Abstract
Severe fever with thrombocytopenia syndrome (SFTS) was firstly discovered in China in 2010, followed by several reports from many other countries worldwide. SFTS virus (SFTSV) has been identified as the causative agent of the disease and has been recognized as a public health threat. This novel Bunyavirus belongs to the Phlebovirus genus in the family Bunyaviridae. This review also describes the different aspects of virology, pathogenesis, epidemiology, and clinical symptoms on the basis of the published article surveillance data and phylogenetic analyses of viral sequences of large, medium, and small segments retrieved from database using mega 5.05, simplot 3.5.1, network 4.611, and epi information system 3.5.3 software. SFTS presents with fever, thrombocytopenia, leukocytopenia, and considerable changes in several serum biomarkers. The disease has 10 ∼ 15% mortality rate, commonly because of multiorgan dysfunction. SFTSV is mainly reported in the rural areas of Central and North-Eastern China, with seasonal occurrence from May to September, mainly targeting those of ≥50 years of age. A wide range of domesticated animals, including sheep, goats, cattle, pigs, dogs, and chickens have been proven seropositive for SFTSV. Ticks, especially Haemaphysalis longicornis, are suspected to be the potential vector, which have a broad animal host range in the world. More studies are needed to elucidate the vector–animal–human ecological cycle, the pathogenic mechanisms in high level animal models and vaccine development. © 2013 The Authors. Reviews in Medical Virology published by John Wiley & Sons, Ltd.
INTRODUCTION
The mysterious agent of emerging infectious disease reported through 2009 in Central and North-Eastern China was confirmed later in 2010 as severe fever with thrombocytopenia syndrome virus (SFTSV) 1. The common manifestations of the disease include severe fever with thrombocytopenia syndrome (SFTS), vomiting, and diarrhea, which progress to multiple organ failure with 12 ∼ 30% mortality rate reported initially 1. Surveillance data indicate that the incidence of SFTS is growing, and dissemination is expanding to at least 15 Chinese provinces in 2010 ∼ 2013 2. Moreover, SFTS cases have been reported in other Asian and Mediterranean countries as well as in the USA 3–6.
This review is based on mining of the data reported in previous studies related to SFTSV and SFTS. This paper systematically reviews SFTSV in terms of its virology, epidemiology, and clinical characteristics, focusing on molecular evolution and epidemiology among different areas. The information described herein might improve the understanding of this novel virus and help to prevent and control it, thereby decreasing the transmission and diffusion of this novel virus worldwide.
VIROLOGY
Classification of Bunyaviridae
Severe fever with thrombocytopenia syndrome virus belongs to the third group of the Phlebovirus genus, which is a member of the Bunyaviridae family 1. The family Bunyaviridae comprises a group of segmented, negative-strand RNA viruses that includes over 350 members classified into five genera, Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus7–10. The genus Phlebovirus currently includes over 70 antigenically distinct serotypes; 68 of the known serotypes are divided into two groups: Phlebotomus fever viruses include 55 members (transmitted by Phlebotominae sandflies) and the Uukuniemi viruses (transmitted by ticks) comprise 13 members 11,12. Only eight species cause human disease, the Alenquer, Candiru, Chagres, Naples, Punta Toro, Rift Valley fever, Sicilian, and Toscana viruses 13,14. SFTSV has been recognized as a novel member of the Phlebovirus genus because it is distantly related to both the existing Uukuniemi virus and Phlebotomus fever viruses 1,2,11.
Morphology
Severe fever with thrombocytopenia syndrome virus, such as other Bunyaviridae, is a spherical virion of 80–100 nm in diameter, covered by a lipid bilayer envelope of 5–7 nm in thickness, with no Ms 1,2,11,15. The lipid bilayer envelope has spikes composed of gps, regularly arranged in genus specific arrays on the envelope exterior 1,2,16,17. As observed by thin-section EM, the interior of the virions has a filamentous or coiled bead appearance, composed of three viral segments wrapped in the viral N in a circular genome 1,2,17,18 (Figure 1).
Figure 1.
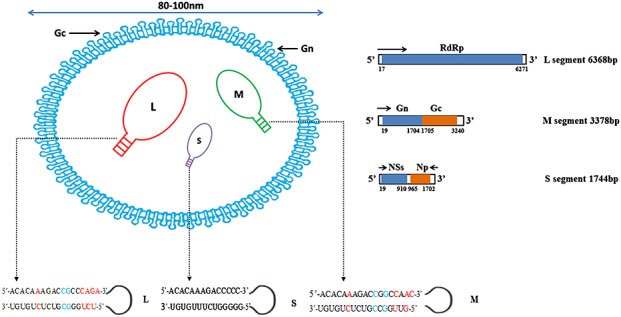
Schematic diagram of the structure and genome of the SFTSV 1,2. SFTSV genome included L, M, and S segments. The antisense RNA and the encoded ORFs are represented in orange and blue boxes respectively. In L, M, and S genomic RNAs, only the first 20 Nts of both the 5′ and 3′ termini of each segment are shown. The first 15 Nts of both the 5′ and 3′ termini of each segment are paired and conserved except for the mismatches A–C/G or C–U in red color and C–C or G–G in blue color. Note: The data were sourced from the report of Li et al. and processed in photoshop 7.0 1
Genome
Comparable with the general genetic composition of Bunyaviridae family members, the genome of SFTSV consists of three single-stranded negative sense RNA segments [large (L), medium (M), and small (S)] 1,2,17,18. The full-length L segment consists of 6368 bp including a single ORF between positions 17–6271 that encodes 2084 aa residues forming the RNA-dependent RNA polymerase (RdRp), which functions as the viral transcriptase/replicase 1,2,17. The M segment is 3378 bp, forming an ORF between positions 19–3240 and encoding 1073 aa of membrane protein precursor, which matures to two envelopes, a glycoprotein N (Gn) [19–1704 nucleotide (Nt)] and a glycoprotein C (Gc) (1705–3240 Nt) 1,2. The S segment is 1744 Nts of ambisense RNA with two oppositely ORFs separated by a 54 bp Nt intergenic region, encoding two proteins, the Np (1702–965 Nt) in antisense orientation and the NS protein (29–910 Nt) in sense orientation 1,2,18. Np facilitates viral RNA encapsidation and is responsible for the formation of RNP complex 19. The 5′ and 3′ termini of the L, M, and S segments possess short noncoding sequences. The 5′ noncoding regions of SFTSV are 16 Nt (L), 18 Nt (M), and 42 Nt (S); and the 3′ noncoding regions are 100 Nt (L), 141 Nt (M), and 28 Nt (S). Similar to other Bunyaviruses, the RNA component of which circularizes because each of the three segments having a consensus 3′-terminal Nt sequence is complementary to the 5′ terminal sequence, forming approximately 30 to 50-bp-long panhandle. The complementary 5′-termini sequences are 5′-ACACAAAGACCGCCCAGA-3′ (L), 5′-ACACAAAGACCGGCCAAC-3′ (M), and 5′-ACACAAAGACCCCC-3′ (S), whereas the 3′-termini complementary sequences are 3′-UGUGUCUCUGCGGGUCU-5′ (L), 3′-UGUGUCUCUGCCGGUUG-5′ (M), and 3′-UGUGUUUCUGGGGG-5′ (S) 1,2. These sequences appear to form panhandle structures that seem likely to play a role in replication and encapsidation facilitated by binding with the viral N (Figure 1).
Cell culture
Acute blood samples in heparin anticoagulant collected within 7 days of onset of this disease are used to isolate the pathogen by inoculating into multiple cell lines of human, animal, or tick origin. Limited types of animal cell lines (Vero E6, Vero, DH82, and L929) can be infected by SFTSV 1,2. The viral nucleic acid can be molecularly detected for 7 days after incubation in the sensitive cell lines, and the viral protein can be tested for 10 days after incubation 2. Interestingly, DH82 cell line presents an obvious cpe after infection, in which the morphologic features of the infected DH82 cells changed from round monocytes to an elongated shape, having granular particles in the cytoplasm; however, these changes were not observed in Vero cell lines 1,2,20. Thus, those cell lines are permissive and can be used for virus isolation and propagation 1,2,20. It needs to further confirm that the other cell lines such animal cell line (BHK 21); human cell lines HL60, A549, 2BS; and tick cell line ISE6 are refractory to this novel virus and could be used for receptor identification and related endeavors.
Receptor
Entry into host cells is thought to occur by attachment of virions to cellular receptors utilized by Bunyaviruses, but the receptors for this family remain poorly characterized and different in the serotypes. Hantaviruses have been shown to use integrin to enter endothelial cells; it is not known whether the integrin serves as a receptor or attachment factor 21. California serogroup of Bunyaviruses shared a common receptor, which is the La Crosse G1 gp 22, whereas DC-specific intercellular adhesion molecule 3-grabbing non-integrin is a Phlebovirus entry receptor for attachment and for promotion of entry and infection. Just like the other Phlebovirus, the C-type lectin DC-specific intercellular adhesion molecule 3-grabbing non-integrin was found to serve as a receptor for SFTSV, Gn/Gc-driven entry into cell lines, and dendritic cells, inducing viral spread and pathogenesis 23,24. Furthermore, sera from convalescent SFTS patients inhibited SFTSV Gn/Gc-driven host cell entry in a dose-dependent fashion 24. SFTSV virions and receptors binding will initiate the subsequent step of life cycle in the cell lines and human cases and animal models 24.
Pathogenesis
The pathogenesis of SFTSV infections is unclear as there is no fully compatible experimental animal model and cell line in vitro to describe it (mice do not seem to acquire severe disease). Jin et al. 25 claimed murine C57BL/6 mice as experimental model for SFTSV infection. In this model, viral RNA was detected in the blood and three organs (spleen, liver, and kidney) but not in the lung, intestine, heart, muscle, or brain. The main histopathological changes in the early stage (<14 days pi) were identified in the spleen, where the number of macrophages and platelets were greatly increased, and SFTSV was co-localized with platelets in cytoplasm of macrophages in that organ. The pathogenic lesions in the later stage (>14 days pi) were found in the liver and kidney, where the cells showed excessive degeneration and necrosis. In addition, Chen et al. 26 reported that the SFTSV infection is lethal in newborn Kunming mice with dispersion of viral antigen and genetic material (RNA) in almost all organs, indicating a systemic infection. In vitro, Li further revealed that SFTSV adhered to platelets and facilitated the phagocytosis of platelets by macrophages 27. Taken together, in vitro and in vivo tests indicate that SFTSV-induced thrombocytopenia is caused by clearance of circulating virus-bound platelets promoted by splenic macrophages, which might resemble the human SFTS disease features.
Koch's postulates in severe fever with thrombocytopenia syndrome virus
Koch's postulates were designed to establish a causal relationship between candidate microbes and diseases. The identification of SFTSV is a prime example of the rapid discovery of a truly emerging infectious disease, which has, mostly, fulfilled Koch's postulates. First, the novel virus has been found in abundance in all hosts suffering from SFTS but not found in healthy people 1. Second, the SFTSV has been isolated from diseased cases and grown in many animal cell line cultures. Third, the cultured novel virus may cause the same disease when introduced into a healthy mouse model. Fourth, SFTS has been be reisolated from the inoculated, diseased experimental mice, and tick model and has been identified as being identical to the original specific causative agent, the identity was 99.99% 26,27.
Evolution
The evolutionary analyses described in this review were based on 43 full-length sequences of SFTSV genes downloaded from genbank (http://www.ncbi.nlm.nih.gov/nuccore/; NIH, Maryland,USA) sourced from different countries/areas, including 15 of L segment, 14 of M segment, and 14 of S segments ( genbank accession numbers are provided in Tables S1, S2, and S3). Phylogenetic tree, plot similarity, and median network were performed using the mega 5.05 (http://www.megasoftware.net/), simplot 3.5.1 (The Johns Hopkins School of Medicine, Maryland, USA), and network 4.611 software (Fluxus Technology Ltd, England) 28–31, respectively. Phylogenetic tree showed that L, S, and M segments from all SFTSV isolates are clustered together but are almost equally distant from the Phlebotomus fever and Uukuniemi viruses. In agreement with Zhang et al. 32, the phylogenies estimated from full-length sequences of SFTSV genes demonstrated four major sublineages, previously named as A, B, C, and E. Furthermore, the aa sequence similarity showed that RdRp (deduced from L gene) proteins in SFTSV have 46.3% similarity to Rift Valley fever virus but only shared 36.1 ∼ 46.1% homology to the other Phleboviruses (Uukuniemi virus, Naples virus). On the contrary, RdRp from different Chinese specimens isolated from different provinces across a wide time range are closely related, sharing 96.2–98.7% aa sequence homology. Similar results are also found in reference to membrane gps (deduced from M gene) and the most highly conserved Np (deduced from S gene) of SFTSV. In general, the data from the molecular evolution and morphology indicate that SFTSV belongs to the prototype of a new member of the genus Phlebovirus, and all Chinese SFTS cases are caused by one genetic lineage of this novel virus. This result agrees with that reported by Yu et al. 1,2 (Figure 2).
Figure 2.
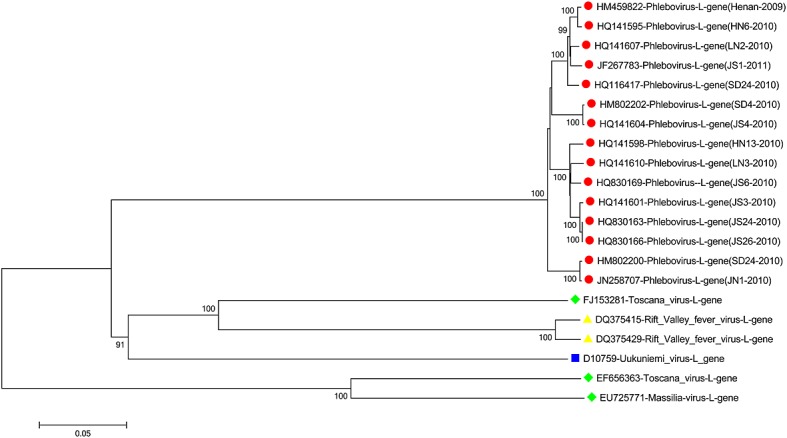
Phylogenetic relationship of Chinese SFTSV and other Phlebovirus strains based on sequences of L gene fragment. Evolutionary relationships of 21 taxa were inferred by the neighbor joining and maximum-likelihood methods using MEGA 5.05 software
The plot similarity versus position was performed by determining the degree of identity of the query sequence (L genes from Chinese strains) to a panel of reference sequences (the other groups of Phleboviruses) in a sliding window (windows 200 bp/step). The results showed that the homology percentage was <25%, 35%, and 0% in the position 1–2000 Nt, 2000–5500 Nt, and 5500–6500 Nt regions, respectively. In addition, the 1–2000 Nt and 5500–6500 Nt regions were highly diverse compared with the 2000–5500 Nt region (Figure 3). In agreement with the results of He and Ding 33, the query sequences from the Chinese isolates and other Phlebovirus reference sequences showed no crossover non-mosaic sites, which might indicate that this new virus undergoes considerable recombination and gene rearrangement processes. In addition, the similarity results provided further support for the separation of this novel SFTSV from other Phleboviruses.
Figure 3.
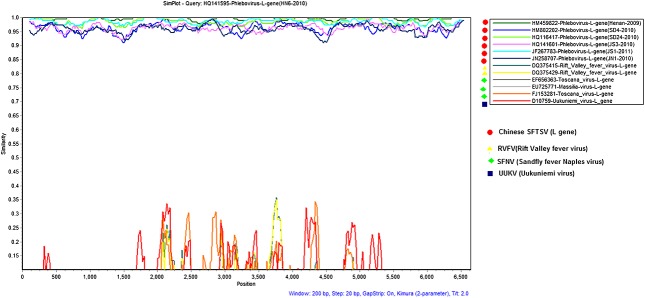
Similarity plot analysis of Chinese SFTSV strains and the other Phlebovirus species, with a window size of 200 bp and a step size of 20 bp. The plot were done using SIMPLOT 3.5.1 software (an interactive 32-bit software program) based on the sequence homology versus position of Chinese SFTSV strains to a panel of other Phleboviruses in database
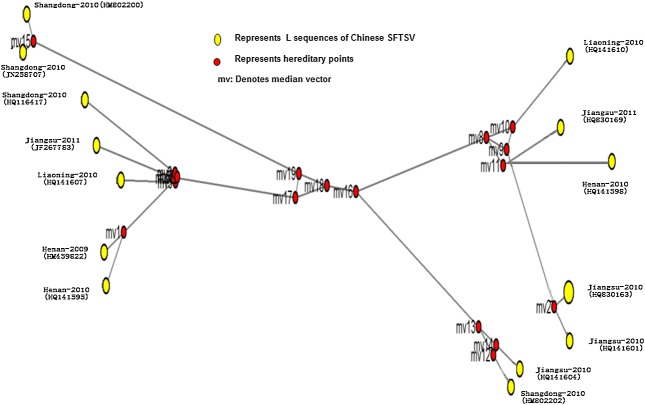
The median-joining network analysis showed that all SFTSV isolated had no common ancestor and can be divided into four subgroups, each of sub-linkage with no regional and timeline cluster (Figure 4).
Figure 4.
Median-joining network depicting the relationship of Chinese SFTSV strains and other Phlebovirus species in database. The common ancestor was inferred based on network of L gene mutations using the NETWORK 4.611 software reported by Bandelt et al. 68
EPIDEMIOLOGY
According to the literature and Chinese surveillance data from 2009 to 2012, we analyzed the epidemical data by epi information software 3.5.3 (USA cdc, Atlanta Botanical, Georgia, USA) and spss 17.0 (SPSS Inc., Chicago, IL, USA), then summarized the epidemiological features in four ways: the disease distribution, host and vector, seroepidemical survey, and life cycle.
Disease distribution
Geographical distribution
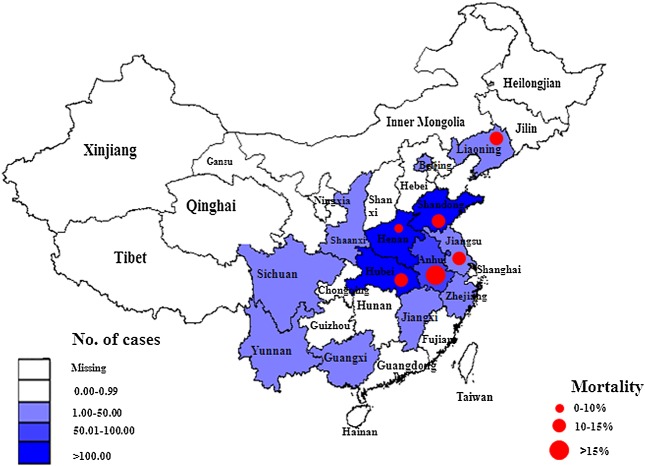
In the past decades, Phlebovirus was mainly circulating in some parts of Africa and Europe, Arabian Peninsula, the regions with the highest incidences of the phlebotomine sandfly transmitted viruses. Phlebovirus has never been reported in China until SFTSV was identified in 2010. Although the SFTS have been found in some countries outside China such as Korea, Japan, and the USA since the SFTSV were identified in 2010, the major affected regions were in China. SFTS mainly occurs in the rural areas of the Eastern, Central, and North-Eastern China 1,2,34,35. According to the national surveillance data in 2011, a total of 571 confirmed cases including 59 deaths from 13 provinces were reported to the Chinese SFTS information reporting system. The high incidence areas (>100 cases/year) were as follows: 191 in Shandong (24 deaths), 138 in Henan (four deaths), and 106 in Hubei (15 deaths); the median incidence areas (50 ∼ 100 cases/year) were listed as follows: 61 in Anhui (10 deaths); the low incidence areas (<50 cases/year) were as follows: 39 in Liaoning (four deaths), 19 in Jiangsu (two deaths), nine in Zhejiang (zero deaths), two in both Yunnan and Jiangxi (zero deaths), and one case in each of Sichuan, Shanxi, Guangxi, and city of Beijing, with no reported deaths. The mortality rate reached 10.33% nationwide although it was as high as 30% in the initial stage. In detail, provincial mortality rates were as follows: Anhui, 16.39%; Hubei, 14.15%; Shandong, 12.57%; Jiangsu, 10.53%; Liaoning, 10.26%; and Henan, 2.90% (Figure 5). In 2012, the number of confirmed cases increased slightly to 600, involved in 15 endemic provinces, but the mortality rate remained roughly 10.30% (61/600). In summary, the high rate of infection was reported among those living in hilly and wooded lands and those working in agricultural fields 1,2,36,37, which might be attributed to the underlying medical conditions, natural circumstances, exposure chances, disease detection, and laboratory testing, as well as consulting situation and medical intervention.
Figure 5.
Geographic distribution of 571 SFTS confirmed cases and 59 deaths from Chinese SFTS surveillance in 2011, as processed in EPI information 3.5 software
Population distribution
On the basis of the data of 649 confirmed cases and 31 deaths, retrieved from the national surveillance data with age range of 2 ∼ 87 years (average 57.6 years), 92.14% (598/649) of the reported cases were older than 35 years of age (35 ∼ 80 years), whereas those younger than 30 years of age accounted for 3.70%, and those older than 80 years were only 2.47% (16/649). Farmers were the major population, accounted for 89.22% of infected cases. Overall, 31 deaths occurred in those aged 42–78 years (the average was 63 years old). Farmers accounted for 87.10% (27/31) of the total deaths (Figure 6). In addition, analysis of data showed that there was no obvious difference in regards of sex between confirmed cases (1:1.02) and deaths (1:1.07) of male to female participants (p > 0.05), which concord with previous reports from different areas 1,2,27.
Figure 6.
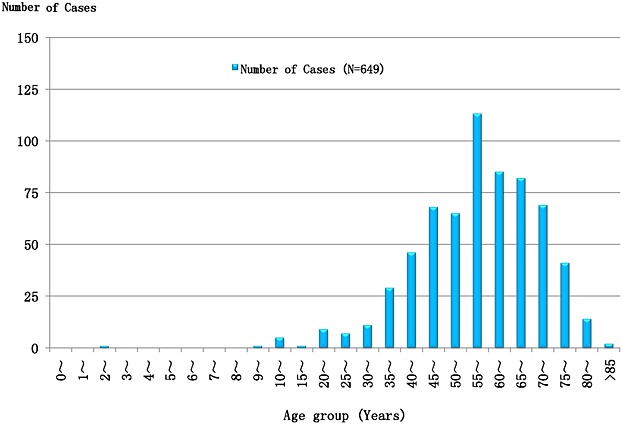
Age distribution of 649 SFTS confirmed cases from Chinese SFTS surveillance in 2012. Note: The data were sourced from Chinese SFTS surveillance and processed in excel 2010 and spss 17.0
Seasonal distribution
Data from highly affected provinces including Hubei, Henan, Jiangsu, and Zhejiang indicated that the epidemic season extended from early Summer to late Autumn with a peak of incidence through May–July 1,29,32. On the basis of the weekly distribution across the nation, occurrence of 275 cases was reported from the 9th to 42nd week, with biphasic distribution. Since the first report on the 9th week, the cumulative cases gradually increased to the peak value at 17th–30th week with some obvious spikes, followed by short decline at 31st and 32nd weeks before burst to the second peak from the 33rd to 36th weeks (Figure 7). Seasonal occurrence may be attributed to the increased activity of the farmers such as harvesting tea, and cutting grasses, subsequently the possibility of more exposure to the potential vectors.
Figure 7.
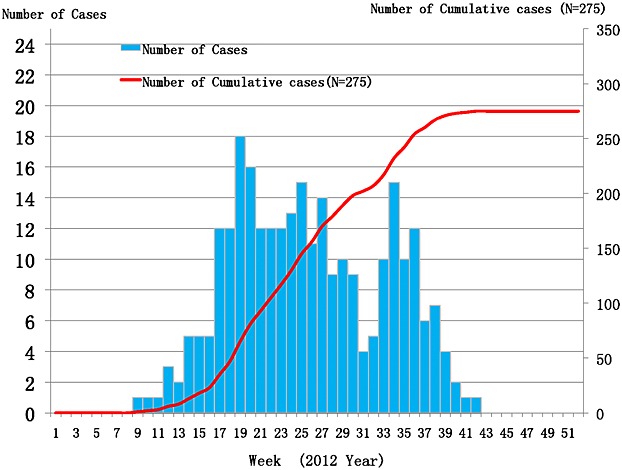
Weekly distribution of 275 SFTS confirmed cases in China during 2012. Note: The data were sourced from Chinese SFTS surveillance and processed in excel 2010 and spss 17.0
Host and vector
The ecological cycle of the SFTSV is not yet known, but it seems most likely that Phleboviruses involve arthropod vectors and mammalian hosts, including cats, mice, hedgehogs, weasels, possums, and yaks. Humans appear to be an accidental host and play no essential role in the life cycle of SFTSV. Current research indicated that ticks, especially Haemaphysalis longicornis, were the major potential insect vector to transmit the virus to humans on the basis of the following evidence: (i) SFTSV has been detected from the ixodid ticks H. longicornis and Rhipicephalus microplus in the endemic regions 32; (ii) the nucleic acid sequence of viruses isolated from ticks has 95 ∼ 100% homology with the SFTSV isolated from patients and other mammalian hosts; (iii) about 52% of patients had a tick exposure history 36,37; (iv) 30 ∼ 70% of mammalian hosts, which likely have exposures to ticks, such as dogs and goats and cattle, had substantial antibody levels to SFTSV. On the other hand, there may be more potential vectors linked with SFTSV life cycles because 48% of patients had no tick exposure history 36,37, and the SFTSV positive rate was very low (0.7 ∼ 5.4%) in the tick population 1,2,37. Viral RNA was not detected in any mosquito population so far 1,38–40, suggesting those mosquitoes, which are commonly found in endemic rural areas, are not potential vectors.
To ascertain the role of domestic animals as reservoir hosts for SFTSV, investigation of different animal species including sheep, cattle, pigs, dogs, and chickens in China showed that only a small fraction of animals (ranging from 1.7% to 5.3%) were found to carry low levels of viral RNA in their sera 37,41. The viral isolates from the aforementioned species shared 95.4% identify with those of the patients and ticks 41.
Although rodents are known as reservoir hosts of Bunyaviruses, there is pronounced discrepancy on SFTSV in rodents. Results of studies on the occurrence of SFTSV in rodents indicated that SFTSV-antigen positive rate was about 4.31% (76/1762) in rats (as detected in heart, liver, spleen, lung, kidney, and brain tissue) captured from 18 Chinese provinces, as well as 11.4% (8/70) in Zhejiang province according to immunofluorescence methods 35,42. On the other hand, Lu et al. could not detect SFTSV RNA in the sera of 81 rodents and 19 canines collected from Beijing 43. In conclusion, SFTSV is a close epizootic emergency infectious disease in the hilly lands; however, there is not enough evidence to confirm which animals are reservoir hosts.
Serological epidemiology
Serological survey in patients and health population
The immune response of the patients varies according to the infection (first exposure versus reexposure) and clinical status (early, acute, convalescent phases), with no distinct pattern associated with the infection. The datasets from different geographic areas indicated that a considerable proportion of patients have showed a seroconversion to SFTSV during acute and convalescent phases of the illness 1,44, in the form of high levels of antibody titers. Of note, neutralizing antibodies to SFTSV is prone to increase 38 and persist.
Furthermore, reports of SFTSV-specific antibodies in healthy populations are sparse and inconsistent. Whereas some studies failed to detect SFTSV-specific antibodies in healthy individuals from both endemic areas and nonendemic areas 1, other studies indicated SFTSV antibody positive rate 0.8 ∼ 3.8% 37,38,44–46. This discrepancy may be attributed to the season of sample collection, age and sex of the subjects, previous exposure or low level of infection, and/or detection method utilized in individual studies.
Serological survey in animals
Although molecular methods could detect viral RNA in the small fraction (1.7–5.3%) of samples from domesticated animals (goat, cattle, dog, and pig), poultry (chicken, duck, etc.), and rodents (rat, etc.), serological assays indicated the presence SFTSV-specific antibodies in high numbers of animals. In a study of small size (<500 samples), 47.7% of domesticated animals were positive for SFTSV antibodies, of which 36.7 ∼ 83% of goat, 31.82 ∼ 80% of cattle, 50.00% of hedgehogs, 6.40 ∼ 55% of dogs, 2.00 ∼ 6.00% of pigs, and 0.98 ∼ 2.00% of chickens were positive according to the report from Hubei, Jiangsu, and Shandong province 37,38,44,45. Serology survey of more than 3000 domestic animals showed that approximately 70% of sheep, 60% of cattle, 38% of dogs, 3% of pigs, and 47% of chickens were SFTSV antibody positive. Another large size investigation of 1454 rodents indicated that 3.03% of rats were SFTSV antibody positive in the main epidemic areas, of which Apodemus agrarius and Rattus norvegicus accounted for 52.27% and 20.45% among the positive rats, respectively 37. Serological findings indicated controversy on the data from different regions, but there were some common features: the incidence of livestock infection was significantly higher than the incidence in poultry, humans, and rats. Therefore, it suggested that livestock plays an important role during the cycle of this novel virus transmission to human.
Transmission model
Until now, human exposure to ticks is one of the most suspected transmission routes of the virus, but some studies have provided evidence that SFTSV can be transmitted from person-to-person. The concept of person-to-person transmission was confirmed through several reports, where the secondary patients are likely to be infected with SFTSV via direct contact with blood of index patients 47, forming confined family and/or contact clusters 48–52. Individuals in close contact with primarily patients but not exposed to index blood did not contract the illness. Complete genomic sequencing indicated that the viral strains isolated from the secondary patients were virtually identical to the sequence of the virus isolated from the index case 49,50. Notably, the viral RNA could be detected in throat, urine, and fecal specimens of a substantial proportion of patients, including all fatal cases 53, throwing light on the potential role of body fluids and excreta of SFTSV patients for person-to-person transmission, posing a great challenge to the patients' companions and health care staff.
CLINICAL CHARACTERISTICS
Clinical symptoms
The clinical course of the SFTS patients could be divided into three major stages starting with fever, followed by multiorgan dysfunction and the convalescent stage. The incubation period ranges between 1 and 2 weeks, leading to fever stage, which ranges from day 1 to day 7 post-onset of illness. This stage is characterized, both in survivors and fatal cases, by flu-like symptoms, thrombocytopenia and leukocytopenia, and slight increase in serum biomarkers alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, creatine phosphokinase, and creatine kinase isoenzyme. These symptoms may resolve after 1 week in survivors, and serum enzymes begin to decline toward normal levels and show signs of recovery after 2 weeks of the disease onset. Later, some cases moved to the multiorgan dysfunction stage, the serum viral load decreased in nonfatal cases but still remained high in fatal cases, while the platelet counts reverted to a normal value in nonfatal cases but continued to decline in fatal cases. The biomarker began to decline at approximately day 9 or day 11 in nonfatal cases but progressively increased in fatal cases. In the third stage (over 14 days), nonfatal cases could recover from the disease with most clinical parameters converting to normal. In contrast, the fatal cases showed the serum enzymes increasing accompanied with the main complication including then multiple organ failure, coagulation, shock, and acute respiratory distress syndrome, and so on. 27,54,55.
Review of clinical symptoms and signs reported in previous studies 1,2,36,55 for surviving and fatal cases, respectively, indicated fever (78.28%, 100.00%), anorexia (63.35%, 64.00%), fatigue (62.44%, 55.00%), body sores [52.71%, no data available (NA)], nausea (52.49%, 45.00%), vomiting (45.93%, 36.00%), diarrhea (45.70%, 27.00%), myalgia (44.12%, 18.00%), chills (41.63%, NA), abdominal pain (40.05%, 36.00%), coarse breathing sounds (38.46%, NA), dizziness (37.10%, NA), headache (36.43%, NA), enlargement of lymph nodes (34.16%, 18.00%), cough (28.73%, 18.00%), confusion (22.00%, 36.00%), sputum production (20.14%, NA), arthralgia (19.91%, NA), throat congestion (12.00%, 18.00%), conjunctive congestion (10.00%, NA), apathy (9.00%, 9.00%), petechiae (7.00%, 27.00%), coma (6.00%, 27.00%), slurred speech (6.00%, 9.00%), and skin rash (4.30%, NA) (Figure 8).
Figure 8.
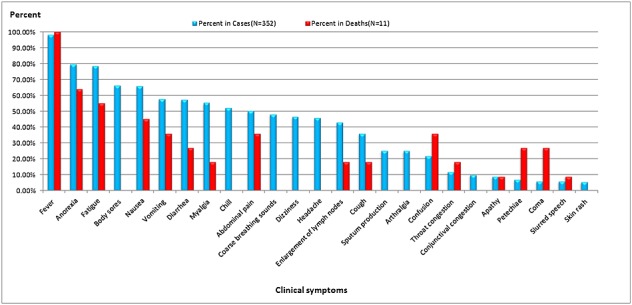
Clinical symptom percentages among 352 SFTS cases and 11 fatal cases from China. Note: The data were sourced from References 1, 38, and 40 and processed in excel 2010 and spss 17.0
Diagnostic methods
Just like most Phlebovirus infections, the diagnosis of SFTSV is based on viral nucleic acid tests and serological detection. In general, viral nucleic acids can be tested in the acute phase serum of patients. One-step TaqMan real-time assays were applied widely in amplifying L, M, and S of SFTSV segments. The molecular techniques improved the detection of SFTSV. For example, Yang et al. 56 and Xu et al. 57 established a modified, low cost, and rapid visualized one-step RT loop-mediated isothermal amplification method for the detection of RNA from the SFTSV. A specific two-tube multiplex real-time RT-PCR assay for the detection of SFTS, along with other closely related viruses revealed high sensitivity (10 copies/μL) with no cross reactivity between tested viruses indicating the high specificity of the assay 58. Furthermore, a highly sensitive one-step real-time RT-PCR method using a minor groove binding probe was developed for detection and quantitation of SFTSV by Li et al. 59. The unique assay of Cui et al. 60 involved a nearly instrument-free, simple molecular method that incorporates RT-cross-priming amplification coupled with a vertical flow visualization strip for rapid detection of SFTSV. The RT-cross-priming amplification coupled-vertical flow assay targets a conserved region of the M segment of the SFTSV genome and has a limit of detection of 100 copies per reaction, with no cross–reaction with other vector-borne Bunyaviruses and bacterial pathogens. The sensitivity and specificity of the assay were 94.1% and 100.0%, respectively. Moreover, potential detection limit of 10 viral RNA copies/µl was achieved using quantitative real-time PCR technique utilizing primer–probe sets to detect L, M, and S genes of SFTSV, with 98.6% sensitivity and over 99% specificity 61. The improved molecular assay was 1000 times more sensitive than the conventional PCR.
Nonetheless, serology testing methods including the in-house Mac-EIA assay, indirect EIA assay, and double-antigen sandwich EIA assay have been also performed in testing virus specific IgM, IgG, and total antibodies in serum samples, respectively. Improvement of EIA system utilizing N of SFTSV was developed by Jiao et al. 62 with high sensitivity and specificity. Additionally, the conventional methods such as indirect immunofluorescence and the serum neutralization assays are still the key, however these methods are costly and need more time and well-trained personnel.
CONCLUSIONS
This review highlights the virology, epidemical, and clinical features of SFTS caused by SFTSV. SFTSV belongs to the third group of Phlebovirus, with transmission by the tick (especially, H. longicornis) and blood/body fluids contact. The fatality rate of the disease is about 10–15% because of multiple organ failure. SFTS has been reported in villagers and farmers older than 50 years of age in rural areas of North, East, and Central China from March to November with peak on May–July. Many domesticated animal, especially goats and cattle might be potential amplifying hosts.
There are still many questions that need clarification. First, other pathogens presented similar symptoms to SFTSV, such as Anaplasma phagocytophilum, Ehrlichia species, and hemorrhagic fever virus should be considered, because SFTSV RNA cannot be detected in many patients 63–66. Furthermore, more studies related to pathogenesis of the disease should be performed in detail using experimental models of different species from murine to higher nonhuman primates. Second, the SFTSV ecologic life cycle (vector–host–human) should be clarified, including potential additional vectors such as mosquitoes, acaruses, and sandflies. It also needs to be determined whether domestic animals play an important role as reservoir. Except by contaminated blood and secretions, it remains unknown whether airborne transmission by aerosols, mother-to-child transmission, or fecal–oral transmission might occur in some cases 67. Third, the endemic areas are expanding and, without specific antiviral treatment, there is an urgent need for the production of an efficient and safe SFTSV vaccine(s) for those at high-risk populations 26 and for the most susceptible animals, which might help to prevent and control this novel disease in the world.
Acknowledgments
This research was supported by the Key Program grant from the Science Technology Department of Zhejiang Province (2012C13016-2) and the Medical Research Program of Zhejiang Province (2012KYA045).
Glossary
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CK
creatine phosphokinase
- CK-MB
creatine kinase isoenzyme
- DC-SIGN
DC-specific intercellular adhesion molecule 3-grabbing non-integrin
- Gc
glycoprotein C
- Gn
glycoprotein N
- HGA
human granulocytic anaplasmosis
- LDH
lactate dehydrogenase
- MOF
multiorgan dysfunction
- NA
no data available
- Np
nucleoprotein
- NS
non-structural
- Nt
nucleotide
- RdRp
RNA-dependent RNA polymerase
- RT-CPA
reverse transcription-cross-priming amplification
- RT-LAMP
reverse transcription loop-mediated isothermal amplification
- RT
reverse transcription
- SFTS
severe fever with thrombocytopenia syndrome
- SFTSV
severe fever with thrombocytopenia syndrome virus
- VF
vertical flow
CONFLICT OF INTEREST
The authors have no competing interests.
REFERENCES
- Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel Bunyavirus in China. New England Journal of Medicine. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DX. Fever with thrombocytopenia associated with a novel Bunyavirus in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25(2):81–84. [PubMed] [Google Scholar]
- Denic S, Janbeih J, Nair S, et al. Acute thrombocytopenia, leucopenia, and multiorgan dysfunction: the first case of SFTS Bunyavirus outside China? Case Reports in Infectious Diseases. 2011;2011:1–4. doi: 10.1155/2011/204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel PhlebovirusBunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne and Zoonotic Diseases. 2009;9:519–530. doi: 10.1089/vbz.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou V, Pardalos G, Athanasiou-Metaxa M, et al. Novel Phlebovirus in febrile child, Greece. Emerging Infectious Diseases. 2011;17(5):940–941. doi: 10.3201/eid1705.101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan LK, Folk SM, Kelly AJ, et al. A new Phlebovirus associated with severe febrile illness in Missouri. New England Journal of Medicine. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- Bishop DH, Calisher CH, Casals J, et al. Bunyaviridae. Intervirology. 1980;14:125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Schmaljohn CS, Nichol ST. Bunyaviridae. In: Knipe D, Howley P, editors. Fields Virology. Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1741–1790. [Google Scholar]
- Walter CT, Barr JN. Recent advances in the molecular and cellular biology of Bunyaviruses. Journal of General Virology. 2011;92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- Guu TS, Zheng W, Tao YJ. Bunyavirus: structure and replication. Advances in Experimental Medicine and Biology. 2012;726:245–266. doi: 10.1007/978-1-4614-0980-9_11. [DOI] [PubMed] [Google Scholar]
- Xiong WY, Feng ZJ, Matsui T, et al. Risk assessment of human infection with a novel Bunyavirus in China. Western Pacific Surveill and Response Journal. 2012;3:69–74. doi: 10.5365/WPSAR.2012.3.4.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot RM. Bunyaviruses and climate change. Clinical Microbiology and Infection. 2009;15:510–517. doi: 10.1111/j.1469-0691.2009.02849.x. [DOI] [PubMed] [Google Scholar]
- Pepin M, Bouloy M, Bird BH, et al. Rift Valley fever virus (BunyaviridaePhlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Veterinary Research. 2010;41:61–71. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo MA. Virus taxonomy—Houston 2002. Archives of Virology. 2002;147:1071–1076. doi: 10.1007/s007050200036. [DOI] [PubMed] [Google Scholar]
- Lu J, Li C, Zhang FS, et al. Expression of structural and non-structural proteins of severe fever with thrombocytopenia syndrome Bunyavirus. Bing Du Xue Bao. 2011;27:515–520. [PubMed] [Google Scholar]
- Lu Z, Fu SH, Lu XJ, et al. Biological and phylogenetic analysis of first isolate of Tahyna virus in China. Bing Du Xue Bao. 2011;27:97–102. [PubMed] [Google Scholar]
- Yu L, Zhang L, Sun L, et al. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One. 2012;7:e38291–e38301. doi: 10.1371/journal.pone.0038291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu Y, Zhao L, et al. An emerging hemorrhagic fever in China caused by a novel Bunyavirus SFTSV. Science China. Life Sciences. 2013;56:697–700. doi: 10.1007/s11427-013-4518-9. [DOI] [PubMed] [Google Scholar]
- Zhou H, Sun Y, Wang Y, et al. The nucleoprotein of severe fever with thrombocytopenia syndrome virus processes a stable hexameric ring to facilitate RNA encapsidation. Protein & Cell. 2013;4:445–455. doi: 10.1007/s13238-013-3901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XL, Wang XJ, Li JD, et al. Isolation, identification and characterization of SFTS Bunyavirus from ticks collected on the surface of domestic animals. Bing Du Xue Bao. 2012;28:252–257. [PubMed] [Google Scholar]
- Gavrilovskaya IN, Shepley M, Shaw R, et al. Beta integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekosz A, Griot C, Nathanson N, et al. Tropism of Bunyaviruses: evidence for a G1 glycoprotein-mediated entry pathway common to the California serogroup. Virology. 1995;214:339–348. doi: 10.1006/viro.1995.0043. [DOI] [PubMed] [Google Scholar]
- Lozach PY, Kühbacher A, Meier R, et al. DC-SIGN as a receptor for Phleboviruses. Cell Host & Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Hofmann H, Li X, Zhang X, et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. Journal of Virology. 2013;87:4384–4394. doi: 10.1128/JVI.02628-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Liang M, Ning J, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57BL6 mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10053–10058. doi: 10.1073/pnas.1120246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Cong ML, Li MH, et al. Infection and pathogenesis of Huaiyangshan virus (a novel tick-borne Bunyavirus) in laboratory rodents. Journal of General Virology. 2012;93:1288–1293. doi: 10.1099/vir.0.041053-0. [DOI] [PubMed] [Google Scholar]
- Li D. A highly pathogenic new Bunyavirus emerged in China. Emerging Microbes and Infections. 2013;2:e1–e6. doi: 10.1038/emi.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. mega 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lole KS, Bollinger RC, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. Journal of Virology. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TT, Hon CC, Pybus OG, et al. Evolutionary and transmission dynamics of reassortant H5N1 influenza virus in Indonesia. PLoS Pathogens. 2008;4:e1000130–e 1000140. doi: 10.1371/journal.ppat.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans-Jurgen B, Peter F, Arne R. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Zhou DJ, Qin XC, et al. The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. Journal of Virology. 2012;86:2864–2868. doi: 10.1128/JVI.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CQ, Ding NZ. Discovery of severe fever with thrombocytopenia syndrome Bunyavirus strains originating from intragenic recombination. Journal of Virology. 2012;86:12426–12430. doi: 10.1128/JVI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of the People's Republic of China. Guideline for prevention and treatment of severe fever with thrombocytopenia syndrome (2010 version) Chinese Journal of Clinical Infectious Diseases. 2011;4:193–194. [Google Scholar]
- Li S, Xue C, Fu Y, et al. Sporadic case infected by severe fever with thrombocytopenia syndrome Bunyavirus in a non-epidemic region of China. Bioscience Trends. 2011;5:273–276. doi: 10.5582/bst.2011.v5.6.273. [DOI] [PubMed] [Google Scholar]
- http://www.cdc.gov/EID/pdfs/ICEID2012.pdf.
- Zhao L, Zhai S, Wen H, et al. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerging Infectious Diseases. 2012;18:963–965. doi: 10.3201/eid1806.111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Liu L, Huang X, et al. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new Bunyavirus. PLoS Pathogens. 2011;7:e1002369–e1002379. doi: 10.1371/journal.ppat.1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang X, Zhang Y, et al. Severe fever with thrombocytopenia syndrome Bunyavirus (SFTSV) infections in Zhejiang Province, China. International Journal of Infectious Diseases. 2012;17:e137–e138. doi: 10.1016/j.ijid.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Zhou DJ, Xiong Y, et al. Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:209–220. [PubMed] [Google Scholar]
- Niu G, Li J, Liang M, et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerging Infectious Diseases. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang YJ, Ding GQ, et al. First confirmation of new Bunyavirus-infected patients in Zhejiang province and molecular identification of the isolated virus. Chinese Journal of Microbiology and Immunology. 2011;31:1107–1111. [Google Scholar]
- Lu Y, Dou X, Wang X, et al. Preliminary investigation on the carriage status of the novel Bunyavirus mong animals and ticks in Beijing area. International Journal of Virology. 2011;18:33–36. [Google Scholar]
- Liu L, Guan XH, Xing XS, et al. Epidemiologic analysis on severe fever with thrombocytopenia syndrome in Hubei province, 2010. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:168–172. [PubMed] [Google Scholar]
- Zhang W, Zeng X, Zhou M, et al. Seroepidemiology of severe fever with thrombocytopenia syndrome Bunyavirus in Jiangsu province. Disease Surveillance. 2011;26:676–678. [Google Scholar]
- Cui F, Cao HX, Wang L, et al. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan County, Shandong Province, China. American Journal of Tropical Medicine and Hygiene. 2013;88:510–512. doi: 10.4269/ajtmh.11-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wu W, Wang H, et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome Bunyavirus through contact with infectious blood. Journal of Infectious Diseases. 2013;207:736–739. doi: 10.1093/infdis/jis748. [DOI] [PubMed] [Google Scholar]
- Gai Z, Liang M, Zhang Y, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome Bunyavirus through blood contact. Clinical Infectious Diseases. 2012;54:249–252. doi: 10.1093/cid/cir776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao CJ, Qi X, Wang H. A novel Bunyavirus in China. New England Journal of Medicine. 2011;365:862–863. doi: 10.1056/NEJMc1106000. [DOI] [PubMed] [Google Scholar]
- Bao CJ, Guo XL, Qi X, et al. A family cluster of infections by a newly recognized Bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clinical Infectious Diseases. 2011;53:1208–1214. doi: 10.1093/cid/cir732. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li Q, Hu W, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne and Zoonotic Diseases. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu K, Zou J, et al. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome Bunyavirus. International Journal of Infectious Diseases. 2013;17:e206–e208. doi: 10.1016/j.ijid.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, He YW, Dai YA, et al. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clinical Infectious Diseases. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of the People's Republic of China. Guideline for severe fever with thrombocytopenia prevention and control (2010 version). Available at http://www.moh.gov.cn/zhuzhan/
- Gai ZT, Zhang Y, Liang MF, et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. Journal of Infectious Diseases. 2012;206:1095–1102. doi: 10.1093/infdis/jis472. [DOI] [PubMed] [Google Scholar]
- Yang G, Li B, Liu L, et al. Development and evaluation of a reverse transcription loop-mediated isothermal amplification assay for rapid detection of a new SFTS Bunyavirus. Archives of Virology. 2012;157:1779–1783. doi: 10.1007/s00705-012-1348-1. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang L, Shen G, et al. Establishment of a novel one-step reverse transcription loop-mediated isothermal amplification assay for rapid identification of RNA from the severe fever with thrombocytopenia syndrome virus. Journal of Virological Methods. 2013;194:21–25. doi: 10.1016/j.jviromet.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Li Z, Qi X, Zhou M, et al. A two-tube multiplex real-time RT-PCR assay for the detection of four hemorrhagic fever viruses: severe fever with thrombocytopenia syndrome virus, Hantaan virus, Seoul virus, and dengue virus. Archives of Virology. 2013;158:1857–1863. doi: 10.1007/s00705-013-1677-8. [DOI] [PubMed] [Google Scholar]
- Li Z, Cui L, Zhou M, et al. Development and application of a one-step real-time RT-PCR using a minor-groove-binding probe for the detection of a novel Bunyavirus in clinical specimens. Journal of Medical Virology. 2013;85:370–377. doi: 10.1002/jmv.23415. [DOI] [PubMed] [Google Scholar]
- Cui L, Ge Y, Qi X, et al. Detection of severe fever with thrombocytopenia syndrome virus by reverse transcription-cross-priming amplification coupled with vertical flow visualization. Journal of Clinical Microbiology. 2012;50:3881–3885. doi: 10.1128/JCM.01931-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liang M, Qu J, et al. Early diagnosis of novel SFTS Bunyavirus infection by quantitative real-time RT-PCR assay. Journal of Clinical Virology. 2012;53:48–53. doi: 10.1016/j.jcv.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zeng X, Guo X, et al. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. Journal of Clinical Microbiology. 2012;50:372–377. doi: 10.1128/JCM.01319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil A, Ksiazek TG, Rollin PE. Hantavirus pulmonary syndrome, United States, 1993–2009. Emerging Infectious Diseases. 2011;17:1195–1201. doi: 10.3201/eid1707.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu Y, Ni D, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. 2008;300:2263–2270. doi: 10.1001/jama.2008.626. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hai R, Li W, et al. Seroprevalence of human granulocytotropic anaplasmosis in Central and Southeastern China. American Journal of Tropical Medicine and Hygiene. 2009;81:293–295. [PubMed] [Google Scholar]
- Kok KH, Jin DY. A novel Bunyavirus causing fever and thrombocytopenia: more questions than answers. Journal of the Formosan Medical Association. 2011;110:669–670. doi: 10.1016/j.jfma.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XC, Zhou DJ, Chen XP, et al. Viruses of the family Bunyaviridae and their associated-diseases. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:1111–1114. [PubMed] [Google Scholar]
- Bandelt H, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]