Abstract
BACKGROUND
Prostate-specific membrane antigen (PSMA) remains an important target for diagnostic and therapeutic application for human prostate cancer. Model cell lines have been recently developed to study canine prostate cancer but their PSMA expression and enzymatic activity have not been elucidated. The present study was focused on determining PSMA expression in these model canine cell lines and the use of fluorescent small-molecule enzyme inhibitors to detect canine PSMA expression by flow cytometry.
METHODS
Western blot and RT-PCR were used to determine the transcriptional and translational expression of PSMA on the canine cell lines Leo and Ace-1. An endpoint HPLC-based assay was used to monitor the enzymatic activity of canine PSMA and the potency of enzyme inhibitors. Flow cytometry was used to detect the PSMA expressed on Leo and Ace-1 cells using a fluorescently tagged PSMA enzyme inhibitor.
RESULTS
Canine PSMA expression on the Leo cell line was confirmed by Western blot and RT-PCR, the enzyme activity, and flow cytometry. Kinetic parameters Km and Vmax of PSMA enzymatic activity for the synthetic substrate (PABGgG) were determined to be 393 nM and 220 pmol min −1 mg protein −1, respectively. The inhibitor core 1 and fluorescent inhibitor 2 were found to be potent reversible inhibitors (IC50 = 13.2 and 1.6 nM, respectively) of PSMA expressed on the Leo cell line. Fluorescent labeling of Leo cells demonstrated that the fluorescent PSMA inhibitor 2 can be used for the detection of PSMA-positive canine prostate tumor cells. Expression of PSMA on Ace-1 was low and not detectable by flow cytometry.
CONCLUSIONS
The results described herein have demonstrated that PSMA is expressed on canine prostate tumor cells and exhibits similar enzymatic characteristics as human PSMA. The findings show that the small molecule enzyme inhibitors currently being studied for use in diagnosis and therapy of human prostate cancer can also be extended to include canine prostate cancer. Importantly, the findings demonstrate that the potential of the inhibitors for use in diagnosis and therapy can be evaluated in an immunocompetent animal model that naturally develops prostate cancer before use in humans.
Keywords: prostate cancer, prostate-specific membrane antigen (PSMA), canine, inhibitor, substrate, flow cytometry, Western blot, RT-PCR
INTRODUCTION
Prostate cancer is the most common diagnosed non-cutaneous cancer for men in the US [1]. Dogs also develop prostate cancer spontaneously [2–4] and, in fact, both canine prostate cancer and human prostate cancer have similar characteristics such as an increased incidence with age [5], the presence of pre-neoplastic PIN lesions [6], and bone metastases [4,5]. In both the canine and human prostate carcinogenesis, there is a strong association between high-grade prostatic intraepithelial neoplasia and carcinoma [7,8]. Although less is known about the expression of characteristic bio-markers in the canine prostate cancer than human prostate cancer, canine prostate tumors are generally considered to be more aggressive and less differentiated than the androgen-responsive human prostate cancers [9]. In fact, the vast majority of canine prostate cancers at the time of diagnosis are end-stage, aggressive, and mostly androgen-independent carcinomas with frequent metastases [5].
Although canine prostate cancer cell lines have been established, few develop bone metastases and no cell lines have been previously reported to cause brain metastases [10]. The Leo cell is the first prostate cancer cell line that consistently develops brain metastases in vivo in a xenogeneic nude mouse model [11]. In contrast, the Ace-1 canine prostate cancer cell line metastasizes almost exclusively to bone and induces mixed osteoblastic and osteolytic lesions in nude mice similar to progression of metastatic prostate cancer in human [12]. These traits make Ace-1 and Leo xenograft models valuable surrogate models for human prostate cancer.
Prostate-specific membrane antigen (PSMA), which is a validated prostate cancer biomarker in humans, has not been examined fully for its relevance to canine prostate cancer [13]. However, PSMA expression has been found in approximately 50% of canine prostate cancer tumors examined using immunohistochemical analysis [9]. More recently, the cloning and preliminary characterization of canine PSMA was reported [14]. In terms of PSMA expression in established canine prostate cancer cell lines, it has only been reported for the DPC-1 cell line [15]. Due to the similarity of canine PSMA to the human ortholog, efforts are growing to develop new model cell lines to advance the development of PSMA-targeted technologies for diagnostic and therapeutic strategies for human prostate cancer.
Recently, we developed both a method to detect (PSMA+) cells by flow cytometry, using a fluorescent PSMA inhibitor as well as a method to selectively isolate such cells from blood using a pre-targeted strategy [16,17]. The focus of this study was to evaluate PSMA expression in two canine prostate cancer cell lines, which are known to be androgen receptor (AR)-negative [11,18], and characterize the PSMA enzymatic activity, especially with respect to our functionalized PSMA inhibitors. Assuming that canine PSMA is functionally equivalent to human PSMA, PSMA-targeted diagnostic and therapeutic strategies for humans could be first screened in a natural canine model of prostate cancer prior to advancing to human studies.
MATERIALS AND METHODS
Cell Lines and Reagents
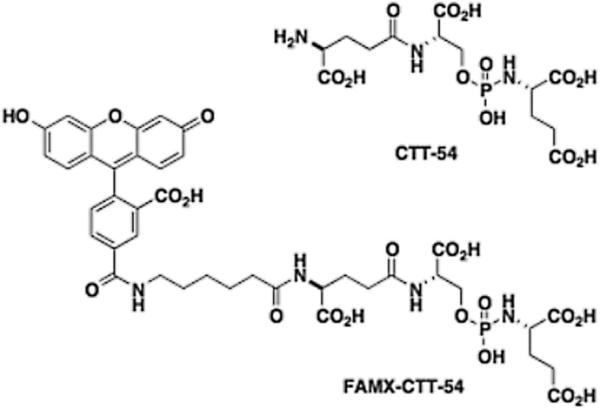
LNCaP and PC3 cells were obtained from the American Type Culture Collection (Manassas, VA). The Ace-1 and Leo cell lines were available from Dr. Rosol's laboratory at The Ohio State University (Columbus, OH) [11,12]. Mouse monoclonal antibodies 3C6 and 4D8 were obtained from Northwest Biotherapeutics (Seattle, WA). All other chemicals and cell-culture reagents were purchased from Fisher Scientific (Somerville, NJ), Pierce (Rockford, IL), Invitrogen (Grand Island, NY), or Sigma-Aldrich (St. Louis, MO). The PSMA inhibitors CTT-54 and FAMXCTT-54 (Fig. 1) were available from prior studies [17,19].
Fig. 1.
Peptidomimetic PSMA inhibitor1CTT-54 and its fluorescentderivative2 FAMX-CTT-54.
RNA Extraction, Reverse Transcription, and Real-Time RT-PCR
Total RNA from each cell line was extracted using the Absolutely RNA RT-PCR Miniprep Kit (Agilent Technologies). Approximately 0.5 mg of each RNA was reverse transcribed with SuperScript II Reverse Transcriptase and oligo(dT)12–18 primer (Invitrogen). Quantitative real-time RT-PCR analysis was performed using QuantiTect SYBR Green PCR Kit (Qiagen, Inc.) along with primers for canine GAPDH: forward CCCACTCTTCCACCTTCGAC and reverse AGCCAAATTCATTGTCATACCAGG; and canine PSMA: forward GCAGGGGACCCTCTCACACCTG and reverse CTCGGAAGACCAACAGCCTCTGTGA. Real-time RT-PCR was repeated twice on three replicate samples. Absolute quantification was achieved using serial 10-fold dilutions of purified PCR products to generate standard curves with LightCycler 480 Software (Roche). From these standard curves, the copy number of PSMA and GAPDH mRNA in each cell line was determined.
Whole Cell Lysate Extraction and Western Blot Analysis
The whole cell lysate extraction was performed as described previously [20]. Briefly, the Leo and Ace-1 cells were collected from flasks by scraping, washed once in ice-cold PBS, resuspended in threefold cell-pellet volumes of lysis buffer (1% NP-40, 20 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol) supplemented with 1× anti-protease cocktail (Pierce) for 15 min on ice, then transferred to 1.5 ml microcentrifuge tubes for centrifugation at 10,000g for 15 min at 4° C. The supernatant was saved as a whole-cell protein extract. Protein concentrations were determined using the BCA protein assay (Pierce). Western blotting was performed as described previously with minor modifications [20]. In brief, detergent soluble proteins (30 μg) were loaded and separated on a NuPAGE™ 4– 12% Bis–Tris Gel (Invitrogen, Carlsbad, CA), electro-phoresed for 40 min at a constant 200 V under reducing conditions, and then transferred to a 0.45 μm PVDF Immobilon-P Transfer Membrane (Millipore Corporation, Bedford, MA) at 400 mA for 100 min in a transfer apparatus-Owl Bandit VEP-2 (Owl, Portsmouth, NH) according to the manufacturer's instructions. Membranes were incubated with primary antibodies (4D8 for PSMA [21]) overnight at 4°C and then with horseradish peroxidase conjugated-second antibody for 1 hr at room temperature. The 4D8 antibody binds to a linear epitope between amino acids 58–133 of human PSMA [22] with which canine PSMA (Human Q04609.1 and Canine XP_533980.3) shares 93% identity. The immunoreactive bands were visualized using Protein Detector TMB Western Blot Kit (KPL, Gaithersburg, MD) following the manufacturer's instructions. The molecular weight marker was SeeBlue® Plus2 Pre-Stained Standard (Invitrogen).
PSMA Enzyme Activity and Inhibition Studies
Km and Vmax determination
Working solutions of the substrate (PABGγG) were made in Tris buffer (50 mM, pH 7.5). A typical incubation mixture (final volume 250 μl) was prepared by the addition of 200 μl Tris buffer (50 mM, pH 7.4) to either 25 μl of a solution of crude PSMA (0.00214 μg) or 25 μl Tris buffer (50 mM, pH 7.4) as a negative control. The enzymatic reaction was initiated by the addition of 25 μl PABGγG (1–20 μM). The final concentration of PABGγG ranged from 0.1 to 2.0 μM. The reaction was allowed to proceed for 15 min with constant shaking at 37°C and was terminated by the addition of 25 μl methanolic TFA (2.5% trifluoroacetic acid by volume in methanol) followed by vortexing and centrifugation (10 min at 7,000g). An 85 μl aliquot of the resulting supernatant was subsequently quantified by HPLC as described above. Under the assay conditions described above, it was noted that the initial substrate concentration was not substantially depleted during the time course of the incubation (e.g., approximately 15% conversion to product was observed for incubations with the lowest substrate concentration, 0.1 nM).
IC50 determinations
PSMA enzyme inhibition studies were performed as described previously [23,24]. Working solutions of the substrate (N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid, PABGγG) and inhibitors were made in TRIS buffer (50 mM, pH 7.4 containing 1% Triton X-100). Working solutions (50 μg/ml) of canine PSMA [25] were diluted in TRIS buffer (50 mM, pH 7.4 containing 1% Triton X-100) to provide approximately 15% conversion of substrate to product in the absence of inhibitor. A typical incubation mixture (final volume 250 μl) was prepared by the addition of either 25 μl of an inhibitor solution or 25 μl TRIS buffer (50 mM, pH 7.4 containing 1% Triton X-100) to 175 μl TRIS buffer (50 mM, pH 7.4 containing 1% Triton X-100) in a test tube. PABGγG (25 μl, 10 mM) was added to the above solution. The enzymatic reaction was initiated by the addition of 25 μl of the PSMA working solution. In all cases, the final concentration of PABGγG was 1 μM while the enzyme was incubated with five serially diluted inhibitor concentrations providing a range of inhibition from 10% to 90%. The reaction was allowed to proceed for 15 min with constant shaking at 37°C and was terminated by the addition of 25 μl methanolic TFA (2% trifluoroacetic acid by volume in methanol) followed by vortexing. The quenched incubation mixture was quickly buffered by the addition of 25 μl K2HPO4 (0.1 M), vortexed, and centrifuged (10 min at 7,000g). An 85 μl aliquot of the resulting supernatant was subsequently quantified by HPLC as previously described [23,26]. IC50 values were calculated using KaleidaGraph 3.6 (Synergy Software).
Mode of inhibition studies
The mode of inhibition study followed the procedure described in our previous study [27]. Briefly the concentration of PSMA (2.5 μg/ml) was 100-fold greater than used in the typical enzyme activity assays. The enzyme was pre-incubated for 10 min with 0.1 μM of inhibitor (40 μl), at approximately 10-fold greater than the IC50 value. The solution was diluted with 1 mM of substrate in 50 mM tris + 1% triton buffer (100-fold, total volume 3,960 μl). The formation of product was monitored every 5 min for 1 hr. A control sample was defined as incubation described here without inhibitor. Progress curves of product formation were generated to monitor the recovery of enzymatic activity for inhibited PSMA and compared to a control sample in which no inhibitor was added.
Detection of PSMA+Cells by Flow Cytometry
LNCaP (PSMA+), PC3 (PSMA-negative; PSMA−), Ace-1, and Leo cells were cultured in T-75 flasks with complete growth medium [RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin and 100 μg/ml streptomycin] in a humidified incubator at 37°C and 5% CO2. The cells were cultured to 70% confluency prior to conducting the following experiments.
Cell preparation
Cells were washed twice in 37°C pre-warmed medium A (phosphate-free RPMI 1640 containing 1% FBS), and then detached with a 0.25% trypsin 0.53 mM EDTA solution (5 μl) for 8 min at 37° C. Medium A (5 ml) was then added to each flask. The cells were distributed into five 2 ml tubes (~100,000 cells/tube). The cells were then centrifuged at 900g at 4°C for 5 min. Following removal of the medium, the cells were resuspended in 2 μl of medium A. Approximately 500,000 cells were predetermined by flow cytometry and were used to perform the following experiments.
In vitro cell labeling
The cells were treated with FAMX-CTT-54 (300 nM in medium A, total volume was 500 μl) or the equivalent volume of medium A as a negative control for cell labeling. The cells were then placed in a shaking water bath (50 rpm) at 37°C in the dark for 30 min. The samples were then subjected to the following post-labeling protocol prior to analysis by FC. In the antibody labeling, the same amount of cells were treated with TAMRA-conjugated antibody 3C6 (50× dilution in medium A, total volume was 500 μl) or the equivalent volume of medium A as a negative control for cell labeling. The cells were then placed in a shaking water bath (50 rpm) at 4°C in the dark for 1 hr. The samples were then subjected to the following post-labeling protocol prior to analysis by FC.
Post-labeling protocol
Following centrifugation (900g at 4°C for 6 min), the medium was removed and the cells fixed in 4% formaldehyde in PBS buffer pH 7.4 for 10 min at room temperature. The cells were pelleted again (900g at 4°C for 10 min), re-suspended in 500 μl of 50 mM of Tris buffer (pH 9.7 at 4°C), and covered with aluminum foil until analyzed by FC.
Flow cytometry
A Beckton-Dickinson FACSCalibur flow cytometer equipped with argon and red lasers, a Macintosh computer, and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA) was used to collect data. FCS Express software (De Novo Software, Thornton, Ontario, CA) was used to analyze the data. Data were collected on 50,000 cells for analysis. The FL-1 channel was used to detect cell labeling as the 5-FAM-X amine-reactive dye is fluorescein-based. The FL-2 channel was used to detect cell labeling by the fluorescent monoclonal antibody 3C6-TAMRA.
Inhibitor-blocking experiments
Cells were pre-incubated with 500 μl of non-fluorescent inhibitor CTT54 (2 mM) for 30 min at 37°C. Cells pre-treated with non-fluorescent inhibitor 1 CTT-54 were then treated with FAMX-CTT54 (100 nM) for another 30 min at 37°C. These cells were washed twice and subjected to the post-labeling protocol prior to analysis by FC.
RESULTS
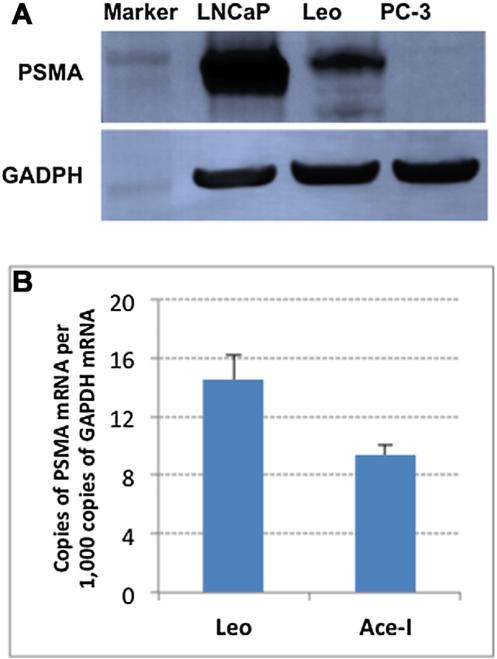
The expression of PSMA on the AR-negative canine cell lines Leo and Ace-1 was first determined by Western blot and RT-PCR. Although the Leo and Ace-I cells expressed similar amounts of PSMA mRNA as determined by RT-PCR (Fig. 2B), the PSMA protein expression, as determined by Western blot, was considerably greater for the Leo cell line but much less than the expression detected in the model human prostate cancer cell line LNCaP (Fig. 2A).
Fig. 2.
Western blot PSMA of LNCaP, Ace-1,Leo, and PC-3 cells (A). Quantitative real-time RT-PCR results for the presence of PSMA in Ace-1and Leo cell lines (B).Data are expressed as copies ofPSMAmRNAper1,000copies of GAPDH mRNA.
After confirming that PSMA was present in the Leo cell line by Western blot, we examined the enzymatic activity of canine PSMA using our standard substrate PABGγG in an HPLC-based assay previously developed by our group [23,26,28]. We confirmed that PSMA enzymatic activity was present in the membrane extracts of the Leo cells but low or absent in the extracts from Ace-1 cells. This finding was consistent with the Western blot data suggesting low levels of PSMA expression on the Ace-1 cells. The Km and Vmax values for the PABGγG substrate with PSMA from Leo cells were determined to be 393 nM and 220 pmol min −1 mg protein −1, respectively.
After confirming the enzymatic activity of canine PSMA, we sought to determine the potency of the inhibitor core 1 and its fluorescent inhibitor conjugate 2 against Leo PSMA. These compounds are known potent inhibitors of human PSMA and have been used for targeted delivery for PET and SPECT imaging as well as fluorescent labeling PSMA+ cells [16,19,29,30]. The IC50 values for these inhibitors against PSMA obtained from Leo cells as a membrane fraction of cell lysates were 13.2 and 1.6 nM, respectively. These values are consistent with those obtained with human PSMA. Mode of binding assays for these inhibitors were also performed, as described previously [27]. The results showed inhibition was reversible with both inhibitors (Supplemental Material Fig. S3). These findings are in contrast to what we have observed with human PSMA as inhibition with human PSMA was irreversible as assessed within the time scale of the inhibition experiments.
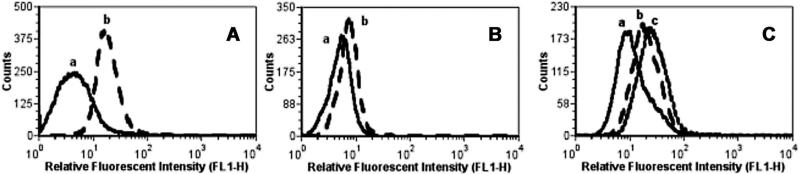
To determine the in vitro performance of the fluorescent inhibitor conjugate 2 for labeling PSMA+ canine prostate cancer cells, Leo cells were incubated with 2 (300 nM) and analyzed by flow cytometry. PSMA-negative (PC-3) cells were used as a control to confirm that 2 did not label cells non-specifically. Flow cytometric analysis revealed that the Leo cells (Fig. 3A) were fluorescently labeled with 2 while no labeling was observed on PC-3 cells (Fig. 3B). Ace-1 cells were also not labeled under these conditions (Supplemental Material Fig. S1), an observation consistent with the Western blot data. The selectivity of 2 for PSMA on Leo cells was confirmed by competitive blocking experiments, which showed the intensity of the fluorescence labeling by 2 was reduced when Leo cells were pre-treated with the PSMA inhibitor core 1 (Fig. 3C).
Fig. 3.
Fluorescent cell labeling of Leo and PC-3 cells with FAM-X-CTT54 (A,B).Leo (A),PC-3 (B) at 37 C 30 min.FAM-X-CTT54 concentration (nM): (a) 0 and (b) 300.Competitive blocking experiment (C). Leo cells pre-incubated with CTT-54 for 30 min prior to incubation with FAM-X-CTT-54 for an additional 30 min.CTT-54 concentration (mM): (a) 0, (b) 2 mM, (c) 0.FAM-X-CTT54 concentration (nM): (a) 0 (b,c):100.
PSMA targeting on live cells was also examined using an antibody (3C6) that recognizes a conformational epitope on the extracellular domain of PSMA [22]. Leo cells were incubated with the fluorescent monoclonal-antibody conjugate 3C6-TAMRA. LNCaP (PSMA+) and PC-3 (PSMA−) were also incubated with 3C6-TAMRA as positive and negative controls, respectively. While LNCaP cells were successfully labeled with 3C6-TAMRA, Leo cells were not (Supplemental Material Fig. S2) indicating the conformational epitope present in PSMA is not conserved on canine PSMA.
DISCUSSION
PSMA expression on the AR-negative canine prostate cancer cell line Leo was confirmed by Western blot using an anti-PSMA mouse antibody 4D8, which recognizes a conserved epitope expressed on canine prostate cancer cells [21]. The 4D8 antibody binds to a linear epitope present in a region of the intracellular domain predicted to be identical with human PSMA, using protein BLAST (NCBI Reference Sequence: FOLH1_Human Q04609.1 and Canis lupus familiaris XP_533980.3). In addition, molecular analysis by RTPCR also confirmed the presence of PSMA expression in the Leo cell line.
With respect to enzymatic, similar Km values for a model γ-glutamate substrate were observed for both human and canine PSMA. These results indicate that the function of PSMA in both humans and dogs is similar. Because the core scaffold of the peptidomimetic PSMA inhibitors 1 and 2 were designed to mimic γ-glutamate, it was anticipated that these compounds would inhibit canine PSMA in addition to human PSMA. Indeed, both the fluorescent conjugate 2 and its parent inhibitor core 1 were potent inhibitors of PSMA from the canine prostate cancer cell line Leo. Consistent with findings from prior studies, the fluorescent inhibitor conjugate 2 exhibited greater potency toward canine PSMA than 1 [19].
Cell-labeling experiments employing flow cytometry confirmed that the fluorescent inhibitor conjugate 2 targets the PSMA expressed on Leo cells as observed previously for human prostate cancer cells [16,19]. The binding of 2 to canine PSMA was also confirmed through competitive blocking experiments that showed pre-treatment with unconjugated inhibitor 1 reduced the labeling with 2 (Fig. 3C). As expected, no labeling was observed with the PSMA-negative Ace-1 cell line (Supplemental Material Fig. S1). It should be noted that the reduced fluorescence labeling observed by flow cytometry using fluorescent probe 2 for Leo cells as compared that typically observed for LNCaP cells [16] was consistent with the reduced level of PSMA expression observed for Leo cells by Western blot.
In previous studies, we found that the binding of anti-PSMA antibody 3C6 to PSMA either blocked access to the active site directly or caused a global conformation change resulting in an indirect blocking of the active site preventing binding of the fluorescent inhibitor 2 to PSMA. The 3C6 antibody did not bind to canine PSMA, indicating the conformational epitope recognized by the antibody is not conserved on canine PSMA.
In summary, the results described herein have demonstrated that PSMA is expressed on AR-negative canine prostate tumor cells and that it exhibits enzymatic characteristics similar to human PSMA. These findings indicate research focused on exploring the potential of the PSMA inhibitors for developing a diagnostic assay and a method for targeted delivery of toxins for prostate cancer can be extended to include their potential for canine prostate cancer. Importantly, the findings indicate the potential of the inhibitors can be evaluated in an immunocompetent animal model. This affords an opportunity to facilitate development a diagnostic assay and methods for targeted delivery of toxins for use in dogs and humans.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Department of Defense Prostate Cancer Research Program (W81XWH-10-PCRP-PCTA, PC102144, predoctoral fellowship for L.Y. Wu); the Marge Crowley Canine Cancer Research Endowment, the Dorothy Shea Brink Memorial Fund, and the Joseph and Barbara Mendel-son Endowment Research Fund of Washington State University College of Veterinary Medicine; and the National Institutes of Health (R21CA135463 and R01CA140617). The authors would also like to extend their gratitude to the WSU Flow Cytometry and Cell Analysis Core. The study sponsors did not have a role in the study design, the collection, analysis and interpretation of data, the writing of the manuscript, nor in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: Dr. Berkman is the inventor of a patent on the PSMA inhibitors described in this report and presently also serves as the CSO of Cancer Targeted Technology.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web-site.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Johnston SD, Kamolpatana K, Root-Kustritz MV, Johnston GR. Prostatic disorders in the dog. Anim Reprod Sci. 2000;60–61:405–415. doi: 10.1016/s0378-4320(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 3.Bell FW, Klausner JS, Hayden DW, Feeney DA, Johnston SD. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987). J Am Vet Med Assoc. 1991;199(11):1623–1630. [PubMed] [Google Scholar]
- 4.Cornell KK, Bostwick DG, Cooley DM, Hall G, Harvey HJ, Hendrick MJ, Pauli BU, Render JA, Stoica G, Sweet DC, Waters DJ. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate. 2000;45(2):173–183. doi: 10.1002/1097-0045(20001001)45:2<173::aid-pros12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Waters DJ, Sakr WA, Hayden DW, Lang CM, McKinney L, Murphy GP, Radinsky R, Ramoner R, Richardson RC, Tindall DJ. Workgroup 4: Spontaneous prostate carcinoma in dogs and nonhuman primates. Prostate. 1998;36(1):64–67. doi: 10.1002/(sici)1097-0045(19980615)36:1<64::aid-pros12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Waters DJ. High-grade prostatic intraepithelial neoplasia in dogs. Eur Urol. 1999;35(5–6):456–458. doi: 10.1159/000019878. [DOI] [PubMed] [Google Scholar]
- 7.Aquilina JW, McKinney L, Pacelli A, Richman LK, Waters DJ, Thompson I, Burghardt WF, Jr, Bostwick DG. High grade prostatic intraepithelial neoplasia in military working dogs with and without prostate cancer. Prostate. 1998;36(3):189–193. doi: 10.1002/(sici)1097-0045(19980801)36:3<189::aid-pros7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Waters DJ, Hayden DW, Bell FW, Klausner JS, Qian J, Bostwick DG. Prostatic intraepithelial neoplasia in dogs with spontaneous prostate cancer. Prostate. 1997;30(2):92–97. doi: 10.1002/(sici)1097-0045(19970201)30:2<92::aid-pros4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, van den Ham R, van Leenders G, van der Lugt J, Mol JA, Teske E. Histopathological and immunohistochemical characterization of canine prostate cancer. Prostate. 2008;68(5):477–488. doi: 10.1002/pros.20720. [DOI] [PubMed] [Google Scholar]
- 10.Thudi NK, Martin CK, Nadella MV, Fernandez SA, Werbeck JL, Pinzone JJ, Rosol TJ. Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate. 2008;68(10):1116–1125. doi: 10.1002/pros.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thudi NK, Shu ST, Martin CK, Lanigan LG, Nadella MV, Van Bokhoven A, Werbeck JL, Simmons JK, Murahari S, Kisseberth WC, Breen M, Williams C, Chen CS, McCauley LK, Keller ET, Rosol TJ. Development of a brain metastatic canine prostate cancer cell line. Prostate. 2011;71(12):1251–1263. doi: 10.1002/pros.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoy BE, Thudi NK, Nadella MV, Toribio RE, Tannehill-Gregg SH, van Bokhoven A, Davis D, Corn S, Rosol TJ. New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate. 2006;66(11):1213–1222. doi: 10.1002/pros.20408. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen JJ, Rajasekaran SA, Moy P, Butch A, Goodglick L, Gu Z, Reiter RE, Bander NH, Rajasekaran AK. Polarity of prostate specific membrane antigen, prostate stem cell antigen, and prostate specific antigen in prostate tissue and in a cultured epithelial cell line. Prostate. 2003;55(1):9–19. doi: 10.1002/pros.10203. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt S, Fracasso G, Colombatti M, Naim HY. Cloning and characterization of canine prostate-specific membrane antigen. Prostate. 2013;73(6):642–650. doi: 10.1002/pros.22605. [DOI] [PubMed] [Google Scholar]
- 15.Anidjar M, Villette JM, Devauchelle P, Delisle F, Cotard JP, Billotey C, Cochand-Priollet B, Copin H, Barnoux M, Triballeau S, Rain JD, Fiet J, Teillac P, Berthon P, Cussenot O. In vivo model mimicking natural history of dog prostate cancer using DPC-1, a new canine prostate carcinoma cell line. Prostate. 2001;46(1):2–10. doi: 10.1002/1097-0045(200101)46:1<2::aid-pros1002>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu LY, Liu T, Grimm AL, Davis WC, Berkman CE. Flow cytometric detection of prostate tumor cells using chemoaffinity labels. Prostate. 2011;71(1):52–61. doi: 10.1002/pros.21221. [DOI] [PubMed] [Google Scholar]
- 17.Wu LY, Liu T, Hopkins MR, Davis WC, Berkman CE. Chemo-affinity capture of pre-targeted prostate cancer cells with magnetic beads. Prostate. 2012;72(14):1532–1541. doi: 10.1002/pros.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senapati S, Chakraborty S, Nath I, Panda SK, Patra RC, Batra SK. Non-human prostate cancer cell lines. Oncol Gastroenterol Hepatol Rep. 2012;1(1):10. [Google Scholar]
- 19.Liu T, Wu LY, Kazak M, Berkman CE. Cell-Surface labeling and internalization by a fluorescent inhibitor of prostate-specific membrane antigen. Prostate. 2008;68(9):955–964. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Wu LY, Berkman CE. Prostate-specific membrane antigen-targeted photodynamic therapy induces rapid cytoskeletal disruption. Cancer Lett. 2010;296(1):106–112. doi: 10.1016/j.canlet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carr A, Mullet A, Mazorra Z, Vazquez AM, Alfonso M, Mesa C, Rengifo E, Perez R, Fernandez LE. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma. 2000;19(3):241–247. doi: 10.1089/02724570050109639. [DOI] [PubMed] [Google Scholar]
- 22.Tino WT, Huber MJ, Lake TP, Greene TG, Murphy GP, Holmes EH. Isolation and characterization of monoclonal antibodies specific for protein conformational epitopes present in prostate-specific membrane antigen (PSMA). Hybridoma. 2000;19(3):249–257. doi: 10.1089/02724570050109648. [DOI] [PubMed] [Google Scholar]
- 23.Maung J, Mallari JP, Girtsman TA, Wu LY, Rowley JA, Santiago NM, Brunelle AN, Berkman CE. Probing for a hydrophobic a binding register in prostate-specific membrane antigen with phenylalkylphosphonamidates. Bioorg Med Chem. 2004;12(18):4969–4979. doi: 10.1016/j.bmc.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Wu LY, Anderson MO, Toriyabe Y, Maung J, Campbell TY, Tajon C, Kazak M, Moser J, Berkman CE. The molecular pruning of a phosphoramidate peptidomimetic inhibitor of prostate-specific membrane antigen. Bioorg Med Chem. 2007;15(23):7434–7443. doi: 10.1016/j.bmc.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Toriyabe Y, Berkman CE. Purification of prostate-specific membrane antigen using conformational epitope-specific antibody-affinity chromatography. Protein Expr Purif. 2006;49(2):251–255. doi: 10.1016/j.pep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Anderson MO, Wu LY, Santiago NM, Moser JM, Rowley JA, Bolstad ES, Berkman CE. Substrate specificity of prostate-specific membrane antigen. Bioorg Med Chem. 2007;15(21):6678–6686. doi: 10.1016/j.bmc.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Toriyabe Y, Kazak M, Berkman CE. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry. 2008;47(48):12658–12660. doi: 10.1021/bi801883v. [DOI] [PubMed] [Google Scholar]
- 28.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. J Nucl Med. 2009;50(12):2042–2048. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nedrow-Byers JR, Jabbes M, Jewett C, Ganguly T, He H, Liu T, Benny P, Bryan JN, Berkman CE. A phosphoramidate-based prostate-specific membrane antigen-targeted SPECT agent. Prostate. 2011;72(8):904–912. doi: 10.1002/pros.21493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.