0.01 | 0.31 | 0.03 | 0.26 | 0.61 | 1.2 | 0.09-0.5 | 1 | 0.33 |
| 51 | Haloperidol | 94 | 0.003 | 0.05 | 0.01 | 0.06 | 0.29 | 0.40 | 0.005-0.017 | 5 | 1 |
| 52 | Hydroxycarbamazepine | 475 | 5/3c | 27 | 22 | 52 | 66 | 92 | 10-35 | 0 | 1 |
| 53 | Hydroxychloroquine | 165 | 1 | 14 | 10 | 30 | 35 | 52 | -0.1 | 14 | 100 |
| 54 | Hydroxyzine | 159 | 0.2 | 0.80 | 0.30 | 1.6 | 2.8 | 5.3 | 0.05-0.1 | 16 | 3.0 |
| 55 | Ibuprofen | 379 | 10 | 26 | 19 | 45 | 62 | 89 | 15-30 | 0 | 1 |
| 56 | Indomethacin | 9 | 0.5 | 1.2 | 1.1 | 1.6 | 1.7 | 1.8 | 0.3-1 | 0 | 1.1 |
| 57 | Ketamine | 118 | 0.1 | 1.1 | 0.50 | 2.2 | 4.1 | 6.3 | 1-6 | 1 | 0.50 |
| 58 | Ketoprofen | 80 | 0.3 | 3.2 | 1.3 | 5.7 | 7.6 | 16 | appr. 3.7 | 0 | 0.34 |
| 59 | Labetalol | 62 | 0.01 | 0.20 | 0.09 | 0.30 | 0.45 | 1.2 | 0.03-0.18 | 0 | 1 |
| 60 | Lamotrigine | 338 | 1/0.5c | 5.7 | 3.8 | 12 | 18 | 25 | 3-14 | 4 | 1 |
| 61 | Levetiracetam | 105 | 3 | 26 | 15 | 55 | 76 | 94 | 10-40 | 1 | 1 |
| 62 | Levomepromazine | 1602 | 0.1 | 0.99 | 0.40 | 1.9 | 3.2 | 5.0 | 0.005-0.025 | 23 | 16 |
| 63 | Lidocaine | 2391 | 0.1 | 0.81 | 0.40 | 1.8 | 2.8 | 4.1 | 1.5-5 | 0 | 0.27 |
| 64 | Lithium | 165 | 0.5 | 3.2 | 2.0 | 6.3 | 9.4 | 13 | 4-8 | 7 | 0.50 |
| 65 | Lorazepam | 802 | 0.01 | 0.06 | 0.03 | 0.10 | 0.20 | 0.30 | 0.08-0.25 | 0 | 0.38 |
| 66 | MDMA | 57 | 0.04 | 0.76 | 0.30 | 2.1 | 3.5 | 4.0 | 0.1-0.35 | 0 | 1 |
| 67 | Melperone | 161 | 0.05 | 1.5 | 0.40 | 3.3 | 9 | 14 | 0.03-0.1 | 32 | 4.0 |
| 68 | Meprobamate | 20 | 10 | 74 | 28 | 121 | 159 | 434 | 5-10 | 5 | 2.8 |
| 69 | Mesoridazine | 85 | 0.2 | 0.75 | 0.50 | 1.3 | 1.6 | 2.7 | 0.15-1 | 0 | 1 |
| 70 | Metformin | 1376 | 1 | 15 | 6 | 36 | 69 | 110 | 0.1-1 | 6 | 5.5 |
| 71 | Methadone | 207 | 0.05 | 0.59 | 0.40 | 1.3 | 1.8 | 2.2 | 0.1-0.5 | 43 | 1 |
| 72 | Methamphetamine | 51 | 0.04 | 1.7 | 0.18 | 1.2 | 2.2 | 25 | -0.1 | 10 | 1.8 |
| 73 | Metoclopramide | 681 | 0.05 | 0.21 | 0.10 | 0.4 | 0.6 | 0.7 | 0.05-0.15 | 0 | 1 |
| 74 | Metoprolol | 2078 | 0.05 | 0.96 | 0.20 | 1.1 | 2.0 | 5.0 | 0.035-0.5 | 2 | 1 |
| 75 | Mianserin | 387 | 0.05 | 0.37 | 0.20 | 0.70 | 0.97 | 1.9 | 0.015-0.07 | 7 | 2.9 |
| 76 | Midazolam | 172 | 0.02 | 0.14 | 0.07 | 0.40 | 0.54 | 0.84 | 0.04-0.1 | 2 | 1 |
| 77 | Mirtazapine | 2179 | 0.05 | 0.49 | 0.20 | 0.80 | 1.7 | 2.9 | 0.03-0.08 | 6 | 2.5 |
| 78 | Moclobemide | 135 | 0.1 | 15 | 1.9 | 41 | 61 | 120 | 0.3-1.0 | 13 | 1.9 |
| 79 | Morphine | 1094 | 0.02 | 0.20 | 0.07 | 0.37 | 0.67 | 1.1 | 0.01-0.1 | 4 | 1 |
| 80 | Naproxen | 202 | 10 | 43 | 33 | 78 | 100 | 120 | 20-50 | 1 | 1 |
| 81 | Nifedipine | 43 | 0.02 | 0.31 | 0.10 | 0.46 | 0.69 | 0.89 | 0.025-0.15 | 2 | 1 |
| 82 | Nitrazepam | 37 | 0.05 | 0.13 | 0.06 | 0.30 | 0.42 | 0.50 | 0.03-0.1 | 11 | 1 |
| 83 | Olanzapine | 1127 | 0.05 | 0.41 | 0.20 | 0.60 | 1.1 | 2.2 | 0.02-0.08 | 5 | 2.5 |
| 84 | Orphenadrine | 284 | 0.1 | 0.95 | 0.30 | 1.6 | 3.5 | 6.2 | 0.1-0.2 | 4 | 1.5 |
| 85 | Oxazepam | 6277 | 0.02 | 0.28 | 0.07 | 0.7 | 1.2 | 1.9 | 0.2-1.5 | 0 | 0.35 |
| 86 | Oxcarbazepine | 71 | 0.3 | 1.2 | 0.60 | 2.4 | 4.0 | 5.3 | 0.4-2 | 1 | 1 |
| 87 | Oxycodone | 1259 | 0.02 | 0.34 | 0.10 | 0.50 | 0.84 | 1.7 | 0.005-0.1 | 8 | 1 |
| 88 | Paracetamol | 3100 | 5/10c | 32 | 15 | 62 | 110 | 190 | 10-25 | 2 | 1 |
| 89 | Paroxetine | 326 | 0.004 | 0.81 | 0.24 | 1.4 | 2.2 | 3.8 | < 0.01-0.05 | 18 | 4.7 |
| 90 | Perphenazine | 283 | 0.005 | 0.08 | 0.01 | 0.08 | 0.18 | 0.36 | 0.001-0.02 | 2 | 1 |
| 91 | Pethidine | 46 | 0.1 | 0.50 | 0.30 | 0.90 | 1.1 | 1.9 | 0.1-0.8 | 2 | 1 |
| 92 | Phenazepam | 20 | 0.03 | 0.13 | 0.09 | 0.20 | 0.22 | 0.41 | 0.02-0.04 | 0 | 2.1 |
| 93 | Phenobarbital | 7 | 10 | 29 | 30 | 44 | 49 | 52 | 10-30 | 0 | 1 |
| 94 | Phenylpropanolamine | 65 | 0.04 | 0.49 | 0.14 | 0.98 | 1.4 | 2.5 | 0.1-0.5 | 0 | 1 |
| 95 | Phenytoin | 37 | 10 | 14 | 13 | 22 | 25 | 31 | 5-15 | 0 | 1 |
| 96 | Pholcodine | 73 | 0.02 | 0.37 | 0.19 | 0.80 | 1.0 | 1.5 | appr. 0.07-0.2 | 3 | 1 |
| 97 | Pregabalin | 380 | 0.2 | 15 | 8.0 | 35 | 52 | 96 | 2-5 | 12 | 1.6 |
| 98 | Promazine | 339 | 0.1 | 4.5 | 1.2 | 11 | 19 | 26 | 0.01-0.05 | 42 | 24 |
| 99 | Propofol | 299 | 0.1/0.5c | 2.5 | 1.3 | 5.0 | 8.2 | 11 | appr. 2-8 | 1 | 0.65 |
| 100 | Propranolol | 1078 | 0.02 | 1.8 | 0.13 | 5.4 | 11 | 16 | 0.02-0.3 | 15 | 1 |
| 101 | Pseudoephedrine | 127 | 0.04 | 0.81 | 0.24 | 1.5 | 2.4 | 3.6 | 0.5-0.8 | 0 | 0.48 |
| 102 | Quetiapine | 505 | 0.2 | 0.62 | 0.90 | 13 | 30 | 44 | 0.1-0.5 | 18 | 1.8 |
| 103 | Quinine | 322 | 0.2 | 3.5 | 1.0 | 4.7 | 23 | 34 | 1-7 | 3 | 1 |
| 104 | Risperidone | 622 | 0.002 | 0.014 | 0.004 | 0.029 | 0.044 | 0.086 | appr. 0.006 | 1 | 1 |
| 105 | Rivastigmine | 56 | 0.003 | 0.03 | 0.01 | 0.05 | 0.08 | 0.12 | 0.008-0.02 | 2 | 1 |
| 106 | Ropivacaine | 44 | 0.1 | 0.90 | 0.70 | 2.1 | 2.4 | 2.9 | -1.5f | 0 | 1 |
| 107 | Salicylic acid | 733 | 5/3c | 48 | 11 | 83 | 150 | 430 | 20-200 | 2 | 0.55 |
| 108 | Sertraline | 445 | 0.1 | 0.55 | 0.30 | 1.0 | 1.7 | 2.3 | 0.05-0.25 | 5 | 1.2 |
| 109 | Sildenafil | 44 | 0.03 | 0.41 | 0.10 | 0.44 | 0.53 | 4.3 | appr. 0.05-0.5 | 2 | 1 |
| 110 | Sitagliptin | 68 | 0.01 | 0.61 | 0.36 | 1.3 | 2.1 | 2.8 | 0.05-0.38 | 0 | 1 |
| 111 | Sotalol | 120 | 0.25 | 2.8 | 2.0 | 5.5 | 8.1 | 11 | 0.5-3 | 1 | 1 |
| 112 | Sulpiride | 95 | 0.02 | 4.2 | 1.1 | 7.5 | 23 | 33 | 0.05-0.4 | 18 | 2.8 |
| 113 | Temazepam | 6283 | 0.02 | 0.34 | 0.09 | 0.90 | 1.4 | 2.3 | 0.02-0.15 | 2 | 1 |
| 114 | Tetrahydrocannabinol | 347 | 0.001 | 0.005 | 0.002 | 0.008 | 0.013 | 0.024 | 0.005-0.01 | 0 | 0.40 |
| 115 | Theophylline | 52 | 10 | 25 | 15 | 34 | 88 | 107 | 8-15 | 4 | 1 |
| 116 | Thioridazine | 283 | 0.1 | 1.3 | 0.70 | 2.9 | 4.3 | 5.3 | 0.1-2 | 6 | 1 |
| 117 | Tizanidine | 107 | 0.003 | 0.12 | 0.02 | 0.27 | 0.48 | 0.90 | appr. 0.015 | 7 | 1 |
| 118 | Topiramate | 56 | 1/3c | 16 | 8.8 | 21 | 27 | 40 | 2-10 | 2 | 1 |
| 119 | Tramadol | 1581 | 0.1 | 3.1 | 0.90 | 6.50 | 13 | 21 | 0.1-1 | 18 | 1 |
| 120 | Trazodone | 58 | 0.2 | 2.1 | 0.40 | 4.7 | 9.6 | 20 | 0.7-1 | 7 | 0.57 |
| 121 | Trimethoprim | 419 | 1 | 3.7 | 2.3 | 6.7 | 9.3 | 13 | 1.5-2.5 | 0 | 1 |
| 122 | Trimipramine | 248 | 0.1 | 1.1 | 0.60 | 2.4 | 3.4 | 4.7 | 0.01-0.3 | 19 | 2.0 |
| 123 | Valproic acid | 956 | 1/3c | 51 | 28 | 75 | 110 | 230 | 40-100 | 2 | 0.70 |
| 124 | Venlafaxine | 824 | 0.1 | 3.7 | 0.60 | 5.7 | 17 | 33 | 0.1-0.4 | 17 | 1.5 |
| 125 | Verapamil | 174 | 0.1 | 2.1 | 0.35 | 5.9 | 9.7 | 13 | 0.02-0.25 | 16 | 1.4 |
| 126 | Warfarin | 425 | 0.5 | 0.81 | 0.70 | 1.1 | 1.6 | 2.4 | 1-3 | 0 | 0.70 |
| 127 | Zolpidem | 287 | 0.1 | 0.59 | 0.30 | 1.4 | 2.0 | 2.9 | 0.08-0.15 | 19 | 2.0 |
| 128 | Zopiclone | 2577 | 0.02 | 0.34 | 0.10 | 0.8 | 1.5 | 2.4 | 0.01-0.05 | 13 | 2.0 |
| 129 | Zuclopenthixol | 171 | 0.005 | 0.10 | 0.05 | 0.20 | 0.30 | 0.58 | 0.004-0.05 | 3 | 1 |
Information derived from Schulz et al.1
Median PM concentration within the established therapeutic range in plasma = 1
Median PM concentration below the therapeutic range divided by the lower limit of the therapeutic range (<1)
Median PM concentration above the therapeutic range divided by the upper limit of the therapeutic range (>1)
Data combined from two successive methods; validation parameters presented in order of appearance
For parent drug metamizole
Information derived from Winek et al.32
Information derived from Dollery33
For each drug, the relationship of the median PM drug concentration in blood to the therapeutic range in plasma (PM blood/plasma relationship) was calculated as follows: drugs with a median PM concentration within the established therapeutic range in plasma1 were given a relationship of 1; for drugs with a median PM concentration below the therapeutic range, the median concentration was divided by the lower limit of the therapeutic range, resulting in a relationship lower than 1; for drugs with a median PM concentration above the therapeutic range, the median concentration was divided by the upper limit of the therapeutic range, resulting in a relationship higher than 1. The midpoint of the volume of distribution range (Vd, L/kg) was determined for each drug based on literature data.4,25
Results
Overview
Table 1 shows characteristics of the 57 903 autopsy cases from the study period between 1 January 2000 and 31 December 2010, for which a comprehensive PM drug analysis was completed. Males represented 75% of the subjects and showed a lower median age than females (p<0.001 Mann-Whitney Test). In 43% of these cases, blood alcohol concentration (BAC) was over the limit of quantification (0.2‰ or 0.2 g/kg), and the median BAC was 1.8‰.
Drug concentrations in PM femoral venous blood
Table 2 shows the PM concentration distributions of the 129 drugs most frequently found in autopsy cases in alphabetical order. Table 2 also gives for each drug the LOQ, proportion of fatal poisonings as the main finding, the established therapeutic concentration range in plasma1 and the relationship of PM median concentration to therapeutic range (PM blood/plasma relationship). For most drugs, the arithmetic means of PM concentrations were much higher than the median values, indicating skewed distributions.
Sixty-one (47%) of the 129 drugs showed a PM blood/plasma relationship of one. For 22 drugs (17%), the relationship was below 1, and for 46 drugs (35%), the relationship was higher than 1. Figure 1 indicates the 36 drugs for which more than 10% of cases were fatal poisonings attributed to this drug as the main finding.
Figure 1.
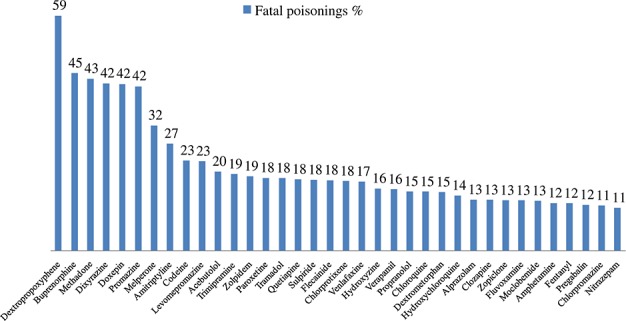
Thirty-six drugs with more than 10% of fatal poisonings attributed to this drug as the main finding
All PM blood/plasma relationships were plotted against the midpoint of the Vd range. No marked correlation was found, and even the drugs with a PM blood/plasma relationship of one showed a wide variation along the y-axis (Vd midpoint).
Discussion
Our study provides reference data to aid interpretation of drug concentrations in PM blood by listing the normal (median) and elevated PM femoral venous blood concentrations of 129 drugs, derived from the extensive Finnish toxicology database. The approach of our study is in accordance with the paper by Jones and Holmgren in 2009 for 25 drugs based on the Swedish database.16 However, the data published here is more comprehensive and the ranking list of the most frequently detected drugs is different, although the age, gender and alcohol statistics are similar. The median concentrations for the majority of drugs included in both studies are very close to each other despite small differences in the LOQs applied (Figure 2). There are some exceptions though: the dextropropoxyphene median concentration in our study is 2.6 mg/L vs. 0.8 mg/L in the Swedish material, codeine 0.16 mg/L vs. 0.05 mg/L, and amphetamine 0.28 mg/L vs. 0.5 mg/L, respectively.16 These differences can be partly explained by the different LOQs and partly by the different abuse practices between the two countries. Information on the proportion of fatal poisonings attributed to each drug was not included in the Swedish study.
Figure 2.
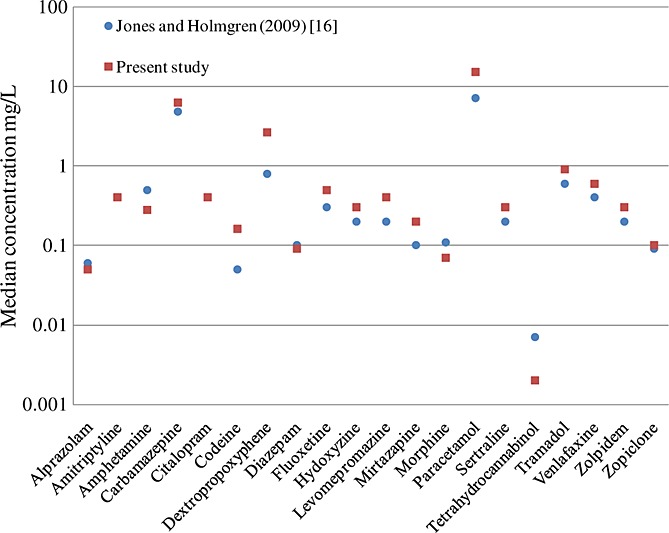
Comparison of median post-mortem concentrations of twenty drugs in common with Jones and Holmgren16
A significant contribution to illustrating the differences between normal and fatal concentrations under well-defined conditions was made by the Swedish scientists Druid and Holmgren.26 They were able to report concentrations of 83 drugs in PM femoral blood from one-substance poisonings, multi-substance poisonings, and from other causes of death without incapacitation due to drugs. The concentrations were compared with blood concentrations detected in drivers suspected of being under the influence of drugs. In the further study by Reis et al.,27 the same strategy was used for 15 anti-depressant drugs but therapeutic drug monitoring material was used for comparison. These elaborate studies provide very useful data but, inevitably, strict inclusion criteria greatly reduce the amount of original data, and for many drugs one-substance poisonings do not exist at all. With the exception of dextropropoxyphene (2.6 mg/L in our study vs. 0.2 mg/L26) and moclobemide (1.9 mg/L vs. 0.6 mg/L26,27) the median concentrations of the present study were in accordance with the results of these two studies when compared with the median concentrations of groups ‘other cause of death without incapacitation due to drugs’26 and ‘certified other cause of death in which the circumstances exclude the possibility of incapacitation by drugs’.27
A PM blood/plasma relationship was introduced to illustrate how the median PM blood drug concentrations relate to the established therapeutic ranges in plasma. In our study, most neutral and acidic drugs, such as benzodiazepines, anti-epileptics, non-steroidal anti-inflammatory drugs, and paracetamol, had a median concentration within or below the therapeutic range, exhibiting a PM blood/plasma relationship ≤1. In particular, drugs that are extensively plasma bound, such as benzodiazepines and warfarin,21,28 had lower PM blood concentrations. For some drugs the low relationship could be explained by their pattern of use, for example, lidocaine in resuscitation. A majority of findings below the LOQ for a certain drug indicates a need to develop a method with better sensitivity. As for basic drugs, a PM blood/plasma relationship >1 was commonly found, and this is thought to be largely due to PMR. Anti-depressants possessed a relatively high relationship, fluoxetine having a value of one but the other anti-depressants ranging from 1.2 for sertraline to 4.7 for paroxetine. Anti-psychotics showed more variation: chlorprothixene, haloperidol, perphenazine, risperidone, thioridazine and zuclopenthixol had a value of one but the other anti-psychotics ranged from 1.8 for clozapine to 24 for promazine.
In a recent review, Linnet17 compared the PM normal concentration intervals of drugs with their therapeutic serum levels. Utilizing predominantly PM concentrations from the Swedish studies26,27 and therapeutic concentrations from the list issued by The International Association of Forensic Toxicologists (TIAFT), Linnet found that the ratio between the upper limits ranged from 0.13 to 11.3 for 57 compounds with a median value of 1.5. Linnet also concluded that highly water-soluble drugs with a low propensity for redistribution, such as meprobamate, carbamazepine, and theophylline, had a tendency towards a low ratio, which is also evident in our study. For anti-depressants, on the other hand, the ratio was relatively high, ranging from 0.6 to 4.7 with a median of 2.4. For anti-psychotics, the ratio ranged from 0.2 to 11.3 with a median of 1.4. These findings are fairly consistent with our present results, although the calculation basis is slightly different.
A high Vd may refer to extensive PMR and a high PM blood/plasma relationship. Hydroxychloroquine has a notoriously high Vd (580–815 L/kg4), and in our study it possessed the highest PM blood/plasma relationship of all (100). However, no straightforward correlation between Vd and PM blood/plasma relationship could be found in our study.
Due to the high prevalence of medico-legal autopsies in Finland, a great proportion of PM toxicological investigations concern non-poisoning cases. Fatal drug poisonings comprise approximately 8% and all fatal poisonings approximately 15% of all toxicologically investigated cases. Consequently, the median drug concentrations reported in Table 2 are generally likely to reflect normal PM concentrations. However, there were 36 drugs with more than 10% of cases being fatal poisonings attributed to this particular drug (Figure 1), and for these drugs the normal concentration is probably lower than the median. The influence of the prevalence of multi-drug and alcohol use cannot be excluded, but in a previous study, significant pharmacokinetic interactions were shown to be rare.29 When assessing the main findings, the forensic pathologists did not have access to the concentration distributions published here, so the results are not biased in that sense.
The drugs frequently associated with fatal poisonings are predominantly opioids, anti-psychotics and anti-depressants. Other drugs include hypnotics and sedatives, some cardiovascular drugs, and amphetamine. As we have shown earlier, for strong opioids of abuse, such as buprenorphine and methadone, their PM concentration plays a somewhat limited role in assessing the cause of death.30,31 The same is partly true for amphetamine and other drugs for which the development of tolerance becomes important. However, these drugs were included in this study to give an insight into their concentration distribution in PM blood.
Proper interpretation of PM concentrations is crucial in many types of expert opinions. As exemplified with the calcium channel blocker amlodipine, a PM concentration of 0.07 mg/L found in a car driver killed in a traffic accident would easily be misinterpreted as serious intoxication resulting in impairment if compared to clinical plasma reference concentrations only (normal/therapeutic 0.003-0.015 mg/L, toxic (from) 0,088 mg/L1). This would in term have insurance judicial implications. However, the present data show that this finding represents the median (normal) PM concentration of the 313 cases analyzed, including only few poisoning cases, and consequently there is no reason to suspect impairment based on the PM concentration alone.
The toxicologist often needs to also consider the role of active metabolites – for example, for tricyclic anti-depressants and codeine – and also the ratio of parent drug to metabolite (active or inactive). Such data is commonly used as an additional tool to differentiate between chronic and acute ingestion and the likelihood of a drug related death. Clinical plasma reference concentrations, let alone post-mortem femoral blood concentrations, are not readily available for many metabolites, but such data would be of obvious interest to generate and collate, notwithstanding that this would be a very laborious task. Of course, such work would also rely on reference standards of these metabolites, many of which are not currently obtainable.
Conclusions
The present study, based on extensive consistent high-quality toxicology data from PM femoral venous blood (59 903 cases), extends our knowledge of how to interpret drug concentrations within the medico-legal context. The median PM concentrations give an idea of the ‘normal’ PM concentration range and the upper percentile concentrations indicate possible overdose levels. As to common drugs, the results were in good agreement with previous studies,16,26,27 suggesting that PM reference concentrations are applicable on an international basis. The proportion of fatal poisonings reported with each drug helps in assessing the concentration distribution further. However, PM toxicology results should always be interpreted in relation to the case as an entity. Our study demonstrates that using clinical therapeutic ranges in plasma to interpret PM toxicology results would commonly lead to misjudgement of a normal PM concentration as a lethal level.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s website.
References
- 1.Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care. 2012;16:R136. doi: 10.1186/cc11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta A. Therapeutic Drug Monitoring: Newer Drugs and Biomarkers. San Diego, CA: Academic Press; 2012. [Google Scholar]
- 3.Osselton MD. A Compendium of pharmacological, therapeutic and toxicological data on 136 drugs and chemicals in humans. Bull. Int. Assoc. Forensic Toxicol. 1983;17:16. [Google Scholar]
- 4.Baselt BC. Disposition of Toxic Drugs and Chemicals in Man. 9th edition. Seal Beach, CA: Biomedical Publications; 2011. [Google Scholar]
- 5.Pounder DJ, Jones GR. Post-mortem drug redistribution--a toxicological nightmare. Forensic Sci. Int. 1990;45:253. doi: 10.1016/0379-0738(90)90182-x. [DOI] [PubMed] [Google Scholar]
- 6.Prouty RW, Anderson WH. The forensic science implications of site and temporal influences on postmortem blood-drug concentrations. J. Forensic Sci. 1990;35:243. [PubMed] [Google Scholar]
- 7.Dalpe-Scott AL, Degouffe M, Garbutt D, Drost M. A comparison of drug concentrations in postmortem cardiac and peripheral blood in 320 cases. Can. Soc. Forensic Sci. J. 1995;28:113. [Google Scholar]
- 8.Hilberg T, Rogde S, Morland J. J. Forensic Sci. 1999;44:3. Postmortem drug redistribution--human cases related to results in experimental animals. [PubMed] [Google Scholar]
- 9.Rodda KE, Drummer OH. The redistribution of selected psychiatric drugs in post-mortem cases. Forensic Sci. Int. 2006;164:235. doi: 10.1016/j.forsciint.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Han E, Kim E, Hong H, Jeong S, Kim J, In S, et al. Evaluation of postmortem redistribution phenomena for commonly encountered drugs. Forensic Sci. Int. 2012;219:265. doi: 10.1016/j.forsciint.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Saar E, Beyer J, Gerostamoulos D, Drummer OH. The time-dependant post-mortem redistribution of antipsychotic drugs. Forensic Sci. Int. 2012;222:223. doi: 10.1016/j.forsciint.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Pelissier-Alicot AL, Gaulier JM, Champsaur P, Marquet P. Mechanisms underlying postmortem redistribution of drugs: A review. J. Anal. Toxicol. 2003;27:533. doi: 10.1093/jat/27.8.533. [DOI] [PubMed] [Google Scholar]
- 13.Yarema MC, Becker CE. Key concepts in postmortem drug redistribution. Clin. Toxicol. 2005;43:235. [PubMed] [Google Scholar]
- 14.Kennedy MC. Post-mortem drug concentrations. Intern. Med. J. 2010;40:183. doi: 10.1111/j.1445-5994.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferner RE. Post-mortem clinical pharmacology. Brit. J. Clin. Pharmacol. 2008;66:430. doi: 10.1111/j.1365-2125.2008.03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones AW, Holmgren A. Concentration distributions of the drugs most frequently identified in post-mortem femoral blood representing all causes of death. Med. Sci. Law. 2009;49:257. doi: 10.1258/rsmmsl.49.4.257. [DOI] [PubMed] [Google Scholar]
- 17.Linnet K. Postmortem drug concentration intervals for the non-intoxicated state - A review. J. Forensic Leg. Med. 2012;19:245. doi: 10.1016/j.jflm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Pelander A, Ojanpera I, Laks S, Rasanen I, Vuori E. Toxicological screening with formula-based metabolite identification by liquid chromatography/time-of-flight mass spectrometry. Anal. Chem. 2003;75:5710. doi: 10.1021/ac030162o. [DOI] [PubMed] [Google Scholar]
- 19.Ojanpera S, Pelander A, Pelzing M, Krebs I, Vuori E, Ojanpera I. Isotopic pattern and accurate mass determination in urine drug screening by liquid chromatography/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1161. doi: 10.1002/rcm.2429. [DOI] [PubMed] [Google Scholar]
- 20.Ojanpera I, Rasanen I, Vuori E. Automated quantitative screening for acidic and neutral drugs in whole blood by dual-column capillary gas chromatography. J. Anal. Toxicol. 1991;15:204. doi: 10.1093/jat/15.4.204. [DOI] [PubMed] [Google Scholar]
- 21.Launiainen T, Sajantila A, Rasanen I, Vuori E, Ojanpera I. Adverse interaction of warfarin and paracetamol: Evidence from a post-mortem study. Eur. J. Clin. Pharmacol. 2010;66:97. doi: 10.1007/s00228-009-0727-3. [DOI] [PubMed] [Google Scholar]
- 22.Rasanen I, Ojanpera I, Vuori E. Quantitative screening for benzodiazepines in blood by dual-column gas chromatography and comparison of the results with urine immunoassay. J. Anal. Toxicol. 2000;24:46. doi: 10.1093/jat/24.1.46. [DOI] [PubMed] [Google Scholar]
- 23.Gunnar T, Ariniemi K, Lillsunde P. Fast gas chromatography-negative-ion chemical ionization mass spectrometry with microscale volume sample preparation for the determination of benzodiazepines and alpha-hydroxy metabolites, zaleplon and zopiclone in whole blood. J. Mass Spectrom. 2006;41:741. doi: 10.1002/jms.1030. [DOI] [PubMed] [Google Scholar]
- 24.Rasanen I, Kontinen I, Nokua J, Ojanpera I, Vuori E. Precise gas chromatography with retention time locking in comprehensive toxicological screening for drugs in blood. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;788:243. doi: 10.1016/s1570-0232(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 25.Liedholm H, Liden A, Kroon L, Melander A, Wahlin-Boll E. Pharmacokinetics of dixyrazine: Low bioavailability, improved by food intake. Drug Nutr. Interact. 1985;3:87. [PubMed] [Google Scholar]
- 26.Druid H, Holmgren P. A compilation of fatal and control concentrations of drugs in postmortem femoral blood. J. Forensic Sci. 1997;42:79. [PubMed] [Google Scholar]
- 27.Reis M, Aamo T, Ahlner J, Druid H. Reference concentrations of antidepressants. A compilation of postmortem and therapeutic levels. J. Anal. Toxicol. 2007;31:254. doi: 10.1093/jat/31.5.254. [DOI] [PubMed] [Google Scholar]
- 28.Jones AW, Larsson H. Distribution of diazepam and nordiazepam between plasma and whole blood and the influence of hematocrit. Ther. Drug Monit. 2004;26:380. doi: 10.1097/00007691-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Launiainen T, Vuori E, Ojanpera I. Prevalence of adverse drug combinations in a large post-mortem toxicology database. Int. J. Leg. Med. 2009;123:109. doi: 10.1007/s00414-008-0261-3. [DOI] [PubMed] [Google Scholar]
- 30.Hakkinen M, Launiainen T, Vuori E, Ojanpera I. Benzodiazepines and alcohol are associated with cases of fatal buprenorphine poisoning. Eur. J. Clin. Pharmacol. 2012;68:301. doi: 10.1007/s00228-011-1122-4. [DOI] [PubMed] [Google Scholar]
- 31.Hakkinen M, Launiainen T, Vuori E, Ojanpera I. Comparison of fatal poisonings by prescription opioids. Forensic Sci. Int. 2012;222:327. doi: 10.1016/j.forsciint.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Winek CL, Wahba WW, Winek CL, Jr, Balzer TW. Drug and chemical blood-level data 2001. Forensic Sci. Int. 2001;122:107. doi: 10.1016/s0379-0738(01)00483-2. [DOI] [PubMed] [Google Scholar]
- 33.Dollery C. Therapeutic Drugs. 2nd edition. Edinborough: Churchill Livingstone; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


