Abstract
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease with no known cure. Current strategies to treat RA, including methotrexate (MTX), target the later inflammatory stage of disease. Recently, we showed that inhibiting indoleamine-2,3-dioxygenase (IDO) with 1-methyl-tryptophan (1MT) targets autoantibodies and cytokines that drive the initiation of the autoimmune response. Therefore, we hypothesized that combining 1MT with MTX would target both the initiation and chronic inflammatory phases of the autoimmune response and be an effective co-therapeutic strategy for arthritis. To test this, we used K/BxN mice, a pre-clinical model of arthritis that develops joint-specific inflammation with many characteristics of human RA. Mice were treated with 1MT, MTX, alone or in combination, and followed for arthritis, autoantibodies, and inflammatory cytokines. Both 1MT and MTX were able to partially inhibit arthritis when used individually; however, combining MTX + 1MT was significantly more effective than either treatment alone at delaying the onset and alleviating the severity of joint inflammation. We went on to show that combination of MTX + 1MT did not lower inflammatory cytokine or autoantibody levels, nor could the synergistic co-therapeutic effect be reversed by the adenosine receptor antagonist theophylline or be mimicked by inhibition of polyamine synthesis. However, supplementation with folinic acid did reverse the synergistic co-therapeutic effect, demonstrating that, in the K/BxN model, MTX synergizes with 1MT by blocking folate metabolism. These data suggest that pharmacological inhibition of IDO with 1MT is a potential candidate for use in combination with MTX to increase its efficacy in the treatment of RA.
Keywords: indoleamine, co-therapy, treatment, autoantibodies, cytokines
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease characterized by severe inflammation of cartilage and bone (1). Disease in RA patients is chronic and progressive and there is no known cure. Currently, four basic types of drugs are used to treat RA; non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), and biologic response modifying drugs (2–4). Often, these drugs are used in combination in an effort to increase their efficacy (5, 6). While these drugs help alleviate the symptoms of RA, there remains an urgent need for new therapeutic strategies to address the underlying causes that drive disease in RA patients.
Results from our laboratory suggest that the tryptophan catabolizing enzyme, indoleamine-2,3-dioxygenase (IDO) is a potential new therapeutic target in the treatment of RA (7, 8). IDO has been linked to immune modulation in a variety of disease settings, including tumor immunology and inflammatory autoimmune disease (9–11). The chief functional paradigm has been that IDO acts by suppressing T cell activation, prompting the general assumption that IDO inhibition would exacerbate autoimmune disorders (12, 13). However, studies using preclinical models of RA, asthma, and allergy suggest that the IDO pathway instead drives inflammation in certain pathological settings (7, 14, 15). Furthermore, RA patients exhibit elevated levels tryptophan catabolism that correlate with disease severity, suggesting that IDO may also contribute to pathogenicity in RA patients (16, 17).
To study the role of IDO in inflammatory autoimmune disease, we made use of 1-methyl-tryptophan (1MT), a small molecule inhibitor of the IDO pathway together with the K/BxN preclinical model of arthritis (7). K/BxN mice share many similarities with RA patients, including high titers of autoantibodies, inflammatory cytokine production, and immune-mediated destruction of cartilage and bone in the synovial joints (18, 19). Joint inflammatory disease develops spontaneously in 100% of K/BxN mice starting around 4 weeks of age and continues chronically throughout the life of the animal. As such, the K/BxN model has been useful for testing therapeutics designed either to prevent the onset or reverse the symptoms of ongoing disease (8, 20–22). Previously, we showed that treatment of K/BxN mice with 1MT reduced autoantibody and inflammatory cytokine levels, resulting in an amelioration of arthritis symptoms (7). We further demonstrated that IDO was necessary for the differentiation of autoreactive B cells into antibody secreting cells, but not for their initial activation or maturation in germinal centers (8). These data demonstrated that IDO is important during the early stages of the autoimmune response, and as such, inhibitors of IDO will be most useful when administered at the initiation of the autoimmune response. In support of this, we recently showed that 1MT is effective at inhibiting the reactivation of autoreactive B cells following their regeneration after B cell depletion therapy (8).
Currently, the most commonly used DMARD in RA patients is methotrexate (MTX) (3, 23, 24). Treatment with MTX has also been used successfully in murine models of inflammatory arthritis, including collagen-induced arthritis and MRL-lpr/lpr mice (25, 26). The mechanism by which MTX alleviates arthritis has been extensively studied, but remains controversial. In some models, MTX has been shown to inhibit inflammation by increasing endogenous adenosine concentrations and altering the production of inflammatory cytokines (27, 28). Other studies have suggested that MTX leads to decreased cell proliferation and increased apoptosis by decreasing polyamine production and increasing intracellular reactive oxygen species (ROS) levels (29). Finally, MTX is a folate antagonist and therefore has also been proposed to inhibit arthritis through its anti-proliferative effects.(30)
Based on its anti-proliferative and anti-inflammatory properties, MTX is thought to act on the effector phase of the response (27, 28). In contrast, our previous data showed that 1MT inhibited arthritis development when administered during the initiation of the autoimmune response, but was ineffective once the inflammatory response was underway (7). Here, we use the K/BxN model to test the hypothesis that combining 1MT with MTX therapy will target both the initiation phase (1MT) and chronic inflammatory phase (MTX) of the autoimmune response. Our data show that the combination of a low dose of MTX with 1MT is significantly more effective than either treatment alone at delaying the onset and alleviating the severity of joint inflammation and suggest that pharmacological inhibition of IDO with 1MT is a potential candidate for use in combination with MTX in the treatment of RA.
Methods
Mice
KRN TCR Tg mice (31) and IDO1 deficient (IDO−/−) mice (32) on a C57BL/6 background have been described. NOD mice were purchased from Jackson laboratories. To obtain arthritic mice, KRN Tg C57BL/6 mice were crossed with NOD mice yielding KRN (C57BL/6 x NOD)F1 mice designated K/BxN or C57BL/6 mice expressing the I-Ag7 MHC Class II molecule, designated KRN B6.g7. IDO−/− arthritic mice were generated by breeding KRN IDO−/− C57BL/6 mice expressing the I-Ag7 MHC Class II molecule (KRN/IDO−/− B6.g7). All mice were bred and housed under specific pathogen free conditions in the animal facility at the Lankenau Institute for Medical Research. Studies were performed in accordance with National Institute of Health and Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the LIMR Institutional Animal Care and Use Committee.
Administration of 1MT, MTX, and inhibitors
Mice were given 400 mg/kg/dose (100µl total volume) of D/L-1MT (Sigma) diluted in Methocel/Tween (0.5% methylcellulose (w/v), 0.5% Tween 80 (v/v) in water) twice daily by oral gavage (p.o.); (33) 1, 10, or 25 mg/kg/dose (100µl total volume) of MTX (Hannah Pharmaceuticals) diluted in Methocel/Tween weekly p.o.; 0.5mg/kg IB-MECA (Sigma) diluted in saline daily i.p.; 10mg/kg theophylline (Sigma) diluted in Methocel/Tween daily p.o.; 1% difluoromethylornithine (DFM0; ILEX oncology) in the drinking water; 1 or 25mg/kg folinic acid (Sigma) diluted in Methocel/Tween daily p.o.; or a combination of 1MT, MTX, and the inhibitors. Folinic acid and MTX were administered 8hr apart to avoid interference with their uptake (28, 30). Control mice were given an equal volume of carrier alone (Methocel/Tween).
Arthritis incidence
The two rear ankles of K/BxN mice were measured starting at weaning (3 wk of age). Measurement of ankle thickness was made above the footpad axially across the ankle joint using a Fowler Metric Pocket Thickness Gauge. Ankle thickness was rounded off to the nearest 0.05mm. At the termination of the experiment, ankles were fixed in 10% buffered formalin for 48h, decalcified in 14% EDTA for 2wks, embedded in paraffin, sectioned, and stained with H&E. Histology sections were imaged using a Zeiss Axioplan microscope with a Zeiss Plan-Apochromat 10x/0.32 objective and Zeiss AxioCam HRC camera using AxioVision 4.7.1 software. The images were then processed using Adobe Photoshop CS2 software.
ELISpot Assay
LN cells were plated at 4 × 105 cells per well and diluted serially 1:4 in Multiscreen HA mixed cellulose ester membrane plates (Millipore) coated with GPI-his (5µg/ml). The cells were incubated on the Ag-coated plates for 4h at 37°C. The Ig secreted by the plated cells was detected by Alkaline Phosphatase-conjugated goat anti-mouse total Ig secondary Ab (Southern Biotechnology Associates) and visualized using NBT/BCIP substrate (nitroblue tetrazolium / 5-bromo-4-chloro-3-indolyl phosphate; Sigma).
ELISA Assay
Serum samples were plated at an initial dilution of 1:100 and diluted serially 1:5 in Immulon II plates coated with GPI-his (5µg/ml). Recombinant GPI-his protein was generated and purified as described previously (34). Donkey anti-mouse total Ig- HRP (Jackson Immunoresearch) was used as a secondary Ab. Ab was detected using ABTS (2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) substrate (Fisher). The serum titer was defined as the reciprocal of the last dilution that gave an OD>3x background.
Cytokine Secretion
Cells from the draining lymph nodes (LNs) of MTX alone or MTX + 1MT treated K/BxN mice were harvested and cultured in PMA (50 ng/ml) + ionomycin (500 ng/ml) for 24h. As a negative control, cells were cultured in media alone. The supernatants were then harvested and analyzed for the levels of IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17, MIP-1α, MIP-1β, RANTES, TNFα, IFNγ, and MCP-1 by cytometric bead array (BD Biosciences). The samples were stained according to manufacturer instructions and analyzed on a FACSCanto II flow cytometer (BD Biosciences) using FACSDiva software (BD Biosciences). Cytokine concentrations were calculated by comparing to standard curves generated using recombinant cytokines provided with the cytometric bead array kit and analyzed with FACS array analysis software (BD Biosciences). The CBA assay is a validated and commonly used assay to measure cytokines (35, 36); however we also confirmed the levels of two cytokines (IL-6 and IL-17) in groups of 1MT and carrier-treated mice using a conventional ELISA assay. Within a treatment group, the levels of both IL-6 and IL-17 were similar whether measured by conventional ELISA or CBA. Likewise, the decrease in IL-6 seen in the 1MT-treated group and lack of difference in IL-17 levels between carrier and 1MT treatment were also seen with both assays. (Supplementary Fig. 1).
Statistical Analysis
Statistical significance was determined using an unpaired Student’s t test or the Mann-Whitney nonparametric test and Instat Software (GraphPad Software, Inc).
Results
1MT synergizes with methotrexate to inhibit arthritis in K/BxN mice
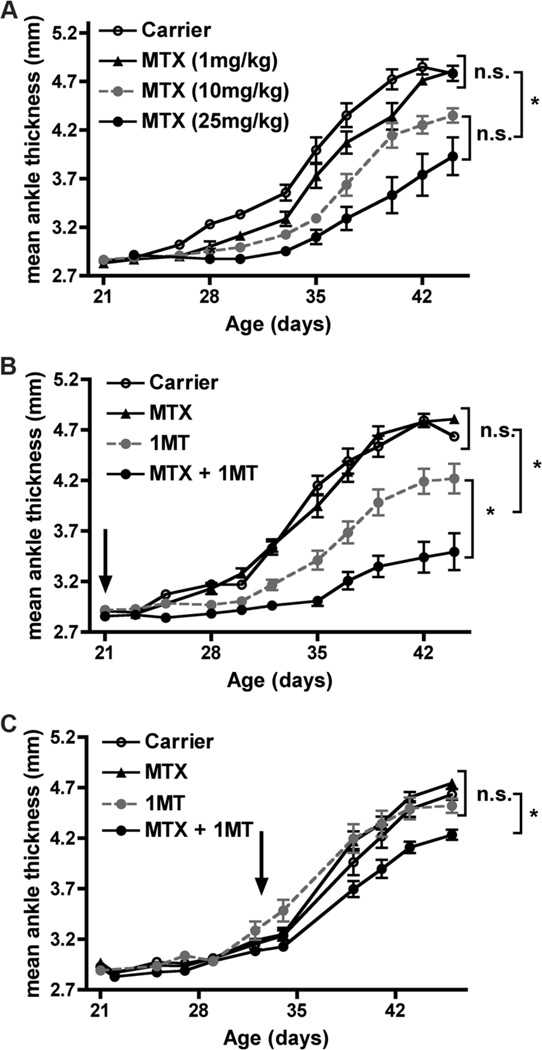
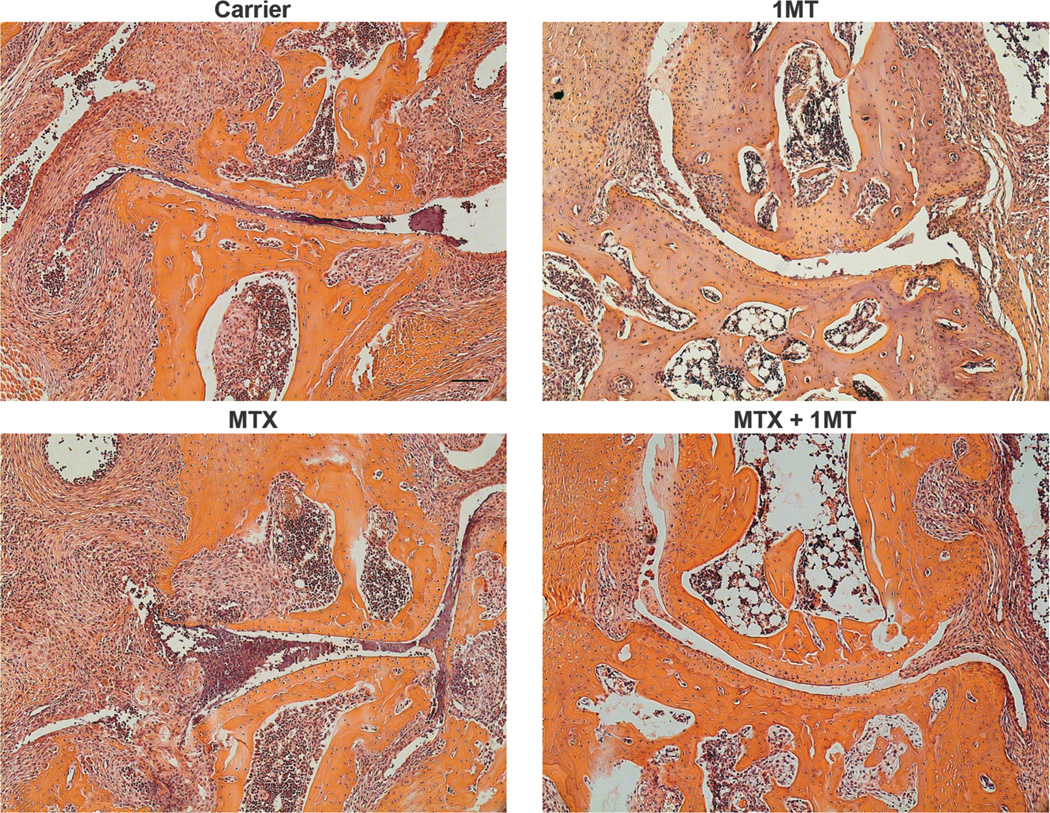
To test the hypothesis that combining 1MT and MTX would target both the initiation and effector phases and therefore be more effective as a co-therapeutic, K/BxN mice were treated with MTX with or without 1MT (D/L racemic mix) starting at 3 weeks of age, before the onset of arthritis (Fig. 1). The treated mice were then followed for the severity of arthritis by measuring inflammation in the ankles and by histological examination of the joints for immune cell infiltrates, synovial hyperplasia, panus formation, and cartilage and bone erosion. MTX was able to partially inhibit arthritis development when used individually at a high dose (10 or 25 mg/kg), but was ineffective at a low dose (1mg/kg, Fig. 1a). Similarly, 1MT was also able to partially inhibit arthritis when administered as a single agent therapy (Fig. 1b and (7)). Because high doses of MTX are associated with severe side effects (28, 37), we tested the efficacy of a low dose of MTX combined with 1MT. Intriguingly, the combination of a low dose of MTX (which had no effect as a single agent) together with 1MT was significantly more effective than either treatment alone at delaying the onset and alleviating the severity of joint inflammation (Fig. 1b). Histological analysis confirmed that ankles from MTX+1MT treated animals had less inflammatory cell infiltrates, synovial infiltration, and cartilage damage than carrier-treated mice or those that received either MTX or 1MT alone (Fig. 2). In this experiment, MTX and 1MT were administered prophylactically, demonstrating that treatment throughout the course of disease can prevent arthritis development. To determine if the combination of MTX and 1MT would also be effective at treating established arthritis, K/BxN mice were allowed to develop arthritis and then treated with MTX alone, 1MT alone, or MTX + 1MT (Fig. 1c). Neither 1MT nor a low dose of MTX affected established arthritis when administered as single agents. The combination of MTX and 1MT did slow the progression of arthritis, albeit not as effectively as when administered prior to arthritis onset (Fig. 1c). These data suggest the combination of MTX and 1MT is an effective strategy to inhibit both the initiation and effector stages of the autoimmune response as a treatment option for alleviating inflammatory arthritis.
Figure 1. MTX synergizes with 1MT to inhibit arthritis development.
(A) Dose-dependent inhibition of arthritis development by MTX. K/BxN mice were treated weekly with MTX (1, 10, or 25mg/kg) or carrier alone p.o. starting at the age of 3wk and followed for arthritis development. The graphs show mean ankle thickness ± SEM for n=8 mice per treatment group pooled from two independent experiments. (B,C) Co-therapy with MTX + 1MT. K/BxN mice were treated with Carrier, MTX alone (1mg/kg), 1MT alone (400mg/kg), or MTX + 1MT starting (B) before (age 3wk) or (C) after arthritis onset (age 4.5wk) and followed for the progression of arthritis. Arrows indicate the start of treatment. The graphs show mean ankle thickness ± SEM for n=9 mice per treatment group pooled from two independent experiments. *p<0.05, n.s., not significant.
Figure 2. MTX synergizes with 1MT to inhibit pathological signs of arthritis.
K/BxN mice were treated with Carrier, MTX alone (1mg/kg), 1MT alone (400mg/kg), or MTX + 1MT starting at the age of 3wk. Ankles were harvested at the age of 6wk, sectioned and stained with hematoxylin and eosin. Images show the metatarsal joint from representative sections from a total of 9 mice for each treatment group. Scale bar = 100µm.
IDO deficient mice are more responsive to MTX treatment
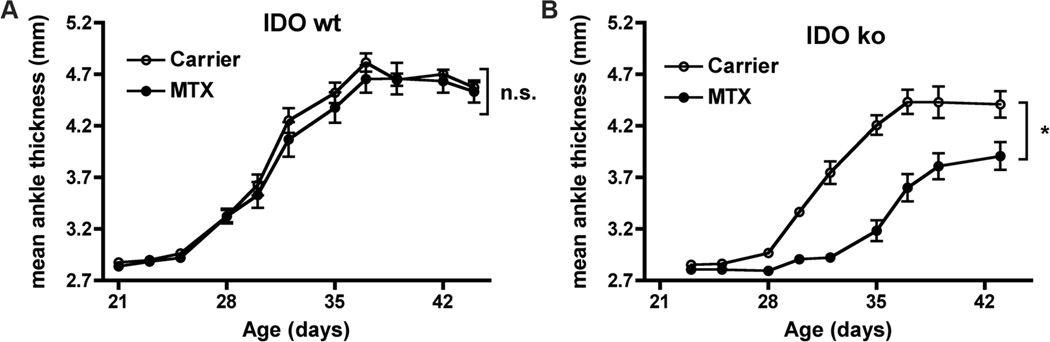
Because pharmacologic inhibitors such as 1MT may have unintended effects on other pathways, we used a genetic “knockout” mouse mutant of IDO. IDO deficient (IDO−/−) arthritic mice were generated by breeding KRN IDO−/− C57BL/6 mice expressing the I-Ag7 MHC Class II molecule (KRN/IDO−/− B6.g7). KRN B6.g7 mice develop arthritis with similar kinetics as the original K/BxN mice without the other immune complications of the NOD genetic background (31). As was demonstrated in the K/BxN model, treatment with a low dose of MTX (1mg/kg) does not inhibit the onset or severity of arthritis in KRN B6.g7 mice (Fig. 3a). In contrast, this low dose of MTX significantly inhibited both the time of onset and severity of arthritis in KRN/IDO−/− B6.g7 mice (Fig. 3b). Therefore, both pharmacological inhibition and genetic knockout approaches demonstrate a synergistic role for inhibition of the IDO pathway together with MTX in the treatment of RA.
Figure 3. MTX inhibits arthritis in IDO ko mice.
(A) KRN B6.g7 (IDO wt) and (B) KRN IDO ko B6.g7 (IDO ko) mice were treated with MTX (1mg/kg) or carrier alone starting at the age of 3wk and followed for the development of arthritis. The graphs show mean ankle thickness ± SEM for n=9 IDO wt and n=6 IDO ko mice per treatment group pooled from two independent experiments. *p<0.05, n.s., not significant.
Combination of MTX and 1MT does not further inhibit autoantibody response
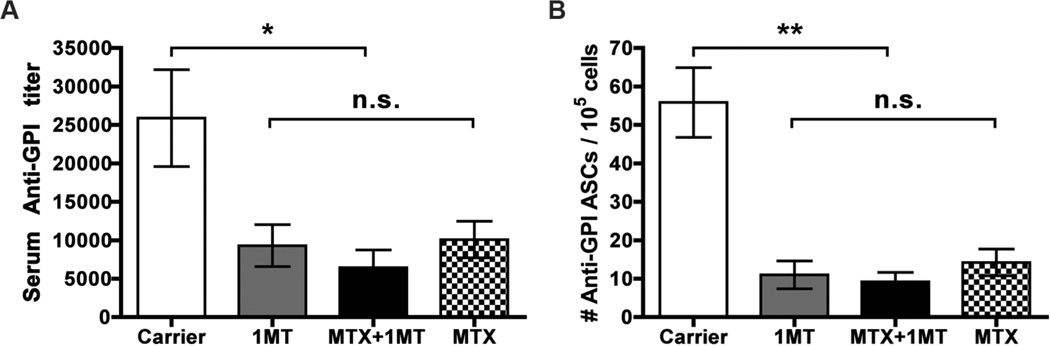
Our results suggest that the combination of MTX + 1MT synergizes to alleviate arthritis by inhibiting both the initiation (1MT) and effector (MTX) stages of the autoimmune response. One mechanism to explain this synergistic effect is that 1MT and MTX are acting on different cellular or molecular targets leading to a more effective inhibition of the overall autoimmune response and reduced arthritis. Arthritis in the K/BxN model has been shown to be dependent on B cells producing pathogenic antibodies to the glycolytic enzyme, glucose-6-phosphate isomerase (GPI) (19). Previously, we demonstrated that the most dramatic effect of 1MT treatment was on this pathogenic anti-GPI B cell response (7). Single agent therapy with 1MT inhibited the autoreactive B cell response, leading to lower serum anti-GPI Ig titers and reduced numbers of anti-GPI antibody secreting cells (ASCs) (Fig. 4 and ref. (7)). To determine if MTX also affects the anti-GPI B cell response and synergizes with 1MT to further reduce pathogenic anti-GPI Ig, the titer of serum anti-GPI Ig and number of anti-GPI antibody secreting cells in the dLNs was compared between mice treated with the combination of MTX + 1MT and control mice treated with MTX alone, 1MT alone, or carrier (Fig. 4). Serum anti-GPI titers were reduced in mice treated with either 1MT or MTX alone, compared to carrier-treated mice; however, no additional reduction was measured in mice treated with both MTX and 1MT (Fig. 4a). Similarly, treatment with MTX alone and 1MT alone both reduced the number of anti-GPI ASCs detected in the dLNs, but the combination of MTX and 1MT provided no additional benefit (Fig. 4b). These data demonstrate that both MTX and 1MT are able to inhibit the pathogenic B cell response when used as single agents, but this does not explain their synergistic effect on inhibiting the overall joint inflammatory response.
Figure 4. MTX and 1MT do not synergize to further inhibit autoantibody production.
K/BxN mice were treated with Carrier, MTX alone (1mg/kg), 1MT alone (400mg/kg), or MTX + 1MT from 3–6wk of age. (A) Serum anti-GPI Ig titers were measured using an ELISA assay. The graph shows the mean titer ± SEM from n=16 Carrier, n=10 1MT, n=14 MTX, and n=14 MTX+1MT treated mice, pooled from 3 independent experiments. (B) Antibody Secreting Cells (ASCs) from the joint draining LN were measured using an ELISpot assay. The graph represents the mean number of ASCs ± SEM from n=13 Carrier, n=10 1MT, n=18 MTX, and n=14 MTX+1MT treated mice, pooled from 3 independent experiments. *p<0.05, **p<0.01, n.s., not significant.
MTX + 1MT combination therapy does not inhibit inflammatory cytokines
Treatment with MTX has been shown to reduce levels of the inflammatory cytokines IL-6, IFNγ, and TNFα in the collagen induced arthritis model (25, 27, 28) and IL-4, IL-6, IL-13, TNFα, IFNγ, and GM-CSF in human RA patients (38–40). Similarly, we have shown that levels of IL-5, IL-6, IL-10, MCP-1, TNFα, and IFNγ are reduced in arthritic mice treated with 1MT (7, 8). To determine if altered cytokine profiles could explain the synergistic effect of MTX + 1MT, the levels of a panel of cytokines in the joint draining lymph nodes were compared in K/BxN mice treated with 1MT alone, MTX alone, or the combination of MTX + 1MT (Table 1). As we showed previously, levels of IL-5, IL-6, IL-10, MCP-1, TNFα, and IFNγ were reduced in mice treated with 1MT alone. Mice treated with a low dose of MTX alone had decreased levels of IL-10, but increased levels of RANTES. Levels of the other cytokines tested were not significantly different from control treated mice. Mice treated with the combination of MTX and 1MT did show lower levels of RANTES compared to MTX alone treated mice; however, the combination of MTX and 1MT did not show any synergistic effect compared to mice treated with 1MT alone. In fact for most cytokines, the beneficial effect of 1MT was lost in the MTX + 1MT group (Table 1). Therefore, the synergistic effect of combined MTX + 1MT treatment cannot be explained by a reduction in inflammatory cytokines.
Table 1. MTX and 1MT do not synergize to inhibit inflammatory cytokine production.
K/BxN mice were treated with Carrier, MTX alone (1mg/kg), 1MT alone (400mg/kg), or MTX + 1MT from 3–6wk of age. At 6wk, cells from the joint dLNs (popliteal, axillary, and brachial LNs) were harvested and cultured overnight in media alone or PMA (50ng/ml) + ionomycin (500ng/ml). Cytokines were measured in culture supernatants by cytometric bead array. Table shows the mean concentration ± SEM from n=20 Carrier, n=13 1MT, n=14 MTX, and n=12 MTX+1MT treated mice, pooled from 3 independent experiments.
| Cytokine (pg/ml) |
Carrier | 1MT | MTX | MTX+1MT |
|---|---|---|---|---|
| IL-4 | 2 ± 1 | 0 ± 0 | 5 ± 3 | 4 ± 3 |
| IL-5 | 77 ± 9 | 46 ± 7* | 117 ± 24 | 102 ± 13 |
| IL-6 | 43 ± 3 | 27 ± 5* | 39 ± 2 | 37 ± 5 |
| IL-9 | 20 ± 4 | 21 ± 5 | 16 ± 5 | 20 ± 7 |
| IL-10 | 144 ± 22 | 75 ± 17* | 51 ± 15* | 59 ± 19* |
| IL-13 | 109 ± 12 | 87 ± 18 | 174 ± 35 | 154 ± 22 |
| IL-17 | 895 ± 140 | 709 ± 284 | 1061 ± 189 | 1226 ± 267 |
| IFNγ | 6107 ± 370 | 4526 ± 727* | 5731 ± 650 | 5999 ± 871 |
| MCP-1 | 159 ± 13 | 104 ± 13* | 156 ± 19 | 144 ± 17 |
| MIP-1α | 662 ± 16 | 650 ± 55 | 655 ± 17 | 630 ± 16 |
| MIP-1β | 160 ± 6 | 175 ± 15 | 161 ± 6 | 153 ± 5 |
| RANTES | 616 ± 38 | 551 ± 89 | 745 ± 37 | 629 ± 45 |
| TNFα | 391 ± 35 | 224 ± 55* | 371 ± 29 | 364 ± 42 |
p<0.05
MTX synergizes with 1MT by inhibition of folate metabolism
To further explore the mechanism by which 1MT synergizes with MTX, we investigated the potential downstream pathways targeted by MTX. The precise mechanism by which MTX acts to inhibit arthritis is unknown and remains controversial (27, 41). In some models, MTX has been shown to inhibit inflammation by increasing endogenous adenosine concentrations and altering the production of inflammatory cytokines (27, 28). Other studies have suggested that MTX leads to decreased cell proliferation and increased apoptosis by decreasing polyamine production and increasing intracellular reactive oxygen species (ROS) levels (29). Finally, MTX is a folate antagonist and therefore has also been proposed to inhibit arthritis through its anti-proliferative effects (30). To determine which, if any, of these mechanisms are responsible for the synergy between MTX and 1MT, we used a combination of inhibitors and agonists to probe the requirement of increased adenosine concentration, polyamine inhibition, and folate antagonism for MTX action.
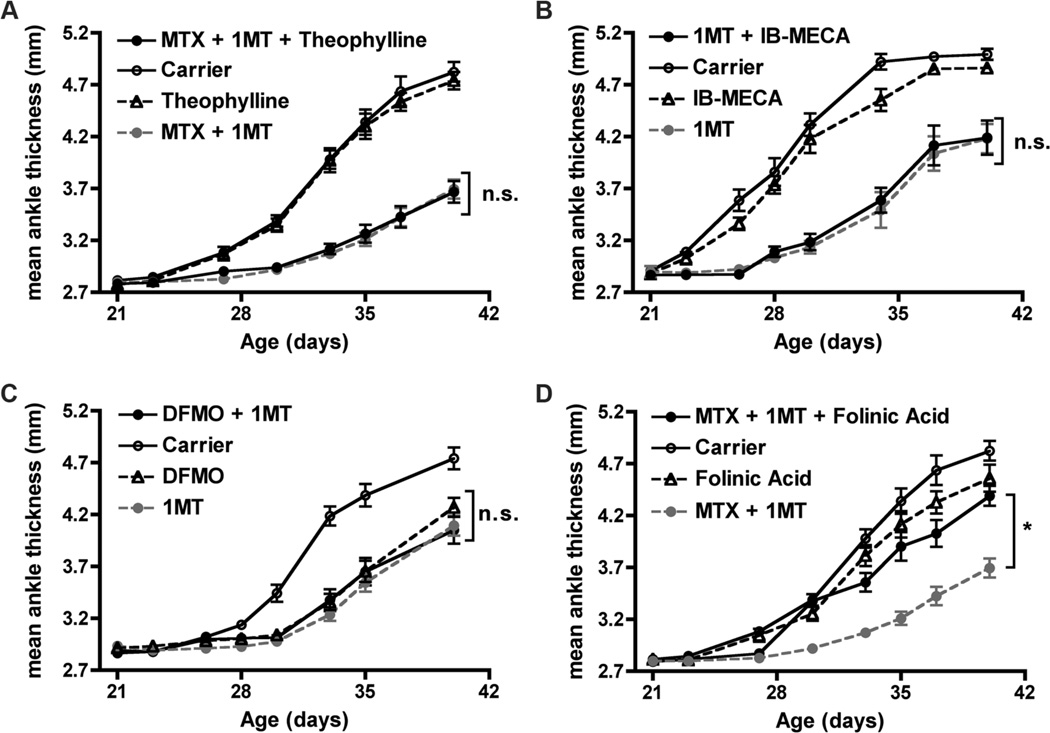
To determine if the anti-arthritic effect of MTX was mediated by increased adenosine concentration, we inhibited adenosine receptors with the adenosine receptor antagonist theophylline in mice treated with the combination of MTX + 1MT (42). If the effect of MTX was due to increased adenosine release, then theophylline should block this effect and lead to increased arthritis in mice treated with MTX and 1MT. Treatment with theophylline alone had no effect on arthritis onset or progression, nor did it reverse the anti-arthritic effect when added to combination therapy of MTX + 1MT (Fig. 5a). Because theophylline antagonizes the A1, A2a, and A2b adenosine receptors, but does not affect the A3 receptor, in a second set of experiments, we combined 1MT with an adenosine receptor agonist (IB-MECA) to stimulate the A3 adenosine receptor directly (43). If adenosine signaling through the A3 receptor was responsible for MTX’s anti-arthritic effect, then IB-MECA should be able to mimic MTX’s synergistic effect when combined with 1MT. Administration of IB-MECA alone had no effect on arthritis, nor did it mimic the synergistic anti-arthritic effect of MTX when combined with 1MT (Fig. 5b). Together, these data demonstrate that the synergistic effect of MTX and 1MT is not due to the release of the anti-inflammatory molecule adenosine.
Figure 5. MTX synergizes with 1MT by inhibition of folate metabolism.
K/BxN mice were treated with Carrier, MTX alone (1mg/kg), 1MT alone (400mg/kg), or MTX + 1MT from 3–6wk of age. To determine if the synergistic effect of 1MT + MTX was due to increased adenosine release, mice were treated with 1MT and MTX in combination with (A) the adenosine receptor A1, A2a, and A2b antagonist theophylline (10mg/kg, p.o.), n=10 mice per treatment group, or (B) the adenosine receptor A3 agonist IB-MECA (0.5mg/kg, i.p.), n=8 mice per treatment group. (C) To determine if inhibition of polyamine synthesis could mimic the effect of MTX, mice were treated with 1MT together with the ODC inhibitor DMFO (1% in drinking water), n=9 mice per treatment group. (D) To test whether inhibition of folate metabolism was responsible for the synergistic effect of 1MT + MTX, 1MT + MTX treatment was supplemented with folinic acid (25mg/kg, p.o.), n=10 mice per treatment group. The graphs show mean ankle thickness ± SEM for the indicated number of mice per treatment group pooled from two independent experiments each. *p<0.05, n.s., not significant.
To determine if MTX is acting by reducing polyamine production, we used DFMO, an inhibitor of ornithine decarboxylase (ODC), a critical enzyme in the polyamine synthesis pathway (44). K/BxN mice were treated with DFMO alone or in combination with 1MT (Fig. 5c). Both 1MT alone and DFMO alone delayed the onset and reduced the severity of arthritis. However, DFMO was not able to mimic the effect of MTX when combined with 1MT, as the combination of DFMO + 1MT did not show an enhanced effect over either agent used individually. These data suggest that polyamine inhibition is not the mechanism by which MTX synergizes with 1MT to alleviate arthritis in K/BxN mice.
Finally, to determine if folate antagonism was required for the action of MTX, mice were supplemented with folinic acid, a technique shown to partially bypass the effect of MTX in the antigen induced arthritis (AIA) model in rats (30). K/BxN mice were treated with the combination of 1MT + MTX in the presence or absence of folinic acid. Treatment with folinic acid alone had no effect on arthritis development. However, addition of folinic acid to 1MT + MTX co-therapy reversed the synergistic anti-arthritic effect, as inflammation in the joints from mice treated with 1MT + MTX + folinic acid approached the levels found in Carrier, MTX alone, or folinic acid alone treated mice (Fig. 5d). Therefore, these data suggest that MTX synergizes with 1MT to alleviate arthritis by antagonism of the folate pathway.
Discussion And Conclusions
Current therapeutic strategies to treat RA primarily focus on controlling or alleviating inflammation and the inflammatory mediators driving chronic disease (2–4). This includes MTX, the most commonly used drug in the treatment of RA (3, 23). In contrast, we have shown that 1MT, an inhibitor of the IDO pathway, blocks the initial stages of the autoimmune response underlying arthritis development in the K/BxN mouse model of RA (7, 8). In this study, we tested the efficacy of combining MTX with 1MT to target both the effector and initiation stages of the autoimmune response. Both 1MT and high doses of MTX were able to partially inhibit arthritis development when used individually; however the combination of 1MT with a low dose of MTX was significantly more effective than either treatment alone at delaying the onset and alleviating the severity of joint inflammation (Fig. 6).
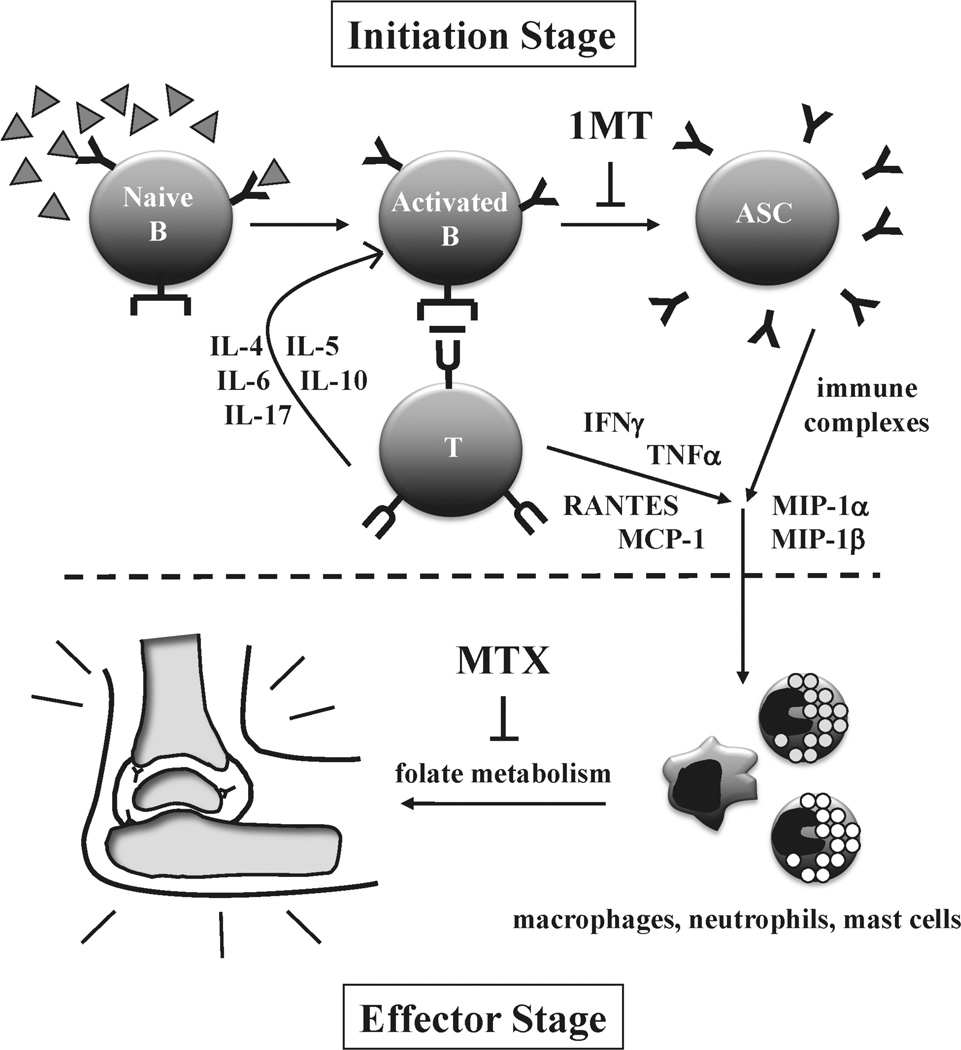
Figure 6. Impact of 1MT and MTX on autoimmune response leading to arthritis.
Arthritis in the K/BxN model can be divided into two stages (1) an initiation stage that is dependent on autoreactive B and T cells and (2) an effector stage that is triggered by immune complexes and inflammatory cytokines and dependent on macrophages, mast cells, and neutrophils. 1MT treatment impacts the initiation stage by inhibiting the differentiation of autoreactive B cells into antibody secreting cells (ASCs) and reducing the levels of several T helper and inflammatory cytokines. In contrast, MTX treatment, through a folate-dependent mechanism, primarily affects the effector stage. By targeting both the initiation and effector stages of the response, cotreatment with 1MT and MTX is more effective than either agent alone at delaying the onset and alleviating the severity of joint inflammation.
Combination therapy is becoming an increasingly popular therapeutic strategy in the treatment of RA (45). Patients who do not experience lowered disease activity in response to monotherapy with a DMARD like MTX often experience better results when it is used in combination with another therapeutic. For example, combining MTX with therapeutics that target the TNFα pathway has been effective for some patients (45). In fact, the latest American College of Rheumatology recommendations for the use of DMARDs and biologics in RA include a prominent role for combination therapies early in the treatment process, a change from previous treatment paradigms (3). The thought is that using drugs that target different mechanistic aspects of the disease process will be more effective than monotherapies that focus on only one target. Our data suggest that 1MT, by targeting the initial stages of the autoimmune response, should also be considered as a potential new therapeutic option for combination therapy in the treatment of RA.
An additional benefit to co-therapy may be in the reduction in the dosage of the component drugs required for efficacy. Preclinical testing of 1MT did not reveal any side effects, even at saturating doses (46, 47). Likewise, results from phase I clinical trials in cancer patients reported that 1MT was generally well tolerated, although a small number of patients who had received prior immunotherapy did develop autoimmune hypophysitis (47–49). Currently, 1MT is in Phase II clinical trials as a therapy for metastatic breast and prostate cancer and primary brain tumors (ClinicalTrials.gov identifier numbers: NCT01792050, NCT01560923, NCT02052648), but toxicity data has not yet been reported. In contrast to 1MT, high doses of MTX are associated with severe side effects including liver toxicity and bone marrow suppression in human RA patients (28, 37). Due to these side effects, some patients are unable to tolerate high enough doses of MTX to achieve a clinical benefit (45). As such, a co-therapeutic strategy that uses 1MT to lower the dose of MTX required for therapeutic effectiveness would be clinically desirable.
The mechanism by which MTX inhibits arthritis is not clear. MTX, a folate antagonist, inhibits purine and pyrimidine synthesis, a mechanism that likely accounts for its effectiveness as a cancer therapeutic (50). Decreased folate metabolism has been shown in RA patients treated with MTX, suggesting that MTX may also inhibit arthritis through its anti-proliferative effects (30, 51). However, data from some preclinical models suggest instead that MTX inhibits inflammation by increasing endogenous adenosine concentrations and altering the production of inflammatory cytokines (27, 28). Still other studies suggest that MTX treatment leads to decreased cell proliferation and increased apoptosis by decreasing polyamine production and increasing intracellular reactive oxygen species (ROS) levels (29). B cell antibody production has also been reported to be diminished by MTX treatment (52, 53). In the current study, we tested each of these mechanisms and found that the synergistic co-therapeutic effect of MTX + 1MT did not lower inflammatory cytokine or autoantibody levels, nor could it be reversed by the adenosine receptor antagonist theophylline or be mimicked by inhibition of polyamine synthesis. However, supplementation with folinic acid did reverse the synergistic co-therapeutic effect, demonstrating that, in the K/BxN model, MTX synergizes with 1MT by blocking folate metabolism.
In contrast to MTX treatment, T helper and inflammatory cytokines are reduced in 1MT-treated K/BxN mice, resulting in a dramatic reduction in both the number of autoantibody secreting cells and titers of autoantibody in the serum (7, 8). However, like MTX, the molecular mechanism by which 1MT exerts its anti-arthritic effect is not entirely clear. Although widely considered an IDO inhibitor, 1MT does not inhibit the IDO enzyme directly, rather it likely inhibits the IDO pathway (54). 1MT inhibition of the IDO pathway has been shown to modulate dendritic cell function, controlling the balance between effector and regulatory T cell populations (55–57). The IDO pathway is complex and the mechanisms controlling its role in other immune functions are only beginning to be established (10). Recent data suggests that IDO may function through tryptophan depletion and sufficiency signals influencing GCN2 and mTOR pathways (54).
In summary, we have identified a potential new co-therapeutic strategy for improving the efficacy of low dose MTX treatment in inflammatory arthritis. We show that targeting both the initiation and chronic inflammatory stages of the autoimmune response with 1MT and MTX, respectively, is an effective strategy to control disease symptoms. Our work suggests that targeting the IDO pathway with 1MT should be considered as an effective co-therapeutic strategy for treating inflammatory autoimmune diseases like RA.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Lisa Laury-Kleintop for help with the imaging studies and Dr. Lauren Merlo for critical reading of the manuscript and thoughtful input.
This project was supported by Grant Number 5-RO1 AR057847 (LM-N) from NIAMS/NIH. J.B.D., A.J.M., and G.C.P. are inventors on issued U.S. patents claiming structure of matter and therapeutic uses of IDO inhibitors. These investigators are shareholders in NewLink Genetics Corporation, which has licensed IDO patents granted to the investigators’ institution for clinical development of the technology. Additionally, G.C.P. receives compensation as an expert consultant for NewLink Genetics Inc.’s scientific advisory board.
Footnotes
Declarations of Interest
The authors have no additional financial interests.
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin. Ther. 2011;33:679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O'Dell J, Winthrop KL, Beukelman T, Bridges SL, Jr, Chatham WW, Paulus HE, Suarez-Almazor M, Bombardier C, Dougados M, Khanna D, King CM, Leong AL, Matteson EL, Schousboe JT, Moynihan E, Kolba KS, Jain A, Volkmann ER, Agrawal H, Bae S, Mudano AS, Patkar NM, Saag KG. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity. Online first. 2014 doi: 10.3109/08916934.2013.873413. [DOI] [PubMed] [Google Scholar]
- 5.Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane database of systematic reviews (Online) 2010 doi: 10.1002/14651858.CD008495. CD008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rath T, Rubbert A. Drug combinations with methotrexate to treat rheumatoid arthritis. Clin. Exp. Rheumatol. 2010;28:S52–S57. [PubMed] [Google Scholar]
- 7.Scott GN, DuHadaway J, Pigott E, Ridge N, Prendergast GC, Muller AJ, Mandik-Nayak L. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J. Immunol. 2009;182:7509–7517. doi: 10.4049/jimmunol.0804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pigott E, Mandik-Nayak L. Addition of an indoleamine 2,3,-dioxygenase inhibitor to B cell-depletion therapy blocks autoreactive B cell activation and recurrence of arthritis in K/BxN mice. Arthritis Rheum. 2012;64:2169–2178. doi: 10.1002/art.34406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 10.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr. Med. Chem. 2011;18:2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oertelt-Prigione S, Mao TK, Selmi C, Tsuneyama K, Ansari AA, Coppel RL, Invernizzi P, Podda M, Gershwin ME. Impaired indoleamine 2,3-dioxygenase production contributes to the development of autoimmunity in primary biliary cirrhosis. Autoimmunity. 2008;41:92–99. doi: 10.1080/08916930701619730. [DOI] [PubMed] [Google Scholar]
- 12.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature reviews. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 13.Williams RO. Exploitation of the IDO Pathway in the Therapy of Rheumatoid Arthritis. International journal of tryptophan research : IJTR. 2013;6:67–73. doi: 10.4137/IJTR.S11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, Ray A. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Bubnoff D, Bieber T. The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy. 2012;67:718–725. doi: 10.1111/j.1398-9995.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 16.Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin. Rheumatol. 2006;25:334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 17.Schroecksnadel K, Kaser S, Ledochowski M, Neurauter G, Mur E, Herold M, Fuchs D. Increased degradation of tryptophan in blood of patients with rheumatoid arthritis. J. Rheumatol. 2003;30:1935–1939. [PubMed] [Google Scholar]
- 18.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- 19.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 20.Kyburz D, Carson DA, Corr M. The role of CD40 ligand and tumor necrosis factor alpha signaling in the transgenic K/BxN mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2571–2577. doi: 10.1002/1529-0131(200011)43:11<2571::AID-ANR26>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Ohmura K, Nguyen LT, Locksley RM, Mathis D, Benoist C. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 2005;52:1866–1875. doi: 10.1002/art.21104. [DOI] [PubMed] [Google Scholar]
- 22.Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, Mahmood U, Weissleder R, Carulli J, Benoist C, Mathis D. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross talk. J. Exp. Med. 2007;204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincus T, Ferraccioli G, Sokka T, Larsen A, Rau R, Kushner I, Wolfe F. Evidence from clinical trials and long-term observational studies that disease-modifying anti-rheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology (Oxford) 2002;41:1346–1356. doi: 10.1093/rheumatology/41.12.1346. [DOI] [PubMed] [Google Scholar]
- 24.Braun J, Rau R. An update on methotrexate. Curr. Opin. Rheumatol. 2009;21:216–223. doi: 10.1097/BOR.0b013e328329c79d. [DOI] [PubMed] [Google Scholar]
- 25.Neurath MF, Hildner K, Becker C, Schlaak JF, Barbulescu K, Germann T, Schmitt E, Schirmacher P, Haralambous S, Pasparakis M, Meyer Zum Buschenfelde KH, Kollias G, Marker-Hermann E. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin. Exp. Immunol. 1999;115:42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asanuma Y, Nagai K, Kato M, Sugiura H, Kawai S. Weekly pulse therapy of methotrexate improves survival compared with its daily administration in MRL/lpr mice. Eur. J. Pharmacol. 2002;435:253–258. doi: 10.1016/s0014-2999(01)01555-2. [DOI] [PubMed] [Google Scholar]
- 27.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 28.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bulletin of the NYU hospital for joint diseases. 2007;65:168–173. [PubMed] [Google Scholar]
- 29.Huang CC, Hsu PC, Hung YC, Liao YF, Liu CC, Hour CT, Kao MC, Tsay GJ, Hung HC, Liu GY. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis. 2005;10:895–907. doi: 10.1007/s10495-005-2947-z. [DOI] [PubMed] [Google Scholar]
- 30.Andersson SE, Johansson LH, Lexmuller K, Ekstrom GM. Anti-arthritic effect of methotrexate: is it really mediated by adenosine? Eur. J. Pharm. Sci. 2000;9:333–343. doi: 10.1016/s0928-0987(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 31.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 32.Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 34.Mandik-Nayak L, Wipke BT, Shih FF, Unanue ER, Allen PM. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14368–14373. doi: 10.1073/pnas.182549099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R, Lowe L, Wilson JD, Crowther E, Tzeggai K, Bishop JE, Varro R. Simultaneous Quantification of Six Human Cytokines in a Single Sample Using Microparticle-based Flow Cytometric Technology. Clin. Chem. 1999;45:1693–1694. [PubMed] [Google Scholar]
- 36.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albrecht K, Muller-Ladner U. Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin. Exp. Rheumatol. 2010;28:S95–101. [PubMed] [Google Scholar]
- 38.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42:1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 39.de Lathouder S, Gerards AH, Dijkmans BA, Aarden LA. Two inhibitors of DNA-synthesis lead to inhibition of cytokine production via a different mechanism. Nucleosides, nucleotides & nucleic acids. 2004;23:1089–1100. doi: 10.1081/NCN-200027365. [DOI] [PubMed] [Google Scholar]
- 40.Kraan MC, Smeets TJ, van Loon MJ, Breedveld FC, Dijkmans BA, Tak PP. Differential effects of leflunomide and methotrexate on cytokine production in rheumatoid arthritis. Ann. Rheum. Dis. 2004;63:1056–1061. doi: 10.1136/ard.2003.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan ES, Cronstein BN. Methotrexate--how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 42.Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 43.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br. J. Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19:1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 45.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology (Oxford) 2012;6(51 Suppl):vi28–vi36. doi: 10.1093/rheumatology/kes278. [DOI] [PubMed] [Google Scholar]
- 46.Jia L, Schweikart K, Tomaszewski J, Page JG, Noker PE, Buhrow SA, Reid JM, Ames MM, Munn DH. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: Absence of toxicity due to saturating absorption. Food Chem. Toxicol. 2008;46:203–211. doi: 10.1016/j.fct.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer journal (Sudbury, Mass) 2010;16:354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soliman HH, Sullivan ASD, Vanahanian N, Link C. Overcoming tumor antigen anergy in human malignancies using the novel indoleamine 2,3-dioxygenase (IDO) enzyme inhibitor, 1-methyl-D-tryptophan (1MT) J. Clin. Oncol. 2009;27 Abstract 3004. [Google Scholar]
- 49.Soliman HH, Minton SE, Han HS, Ismail-Khan R, Mahipal A, Janssen W, Streicher H, Vahanian NN, Link CJ, Ramsey WJ, Antonia SJ, Sullivan D. A phase I study of ad.p53 DC vaccine in combination with indoximod in metastatic solid tumors. J. Clin. Oncol. 2013 suppl abstract 3069. [Google Scholar]
- 50.Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2012;15:183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Blits M, Jansen G, Assaraf YG, van de Wiel MA, Lems WF, Nurmohamed MT, van Schaardenburg D, Voskuyl AE, Wolbink GJ, Vosslamber S, Verweij CL. Methotrexate normalizes up-regulated folate pathway genes in rheumatoid arthritis. Arthritis Rheum. 2013;65:2791–2802. doi: 10.1002/art.38094. [DOI] [PubMed] [Google Scholar]
- 52.Salinas-Carmona MC, Perez LI, Galan K, Vazquez AV. Immunosuppressive drugs have different effect on B lymphocyte subsets and IgM antibody production in immunized BALB/c mice. Autoimmunity. 2009;42:537–544. doi: 10.1080/08916930903019119. [DOI] [PubMed] [Google Scholar]
- 53.Berti E, Vannucci G, Lunardi C, Bianchi B, Bason C, Puccetti A, Giani T, Pagnini I, Cimaz R, Simonini G. Identification of autoantibodies against inner ear antigens in a cohort of children with idiopathic sensorineural hearing loss. Autoimmunity. 2013;46:525–530. doi: 10.3109/08916934.2013.822074. [DOI] [PubMed] [Google Scholar]
- 54.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 56.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int. Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 57.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.