Abstract
Patients with Lesch‐Nyhan disease (LND) often engage in self‐injurious biting. This problem requires difficult management choices, sometimes including removal of the teeth. Although many health care professionals are reluctant to remove teeth in a child because of the permanent negative cosmetic consequences of the edentulous state, disfigurement of the face and tongue from self‐biting can be worse. We analyzed the records of 5 LND patients who used mouth guards to spare the teeth. Success was variable, and dental extraction ultimately was required in 4 cases. We also reviewed previously published cases on the use of dental devices to spare teeth in LND. Various devices have been recommended, but failure rates are high, and tooth extraction often is still needed. Although dental extraction is not required in all cases, it should not be delayed when biting is severe.
Keywords: Lesch‐Nyhan disease, developmental disorders, inherited metabolic disease, self‐injurious behavior, self‐biting, review
Lesch‐Nyhan disease (LND) is a rare X‐linked recessive disorder.1, 2 It is caused by deficiency of hypoxanthine‐guanine phosphoribosyltransferase (HGprt), a purine recycling enzyme.3 Clinically, LND is characterized by overproduction of uric acid, neurological and intellectual disability, and characteristic behavioral problems. The hallmark behavioral problem is severe and recurrent self‐injurious behavior (SIB). In LND, the most common manifestation of SIB is compulsive biting of the lips, tongue, fingers, and shoulders.4, 5, 6, 7 Many patients also engage in hitting, scratching, kicking, and head banging. Though individuals experience pain from these behaviors, they cannot stop the behavior on their own.
Appropriate protective devices can control SIB from hitting or kicking the limbs, whereas head banging can be addressed with helmets. Patients are relieved and are more comfortable when they are prevented from injuring themselves.8, 9, 10, 11 However, control of biting is more challenging, especially when the biting is directed toward the lips or tongue, because restraint of the teeth is not feasible (Fig. 1A,B).
Figure 1.
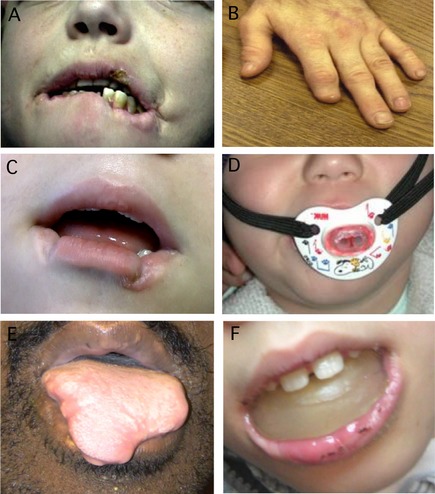
Oral self‐injurious behavior in LND. (A) Typical appearance of chronic lip biting with tissue loss, hypertrophic scarring, and fresh lesions. (B) Partial amputation of a digit by a patient who got free of his protective straps while unrestrained. (C) Consequences for a patient where dental extraction was delayed. (E) Permanent loss of tongue tissue from severe biting during a period of device failure. (D and F) Typical examples of the cosmetic appearance of some devices recommended to prevent SIB. Whether these devices have a better cosmetic appearance than the edentulous state seems a matter of debate.
Oral SIB often begins with the eruption of temporary teeth.9, 12, 13 Although dental extraction is a reliable means for definitive control of self‐biting,8, 9, 14, 15 many health care professionals are reluctant to remove teeth in a child, because of the permanent negative cosmetic appearance. Management of this problem often falls to the treating neurologist or psychiatrist, when primary physicians unfamiliar with the disorder delay making this difficult decision. The cases described here demonstrate the consequences of failing to provide quick, decisive intervention when self‐biting emerges.
Case Reports
Case PM
PM carried a diagnosis of cerebral palsy until 2 years of age, when the development of SIB led to suspicion for LND. His diagnosis was confirmed by finding high uric acid in his blood and documenting a IVS4 + 1G>A splice‐site mutation in the HPRT1 gene.
By 5 years of age, he frequently bit his cheeks, lips, and arms. Later, he developed head banging and flinging movements of the limbs. Protective straps prevented limb injury, and a helmet prevented head injury. Uncontrolled biting led to injury of the lower lip (Fig. 1C). His mother was advised to proceed with dental extraction, and the medical team called her dentist and advised of the situation and the need for urgent action. Although the dentist initially agreed to proceed, he later expressed concerns about the negative cosmetic consequences of removing teeth in a young child and wondered if there might be other options. After 5 months of delays, where ongoing biting led to further facial disfigurement, the family sought an alternative dentist, who removed eight incisor and four cuspid teeth. Three months after dental extraction, the boy stopped biting completely and he and his family both said they were more comfortable. The facial disfigurement resulting from delayed dental extraction required reconstructive surgery of his lips and the area around his mouth.
Case MA
MA had sandy red urine in his diapers at 1 month of age. Psychomotor delay was apparent by 9 months and his serum urate level was found to be elevated to 11.2 mg/dL. The diagnosis of LND was established by showing nondetectable HGprt activity in hemolysates and finding a deletion of exon 1 in the HPRT1 gene.
At age 13 months with the eruption of teeth, he started to bite his lips, tongue, and cheeks. Within a year, he had caused serious damage requiring reconstructive surgery. Botulinum toxin was injected into the muscles of mastication based on positive experience in one report,16 but it was not effective. An oral device designed to protect the teeth was broken by forceful biting. Another oral device was made, but he was unable to eat while using it (Fig. 1D). When it was removed, he rapidly began to bite his lips and tongue. The device was abandoned within a year and his teeth were removed, starting with the upper teeth and later the lower ones. Subsequent to this procedure, he and the family both indicated that distress from biting was completely alleviated.
Case DN
DN had developmental delay and was diagnosed with cerebral palsy, until 3 years of age, when the emergence of SIB led to suspicion for LND. Biochemical studies revealed no residual HGprt enzyme activity, although a mutation could not be found in the coding region or splice sites of the gene.2
At 6 years of age, he was regularly engaging in frequent biting of the fingers, hands, arms, lips, and tongue. He was given upper and lower mouth guards. At one point, the lower guard broke, and significant lower lip damage occurred before it could be fixed. After repair, both guards were cemented in place to avoid further risk of breaking, requiring frequent visits to the dentist for oral hygiene. At 18 years of age, his lip guards were removed during a period of inpatient behavior modification therapy, and he chewed off the anterior portion of his tongue before the guard could be replaced (Fig. 1E). Because of repeated failures of the dental guards and the precipitous nature of his biting, he underwent total tooth extraction. After the extraction, self‐biting declined and no longer was a major concern. Unfortunately, extensive damage to the tongue could not be repaired.
Case JC
This patient had a diagnosis of psychomotor delay of unknown cause at 2 years of age. At age 3, he developed pyelonephritis with hyperuricemia and hyperuricosuria, leading to tests for HGprt enzyme deficiency. He was found to have a c.100insGG in exon 2 of the HPRT1 gene.17
At 8 years of age, he engaged in occasional biting of the lips and cheeks. When this behavior became more frequent, he was given upper and lower mouth guards. He wore these guards intermittently for several years, with frequent breakage and need for replacement. Significant damage to the lips occurred when the guards were not in use, requiring lengthy healing periods. At 19 years of age, he continues to wear the guards, despite intermittent failures (Fig. 1F).
Case DB
This boy had developmental delay, and an elevated uric acid level at 2 years of age led to biochemical and genetic testing for LND. He had no residual HGprt enzyme activity associated with a c.602A>T mutation in the HPRT1 gene.
Self‐biting emerged at 3 years, and he used a lip bumper and tooth guard to spare the teeth. They were effective in preventing facial disfigurement, but they broke four times over the years, and, at one point, he swallowed a piece that led to hospitalization for removal. At 22 years of age, his teeth were removed because the appliances were judged to be too difficult to maintain.
Discussion
The management of self‐biting in LND is a challenging problem. There are numerous published reports showing profound disfigurement of the face and limbs from self‐biting in LND.8, 10, 11, 15, 18, 19, 20 Although dental extraction is a reliable means for preventing this problem, many health care professionals are reluctant to remove teeth in a child because of the negative cosmetic consequences of being edentulous. The current cases demonstrate how a delay in decisive treatment also can have negative cosmetic consequences, either from the appliance itself or the facial disfigurement that results when they fail. In some cases, reconstructive surgery is possible because damage is limited. In other cases, extreme facial and oral disfigurement cannot be corrected.
In an effort to spare teeth, several investigators have proposed the use of intraoral protective appliances, such as lip shields, lip bumpers, occlusal bite plates and splints, and bite blocks. A recent review of these devices concluded that they should be considered and attempted before dental extraction.21 Though these devices may be useful for short‐term control of self‐biting, our experience is that they are rarely useful for long‐term management. There are many reasons for their failure. First, SIB is a chronic disorder and long‐term use of such appliances is impractical. Several investigators have commented on poor tolerability of the appliances, related to the difficulty they create with daily dental hygiene and eating that require frequent removals and reinsertions, as in cases DN and DB.9, 11, 14, 15, 20, 22, 23 A related problem is the need for frequent revisions as the child's mouth grows and additional teeth erupt. It is sometimes impossible to acquire good oral impressions needed for the devices because of the patient's refusal to open the mouth.9, 10, 11 Because oppositional behavior is common in LND,4 creating and maintaining the devices is challenging. Breakage of the devices also is frequent, because of forceful biting or intentional misuse by the patient, as in cases MA and DB.10, 14, 20, 24 Furthermore, self‐biting, as in case DN and others, may be sudden and severe with extreme tissue damage or even anemia from blood loss.9, 15, 25 Serious damage may occur very rapidly when the device falls out, when it is taken out for oral hygiene or eating, or when it is taken out for repairs. Additionally, the dental devices do not address the motivation for SIB, so there is the risk that it may still occur.25, 26, 27 The child may learn to remove or reposition the appliance in his or her mouth. In some of these cases, injury is more serious.9, 14, 28 There is general agreement that the control of difficult behaviors, such as biting, in LND is best managed by behavioral methods that involve distraction, redirection, and eventual extinction.29, 30 The existence of an oral appliance interferes with behavior modification by calling attention to the behavior to be extinguished. Finally, a major argument against dental extraction is the negative cosmetic appearance of an edentulous child. The facial disfigurement that may result from their failure (Fig. 1C,E), or the dental appliances themselves (Fig. 1D,F), often have a worse cosmetic appearance.
In our experience with more than 100 cases, more than half ultimately have some teeth removed, and both patient and family are relieved that self‐biting is no longer possible. The cases described here all provide examples that tooth extraction is beneficial. This experience is similar to that reported from other centers where many LND patients are followed.5, 31 However, dental extraction is not required for all cases, and it should not be applied routinely in all patients as a preventive measure. Sparing of all teeth is possible when biting does not occur, or when biting is mild or infrequent. Full‐mouth extraction also is not always required, especially if the biting behavior relies exclusively on only some of the teeth. When self‐biting emerges, we advocate temporary use of soft mouth guards and behavioral methods to determine whether the behavior can be extinguished. In parallel with these conservative measures, consultation with a dental surgeon is needed, in the event that extraction of some of the teeth becomes urgent. We do not believe that multiple revisions of elaborate dental devices over a long period of time are useful in the long‐term care of this patient population.
Dental extraction in LND should not be viewed as a treatment of last‐resort. Although behavioral modification methods are helpful, they do not guarantee elimination of all self‐injurious behaviors. Because of the severe and chronic nature of self‐biting, and the possibility of precipitous damage from biting, expeditious removal of teeth should be considered part of the standard of care when it becomes necessary.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
E.M.G.: 1B, 1C, 3A
R.J.T.: 1B, 1C, 3B
J.G.P.: 1B, 1C, 3B
H.A.J.: 1A, 1B, 1C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported, in part, by grant HD 053312 from the National Institutes of Health (NIH; Bethesda, MD), a grant from Fondo de Investigaciones Sanitarieas (Health Research Fund, FIS) 11/00598 from Spain, and the Lesch‐Nyhan Syndrome Children's Research Foundation. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: E.M. Goodman reports nothing to disclose. R.J. Torres has received research grant support from Fondo de Investigaciones Sanitarieas (Health Research Fund, FIS) 11/00598 from Spain. J.G. Puig has received research grant support from Fondo de Investigaciones Sanitarieas (Health Research Fund, FIS) 11/00598 from Spain. H.A. Jinnah has received research grant support from the NIH, the Atlanta Clinical & Translational Science Institute, the Emory University Research Council, the Lesch‐Nyhan Syndrome Children's Research Foundation, the Dystonia Medical Research Foundation, the Bachmann‐Strauss Dystonia & Parkinson's Foundation, and the Benign Essential Blepharospasm Research Foundation. He also is principal investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Sciences and National Institute of Neurological Disorders and Stroke. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc., Ipsen Biopharm, Medtronics Inc, and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, The Bachmann‐Strauss Dystonia and Parkinson Foundation, BeatDystonia, The Benign Essential Blepharospasm Foundation, Dystonia Europe, Dystonia Ireland, The Dystonia Medical Research Foundation, The Dystonia Society, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). Dr. Jinnah serves on the Scientific Advisory Boards for Cure Dystonia Now, the Dystonia Medical Research Foundation, Tyler's Hope for a Dystonia Cure, the Lesch‐Nyhan Syndrome Children's Research Foundation, and Lesch‐Nyhan Action France.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med 1964;36:561–570. [DOI] [PubMed] [Google Scholar]
- 2. Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch‐Nyhan disease. Brain 2006;129:1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jinnah HA, Friedmann T. Lesch‐Nyhan disease and its variants In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease, 8th ed New York: McGraw‐Hill; 2001:2537–2570. [Google Scholar]
- 4. Schretlen DS, Ward J, Meyer SM, et al. Behavioral aspects of Lesch‐Nyhan disease and it variants. Dev Med Child Neurol 2005;47:673–677. [DOI] [PubMed] [Google Scholar]
- 5. Anderson LT, Ernst M. Self‐injury in Lesch‐Nyhan disease. J Autism Dev Disord 1994;24:67–81. [DOI] [PubMed] [Google Scholar]
- 6. Nyhan WL. Behavior in the Lesch‐Nyhan syndrome. J Autism Child Schizophren 1976;6:235–252. [DOI] [PubMed] [Google Scholar]
- 7. Robey KL, Reck JF, Giacomini KD, Barabas G, Eddey GE. Modes and patterns of self‐mutilation in persons with Lesch‐Nyhan disease. Dev Med Child Neurol 2003;45:167–171. [DOI] [PubMed] [Google Scholar]
- 8. LaBanc J, Epker BN. Lesch‐Nyhan syndrome: surgical treatment in a case with lip chewing: a case report. J Maxillofac Surg 1981;9:64–67. [DOI] [PubMed] [Google Scholar]
- 9. Dicks JL. Lesch‐Nyhan syndrome: a treatment planning dilemma. Pediatr Dent 1982;4:127–130. [PubMed] [Google Scholar]
- 10. Arhakis A, Topouzelis N, Kotsiomiti E, Kotsanos N. Effective treatment of self‐injurious oral trauma in Lesch‐Nyhan syndrome: a case report. Dent Traumatol 2010;26:496–500. [DOI] [PubMed] [Google Scholar]
- 11. Chen LR, Liu JF. Successful treatment of self‐inflicted oral mutilation using an acrylic splint retained by a head gear. Am Acad Pediatr Dent 1996;18:408–410. [PubMed] [Google Scholar]
- 12. Sugahara T, Mishima K, Mori Y. Lesch‐Nyhan syndrome: successful prevention of lower lip ulceration caused by self‐mutilation by use of mouth guard. Int J Oral Maxillofac Surg 1994;23:37–38. [DOI] [PubMed] [Google Scholar]
- 13. Evans J, Sirikumara M, Gregory M. Lesch‐Nyhan syndrome and the lower lip guard. Oral Surg Oral Med Oral Pathol 1993;76:437–440. [DOI] [PubMed] [Google Scholar]
- 14. Smith BM, Cutilli BJ, Fedele M. Lesch‐Nyhan syndrome: a case report. Oral Surg Oral Med Oral Pathol 1994;78:317–318. [DOI] [PubMed] [Google Scholar]
- 15. Benz CMK, Reeka‐Bartschmid AMT, Agostini FG. Case report: the Lesch‐Nyhan syndrome. Eur J Paediatr Dent. 2004;5:110–114. [PubMed] [Google Scholar]
- 16. Dabrowski E, Smathers SA, Ralstrom CS, Nigro MA, Leleszi JP. Botulinum toxin as a novel treatment for self‐mutilation in Lesch‐Nyhan syndrome. Dev Med Child Neurol 2005;47:636–639. [PubMed] [Google Scholar]
- 17. Puig JG, Torres RJ, Mateos FA, Ramos TH, Arcas JM, Buño AS, O'Neill P. The spectrum of hypoxanthine‐guanine phosphoribosyltransferase deficiency: clinical experience based on 22 patients from 18 Spanish families. Medicine 2001;80:102–112. [DOI] [PubMed] [Google Scholar]
- 18. Marmattom BV. Self‐mutilation in the Lesch‐Nyhan syndrome. Neurology 2005;65:25. [DOI] [PubMed] [Google Scholar]
- 19. Lee JH, Berkowitz RJ, Choi BJ. Oral self‐mutilation in the Lesch‐Nyhan syndrome. ASDC J Dent Child 2002;69:66–69. [PubMed] [Google Scholar]
- 20. Fardi K, Topouzelis N, Kotsanos N. Lesch‐Nyhan syndrome: a preventive approach to self‐mutilation. Int J Paediatr Dent 2003;13:51–56. [DOI] [PubMed] [Google Scholar]
- 21. Limeres J, Feijoo JF, Baluja F, Seoane JM, Diniz M, Diz P. Oral self‐injury. An update. Dent Traumatol 2012;29:8–14. [DOI] [PubMed] [Google Scholar]
- 22. Rashid N, Yusuf H. Oral self‐mutilation by a 17‐month‐old child with Lesch‐Nyhan syndrome. Int J Paediatr Dent 1997;117(7):115. [DOI] [PubMed] [Google Scholar]
- 23. Jeong TS, Lee JH, Kim S, Kim JH, Tootla RG. A preventive approach to oral self‐mutilation in Lesch‐Nyhan syndrome: a case report. Pediatr Dent 2006;28:341–344. [PubMed] [Google Scholar]
- 24. Shapira J, Zilberman Y, Becker A. Lesch‐Nyhan syndrome: a non‐extracting approach to prevent mutilation. Dent Health 1987;25:6–7. [PubMed] [Google Scholar]
- 25. Fenton SJ. Management of oral self‐mutilation in neurologically impaired children. Spec Care Dent. 1982;2:70–73. [DOI] [PubMed] [Google Scholar]
- 26. Davila JM, Aslani MB, Wentworth E. Oral appliance attached to a bubble helmet for prevention of self‐inflicted injury. ASDC J Dent Child 1996;63:131–134. [PubMed] [Google Scholar]
- 27. Eguchi S, Tokioka T, Motoyoshi A, Wakamura S. A self‐controllable mask with helmet to prevent self finger‐mutilation in the Lesch‐Nyhan syndrome. Arch Phys Med Rehabil 1994;75:709–710. [DOI] [PubMed] [Google Scholar]
- 28. Scully C. The orofacial manifestations of the Lesch‐Nyhan syndrome. Int J Oral Surg 1981;10:380–383. [DOI] [PubMed] [Google Scholar]
- 29. Olson L, Houlihan D. A review of behavioral treatments used for Lesch‐Nyhan syndrome. Behav Modif 2000;24:202–222. [DOI] [PubMed] [Google Scholar]
- 30. Anderson LT, Dancis J, Alpert M, Herrmann L. Punishment learning and self‐mutilation in Lesch‐Nyhan disease. Nature 1977;265:461–463. [DOI] [PubMed] [Google Scholar]
- 31. McCarthy GT, Green EM, Ogunbona O, Simmonds HA, Fairbanks L, Pountney T, Bryant E. A population study of Lesch‐Nyhan disease in the United Kingdom. Dev Med Child Neurol 2011;53:34–39. [DOI] [PubMed] [Google Scholar]


