Abstract
Observations of harmful algal blooms (HABs) of the dinoflagellate Alexandrium fundyense in an estuary over multiple years were used to assess drivers of their spatial and temporal variability. Nauset Estuary on Cape Cod, Massachusetts has a recurrent, self-seeding A. fundyense population that produces paralytic shellfish poisoning toxins and leads to nearly annual closure to shellfishing. Weekly surveys of the entire estuary were made in 3 of 4 consecutive years, with surveys of a subembayment during the intervening year. Major A. fundyense blooms were observed all 4 years, with maximum concentrations >106 cells L−1. Concentrations were greatest in three salt ponds at the distal edges of the estuary. The bloom timing varied among the salt ponds and among years, although the blooms had similar durations and maximum cell concentrations. Nutrient concentrations did not correlate with the growth of the bloom, but differences in water temperature among years and ponds were significant. Net growth rates inferred from the surveys were similar to those from laboratory experiments, and increased linearly with temperature. A growing degree day calculation was used to account for effects of interannual variability and spatial gradients in water temperature on population development. The approach collapsed variability in the timing of bloom onset, development, and termination across years and among ponds, suggesting that this relatively simple metric could be used as an early-warning indicator for HABs in Nauset and similar areas with localized, self-seeding blooms.
Introduction
Harmful algal blooms (HABs) cause significant economic, public health, and ecological effects in coastal and estuarine settings around the world (Anderson 1989; Hallegraeff 1993; Anderson et al. 2012). Future effects will depend on the response of marine ecosystems to changing climate conditions, and prediction of those responses first requires a better understanding of the response of organisms and ecosystems to shorter time-scale environmental variability (Hallegraeff 2010). In the United States, one of the most significant HAB problems occurs in the northeast where paralytic shellfish poisoning (PSP) toxins are produced by the dinoflagellate Alexandrium fundyense (synonymous with A. tamarense Group I, a provisional species name (Lilly et al. 2007)). In coastal New England, A. fundyense blooms occur regularly both in the open waters of the Gulf of Maine and in smaller coastal embayments (Anderson 1997). The blooms contaminate shellfish resources, and a monitoring network is maintained throughout the region to safeguard human health. The large-scale Gulf of Maine bloom has been studied extensively and a complex set of factors including cyst distributions, coastal circulation, and freshwater input have been shown to control bloom development (Franks and Anderson 1992; Anderson et al. 2005b, in press). These efforts have led to the development of mechanistic numerical models for hindcast (McGillicuddy et al. 2005; He et al. 2008; Li et al. 2009) and forecast (McGillicuddy et al. 2011) assessments of bloom development in the Gulf of Maine.
Independent of the large-scale Gulf of Maine bloom, HABs also occur in numerous estuaries and embayments along the Northeast coast, with similar effects on commercial and recreational shellfisheries. One example is Nauset estuary on Cape Cod, Massachusetts, where A. fundyense blooms occur annually (Anderson et al. 1983; Crespo et al. 2011). The blooms in Nauset caused shellfishing closures due to PSP toxin in 20 of 21 years from 1992 to 2012. The blooms most likely originate within the estuary rather than coming from the Gulf of Maine because Nauset blooms occur earlier in the year and are concentrated in the upper reaches of the estuary rather than near the inlet (Anderson 1997; Crespo et al. 2011). The potential for cells to be exported from Nauset and transported downcoast to seed HABs in other nearby embayments remains uncertain.
A. fundyense blooms were first investigated in Nauset and a similar Cape Cod estuary over 30 years ago (Anderson et al. 1983; Crespo et al. 2011), and after a long interval with little study, detailed examination of the bloom dynamics resumed recently (Crespo et al. 2011). A. fundyense has the ability to migrate vertically, and observations in Salt Pond, a subembayment in the northwest of the Nauset estuary, found that retention of cells within the pond was enhanced by a combination of the cells’ vertical migration and tidally asymmetric flows (Anderson and Stolzenbach 1985). A. fundyense cells swim higher in the water column during the day and lower at night, but the shallowest point within this ambit is 2–3 m deep rather than at the surface. Surface avoidance during the day and downward migration at night reduces cell concentrations in the near-surface layer and limits the rate of tidal flushing from the salt ponds. This selective retention enhances cell accumulation in the ponds and cyst deposition in the estuary at the end of blooms, perpetuating self-seeding A. fundyense populations (Anderson et al. 1983; Crespo et al. 2011). In this way, A. fundyense life history and behavior seem well matched to the estuary, consistent with the recurrence of localized blooms in the ponds of the Nauset system.
The presence of recurrent, self-seeding blooms concentrated in the upper reaches of the estuary and distinct from influences from the coastal ocean makes Nauset a natural laboratory for study of A. fundyense. Extensive field observations have been made in the past several years to characterize the spatial and temporal evolution of the bloom and water properties. Multiple realizations of the bloom documented natural variability in the factors driving cell growth, including water temperature, salinity, nutrient concentrations, and the grazing community. Previous work in Nauset found that a temperature-dependent exponential growth model represented well the growth phase of the bloom in the ponds (Watras et al. 1982). Another coastal pond in that study (Perch Pond had a bloom that occurred about 35 days earlier than in Nauset, and the difference in bloom timing was attributed to a combination of higher temperatures (~3°C) and lower salinities (~26 vs. 32). Here we present observations from multiple years and multiple ponds to assess of the response of the A. fundyense population to temperature variability, resulting in a simple model for prediction of bloom development based on growing degree days that is consistent with the observed interannual and spatial variability. The results have relevance for other estuaries and coastal embayments that currently have, or in the future may develop HAB problems, and also help inform development of models of A. fundyense blooms occurring in the coastal ocean.
Methods
Study location
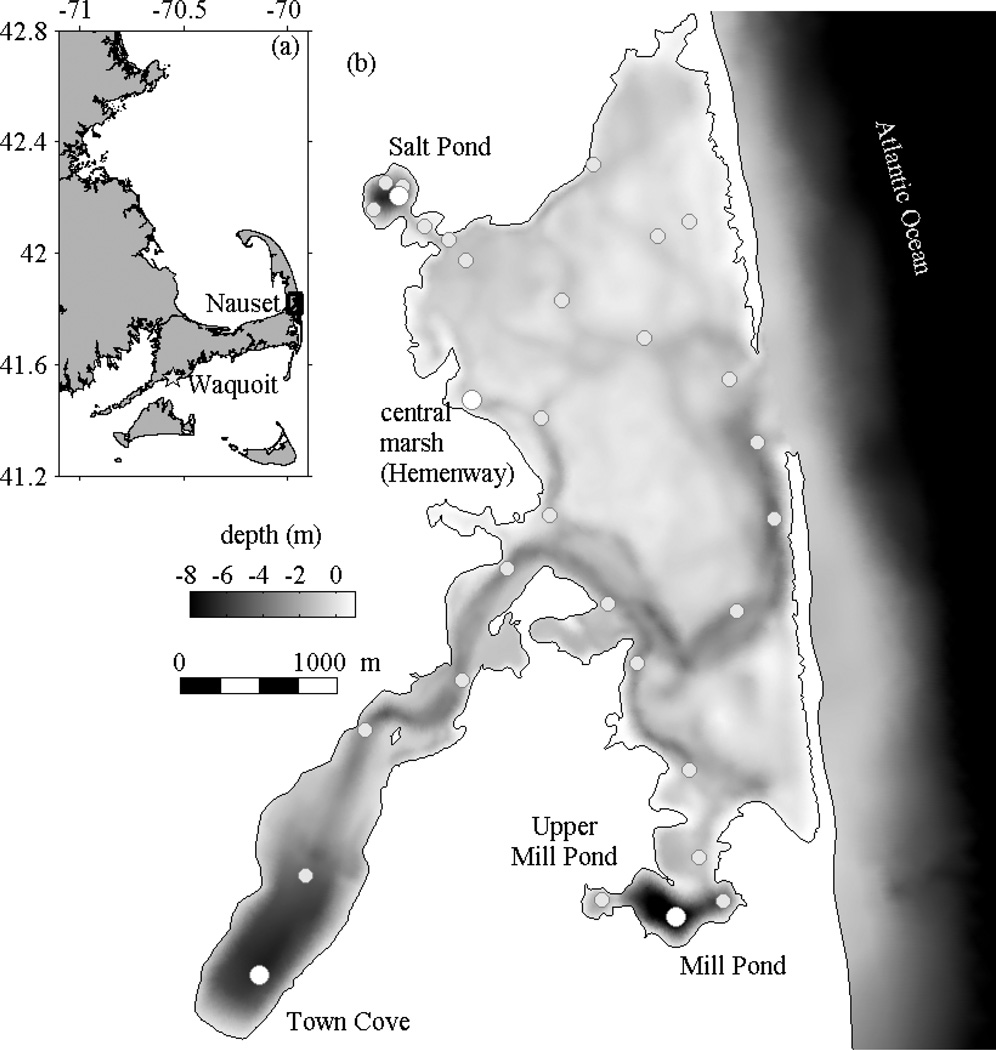
Studies were conducted in Nauset estuary, a complex of marshes and submerged kettle ponds (or salt ponds) on Cape Cod, Massachusetts connected by an inlet through a dynamic barrier beach to the Atlantic Ocean (Fig. 1). The central part of the estuary is a network of tidal channels through a vegetated marsh platform. Three salt ponds are connected to the central marsh through tidal channels: Salt Pond, Town Cove, and Mill Pond (north-to-south). The maximum depths of the salt ponds are 6 to 11 m, significantly deeper than the marsh tidal channels that range from 1 to 3 m in depth.
Figure 1.
Location map and bathymetry of Nauset estuary. (a) The box outlines Nauset on Cape Cod; the star marks the location of Waquoit Bay. (b) Station locations for the weekly surveys are shown on the bathymetry, highlighting selected stations at the deepest points in Salt Pond, Town Cove, and Mill Pond, and the Hemenway station in the central marsh.
The tides at the mouth of the estuary are semi-diurnal and typically range from 1 to 2 m. Tidal propagation through the shallow central marsh produces frictional non-linearities that make the tidal cycle in the estuary highly asymmetric, with a brief flood period (~3–4 h) and a longer ebb (~8–9 h) (Aubrey and Speer 1985). Nauset estuary has no river input, so the primary sources of freshwater to the system are groundwater and direct precipitation. Groundwater discharge from the Nauset and Monomoy lenses also provides a major source of nutrient loading to the estuary from a predominantly residential watershed (Giblin and Gaines 1990; Portnoy et al. 1998; Colman and Masterson 2008). The freshwater input is sufficient to create stratification in the salt ponds due to both salinity and temperature gradients. Surface-to-bottom salinity gradients in the ponds were typically ~1–2, and thermal stratification ranged from about 1°C at the beginning of the bloom to greater than 10°C toward the end.
Large-scale weekly surveys
Approximately weekly during the spring months of 2009, 2011, and 2012, large-scale surveys were made of Nauset estuary to measure physical and biological conditions. The 2009 survey data and details on the survey methodology are described in (Crespo et al. 2011), but here we recap the methods and extend the observations to additional years. Surveys were conducted around high water slack using two vessels with cross-calibrated instruments to sample approximately 30 stations during a 2 hour period (Fig. 1). At each station, continuous vertical profiles of salinity and temperature were measured with a conductivity-temperature-depth (CTD) sensor. Water samples for A. fundyense abundance and nutrient measurements were collected at each station with 2.5 L Niskin bottles at multiple depths: the surface and, where possible, at 3, 5, 7, and 10 m depths.
A. fundyense abundance was quantified using a whole cell oligonucleotide probe hybridization method as described by Anderson et al. (Anderson et al. 2005a). The hybridization assay labels A. fundyense with Cy3-conjugated NA1 ribosomal probe (5’- AGT GCA ACA CTC CCA CCA-3’) so that the cells are easily distinguished from other, similarly sized dinoflagellate species. Staining was performed on sub-samples from >20 µm fraction concentrates of 2 L samples from the field. Subsample volumes were adjusted such that at least 400 A. fundyense were filtered onto 25 mm 5.0 µm Cyclopore filters (Whatman) for NA1 staining. Filters were mounted on microscope slides using an 80% glycerol solution to preserve fluorochrome brightness and stored at 4°C until their A. fundyense cells could be counted using a Zeiss Axioskop epifluorescence microscope outfitted with a Cy3 filter set (Chroma No. 41032). Control samples of cultured cells of A. fundyense were processed simultaneously to confirm the consistency of the staining procedure. Cell counts were typically completed within 2 days of probe treatment.
Nutrient samples were collected in acid-washed, sample-rinsed bottles, transported to the lab on ice and immediately frozen at −20°C. In the laboratory, each sample was analyzed within 2 months from collection for inorganic nitrate plus nitrite (hereafter termed ‘nitrate’), ammonium, and phosphate using a Lachat Instruments QuickChem 800 four-channel continuous flow injection system. This method is United States Environmental Protection Agency approved for nutrient analysis ranging from groundwater to the open ocean.
In 2009, 2011, and 2012, the surveys were conducted approximately weekly, with the sampling period each year dependent on the status of the A. fundyense bloom. In 2009, 12 surveys were conducted from 24 March to 17 June; in 2011, 13 surveys were conducted from 23 March to 16 June; and in 2012, 11 surveys were conducted from 15 February to 08 May. Each survey included stations throughout the estuary, with a few exceptions when weather conditions limited small-boat operations and the sampling was restricted to stations in the ponds. Surveys occurred around day-time high tides to maximize navigability of the central marsh for the sampling vessels.
In addition to the full-estuary surveys in 2009, 2011, and 2012, a similar set of surveys using CTD profiles and Niskin water samples occurred in Salt Pond in the spring of 2010. Sampling was conducted weekly at the deepest station in Salt Pond from 25 March to 20 May. Water samples were processed for A. fundyense abundance as described above, but nutrient concentrations were not analyzed. Navigational constraints did not require the Salt Pond sampling to occur around high tide, so surveys typically occurred mid-morning regardless of the tide.
Moored instruments
Moored, internally recording instruments were deployed in Nauset during the survey periods to provide continuous records of water properties during the blooms. The configuration of the moored sensors varied among the years, but generally included water level and near-surface and near-bottom sensors for temperature and salinity in each of the ponds (Mill Pond, Salt Pond, and Town Cove) and the central marsh (Hemenway) (Fig. 1). In 2009, moorings that recorded water level and water temperature were deployed at these four locations. In 2010 and 2011, moorings in the deep parts of Salt Pond and Mill Pond had near-surface and near-bottom sensors for temperature and salinity, while moorings in Town Cove and at Hemenway were deployed in shallower water with a near-bottom temperature sensor; all stations also recorded water level. In 2012, near-surface and near-bottom temperature and salinity sensors were deployed in Town Cove, Salt Pond, and Mill Pond, and again temperature was recorded in the central marsh at Hemenway. The moored time-series were compared with the weekly survey data at adjacent stations to identify when fouling affected the data quality, and those data were removed.
As a proxy for conditions during periods when instruments were not deployed in Nauset, additional data were retrieved from sensors maintained at the nearby Waquoit Bay National Estuarine Research Reserve (http://cdmo.baruch.sc.edu/). Waquoit Bay is also a tidal embayment on Cape Cod, connecting several small rivers to Nantucket Sound (Fig. 1). The Waquoit Bay temperature time series were used to extend the temperature analyses, so the stations that most closely tracked the observed temperatures in Nauset were selected as representative proxies: Menauhant, which was typically slightly cooler than stations in Nauset, and Metoxit Point, which tended to be slightly warmer. The average temperature from these two stations was used to represent water temperatures during periods when instruments were not deployed in Nauset.
Degree day calculation
To link the population growth with the water temperatures in the ponds, a growing degree day approach was utilized. Growing degree days have been used extensively to predict development phases of terrestrial plants and insects, and more recently have been applied to zooplankton and fisheries (Mackas et al. 1998; Gillooly 2000; Neuheimer and Taggart 2007). Degree days (DD) are calculated as the integral over time (t) of temperature above a threshold value:
| (1) |
where T is the water temperature, Tbase is a lower physiological limit below which growth does not occur, and t0 is the starting time for the growth phase.
To calculate growing degree days, water temperature time series from the moored sensors in each pond and in the central marsh were used. The moored sensors recorded temperature variability at tidal and diurnal time scales, which the weekly survey data did not resolve. The moored sensors had sampling intervals (Δt) of 1 to 10 minutes and were deployed during the weekly surveys, typically beginning in late February or early March. To represent growing conditions prior to instrument deployment, temperature time series from the Waquoit Bay sensors were used instead (Δt = 30 min).
The degree day (or heat unit) scaling of population growth is consistent with a linear dependence of growth rate on temperature (Wang 1960; McMaster and Wilhelm 1997). A. fundyense growth rates in lab experiments from several sources increase approximately linearly to about 16°C, level off at 16–20°C, and decline for temperatures greater than 20°C (Watras et al. 1982; Etheridge and Roesler 2005; D.M. Anderson unpubl. data). Previous studies used A. fundyense cultures from Mill Pond grown at two salinities (25.5 and 35.5) over a range temperatures (Watras et al. 1982) and isolates from the Gulf of Maine grown at salinity of 30 over a range of temperatures (Etheridge and Roesler 2005). More highly resolved data comes from temperature bar growth rate experiments for an A. fundyense isolate from the Gulf of Maine (D.M. Anderson unpubl. data). Considering only the typical range of water temperatures during the growth phase of the Nauset bloom (2–12°C), linear fits of the growth rates to temperature were highly correlated and significant (r2 = 0.98, p < 0.001). Observations spanning multiple phytoplankton species over wider temperature ranges have found that an exponential dependence of growth on temperature can be more appropriate than a linear dependence, particularly to represent growth at higher temperatures (Eppley 1972; Rose and Caron 2007). However, over the temperature range observed in Nauset an exponential fit to the A. fundyense lab growth rates yielded a slightly lower correlation (r2 = 0.95, p < 0.001) than the linear fit.
The degree day calculation requires definition of a threshold temperature above which growth occurs and a starting date for the growing season. The field and laboratory data for A. fundyense growth are too coarse at present to exhibit a clear threshold below which there is no growth. Laboratory results found growth rates of to 0.1 to 0.2 d−1 at 5°C (Watras et al. 1982; Etheridge and Roesler 2005; D.M. Anderson unpubl. data), while other lab studies report cell report cell survival, but no growth, at 2.5°C and 5°C (Anderson et al. 1984). In the calculations presented here we assume Tbase = 0°C. Alternatively, assuming Tbase = 2°C yields qualitatively similar results for the dependence of the population on degree day, just with different degree day values. The starting date for calculation was set arbitrarily to 01 January of each year, but a lack of precision in this start of the growing season is unlikely to be a significant source of error given the cold (< 2°C) temperatures during the pre-bloom winter months (December–February).
Net growth rates in each of the ponds were calculated from the rates of change of the populations in the large-scale surveys. Net growth rates (μnet) were calculated by fitting an exponential growth curve to subsets of the survey data using a moving window of three consecutive surveys: log(C/C0) = μnett, where C is cell concentration, C0 is the concentration at the start of the three-survey window, and t is elapsed time. This net growth rate combines both growth and loss factors including cyst germination, cell division, encystment, grazing and advective losses. The moving window approach was necessary because discrete, weekly samples make the calculation noisy, as they do not resolve processes like tidal advection and spatial patchiness that can affect the observed cell populations. The linear dependence of net growth on temperature was assessed directly from Nauset survey data and compared to A. fundyense growth rates observed in prior laboratory experiments (Results section).
Results
Spatial and temporal evolution of the bloom
Full-estuary surveys extensively documented the A. fundyense bloom in Nauset in 3 of 4 consecutive years (2009, 2011, 2012), and observations in Salt Pond provided similar data in the intervening year (2010). From these surveys, several clear trends in the spatial and temporal distribution of the A. fundyense population emerged. The 2011 large-scale survey data is used as an example to describe physical and biological conditions in the estuary during one of the blooms, and subsequently data from multiple years are compared to evaluate interannual variability.
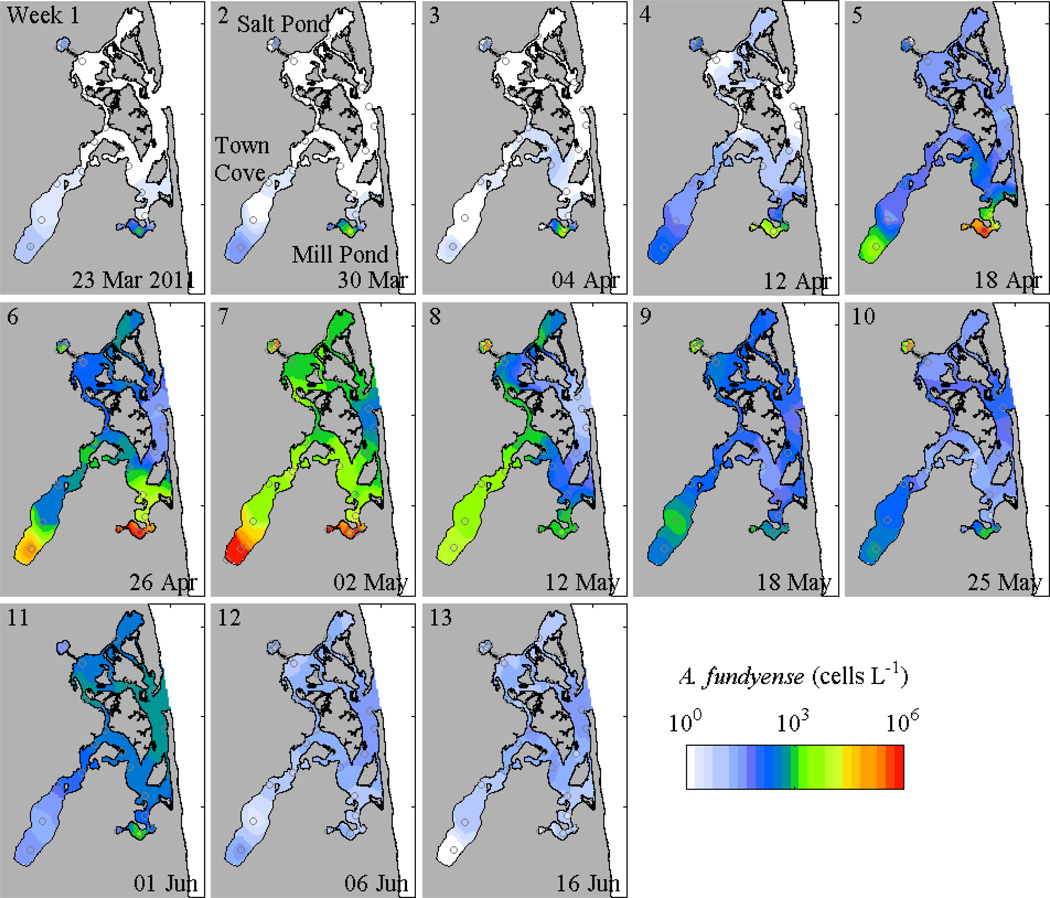
In 2011, A. fundyense cell concentrations began to increase in Mill Pond before the rest of the estuary, with greater than 103 cells L−1 found in the first survey, compared with less than 102 cells L−1 in Town Cove and Salt Pond and concentrations below detection in most of the central marsh (Fig. 2). Cell concentrations subsequently began to increase in Town Cove, and then Salt Pond, but the bloom in Mill Pond continued to lead the other ponds. The maximum concentrations in the ponds ranged from 3 × 105 to 1 × 106 cells L−1 and occurred 30 to 60 days after the start of sampling. In the central marsh, the maximum concentrations were generally around 5 × 103 cells L−1.
Figure 2.
Contours of maximum Alexandrium fundyense cell concentrations during 2011 weekly surveys (log scale); station locations are marked.
In most surveys, maximum cell concentrations were found in the ponds rather than in the central marsh. A lone exception was the 01 June survey, when the peak concentrations occurred in the central estuary near the inlet. In this case, the cells were most likely imported from the Gulf of Maine bloom rather than from within Nauset, consistent with higher salinity and lower temperature in the central marsh (Fig. 3). A similar influx from the Gulf of Maine population was noted in 2009 (Crespo et al. 2011), and in both years, the elevated cell concentrations in the central marsh occurred about a month after the peaks of the blooms in the ponds and had cell concentrations that were several orders of magnitude lower.
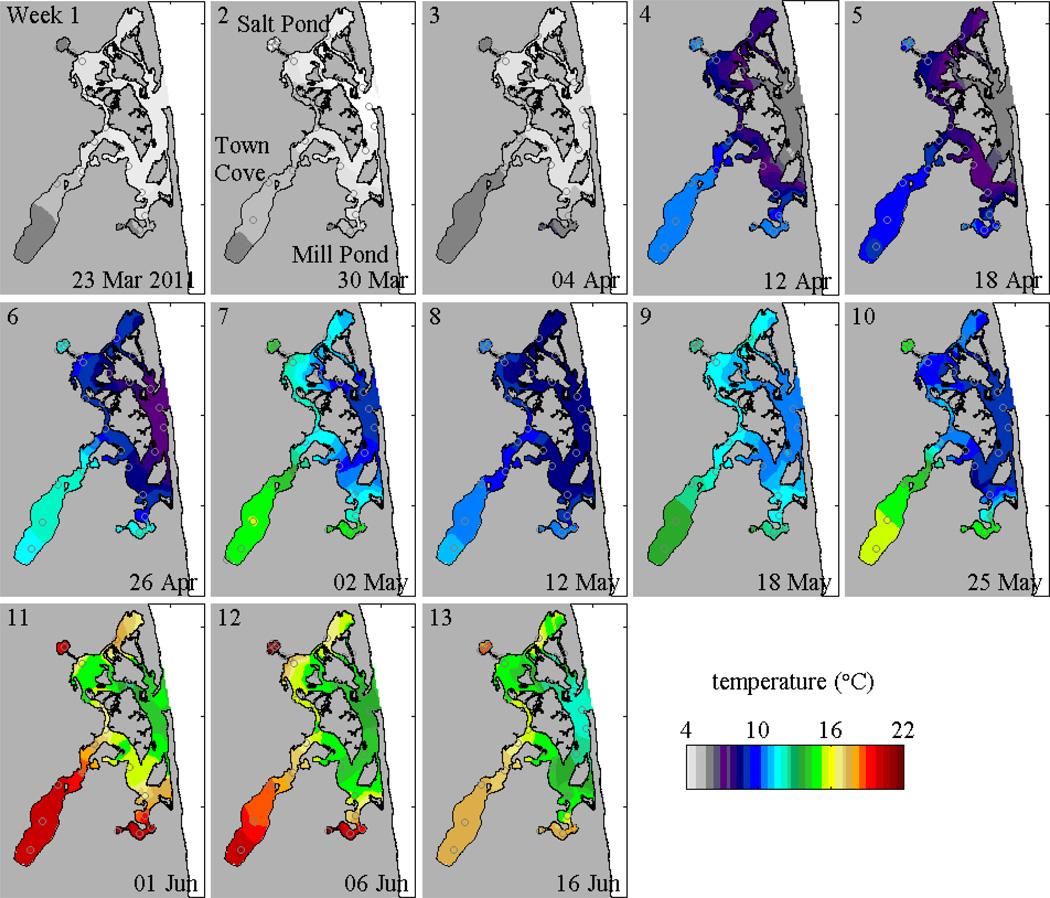
Figure 3.
Contours of near-surface temperature during 2011 weekly surveys; station locations are marked.
Water temperatures during the 2011 bloom reflected the seasonal warming through the spring, but also featured strong gradients between the ponds and the central marsh (Fig. 3). Throughout the bloom, surface waters in the ponds were typically 1–3°C warmer than the central marsh, and the differences were ~5°C by the end of the survey period. The temperature gradients were dramatic in part because the large-scale surveys occurred around high tide, when the intrusion of coastal ocean water into the estuary was maximal. Conditions in the ponds at the edges of the estuary were less influenced by the tidal influx than the central marsh, and thus retained higher temperatures in near-surface water. The extent of intrusion of cooler ocean water depended on the tidal forcing, with a greater influx during spring tides than neaps. Increase in tidal exchange and mixing during spring tides made the surface temperature trends from the weekly surveys non-monotonic. Data from the moored instruments showed that surface temperatures increased during neap tides and decreased during springs, on top of the overall seasonal warming.
Surface salinities in Nauset decreased through the bloom period and the salinity distribution corresponded with an estuarine gradient from the ponds to the ocean (not shown). Freshwater input to Nauset from groundwater and direct precipitation was modest compared to many estuaries with river inputs, but it was sufficient to create stratification in the deep regions of the ponds. The relative contribution of salinity and temperature to the stratification in the ponds changed through the bloom period – salinity was the dominant source of stratification until mid-April, but warming of the surface layer ultimately made temperature an equal, and eventually greater driver of density structure. The freshwater input to the ponds provides an initial stratification and reduction in vertical mixing that accelerates the warming of the surface layer by solar heating, and thus contributes to the temperature gradient between the ponds and the central marsh.
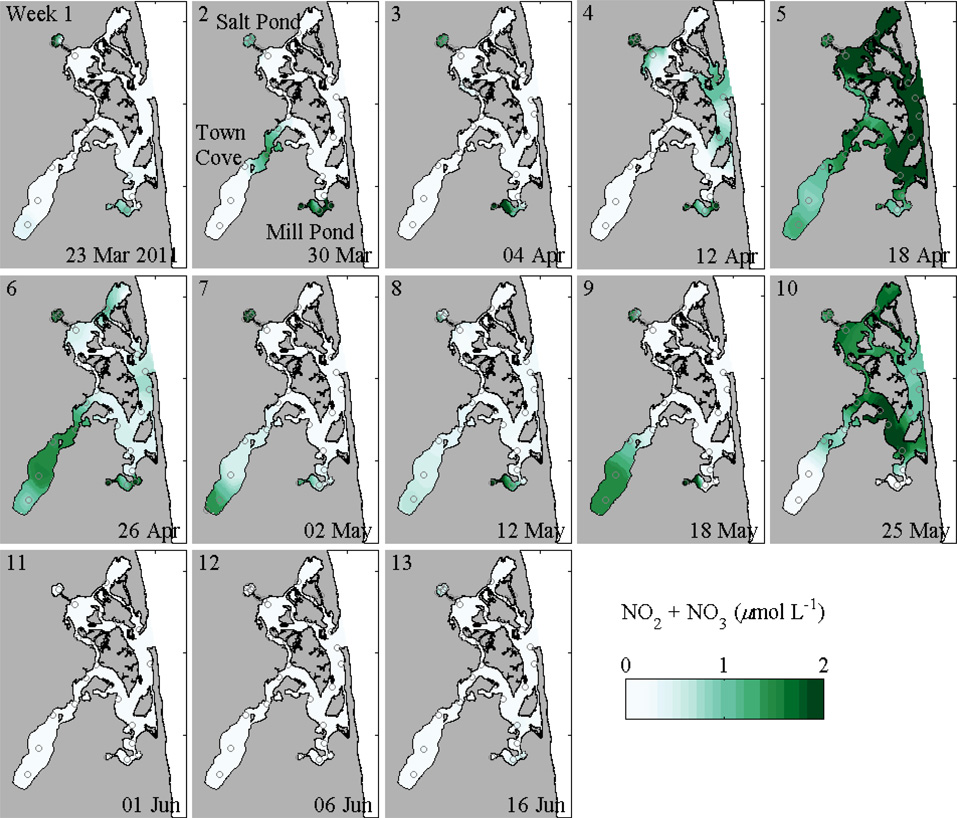
Groundwater fluxes also provide a major source of nutrients to the estuary. In general, nitrate concentrations were elevated in the estuary, but without clear spatial patterns (Fig. 4). At times, the maximum nitrate concentrations occurred in the ponds as would be expected from a groundwater source (e.g., 23 March and 18 May surveys), but in other surveys nitrate concentrations were greater in the central marsh and near the inlet (e.g., 18 April and 25 May). The variable nitrate in the central marsh depended on changes in the coastal ocean, for example due to upwelling events. Groundwater influx may have a more direct effect on the ammonium distributions (not shown). In many surveys, ammonium concentrations were extremely high in the deep parts of Mill Pond and Salt Pond, often exceeding 10 µmol L−1 below the pycnocline and reaching as high as 150 µmol L−1; surface layer concentrations in these ponds were much lower (< 10 µmol L−1). Town Cove is shallower and more weakly stratified, and the maximum concentrations there were ~4 µmol L−1, while in the central marsh ammonium concentrations were typically 1–2 µmol L−1. For both nitrate and ammonium, clear temporal trends were not apparent corresponding either with seasonal changes in forcing or with cell concentrations.
Figure 4.
Contours of maximum nitrate plus nitrite concentrations during 2011 weekly surveys; station locations are marked.
Comparison among years: 2009 – 2012
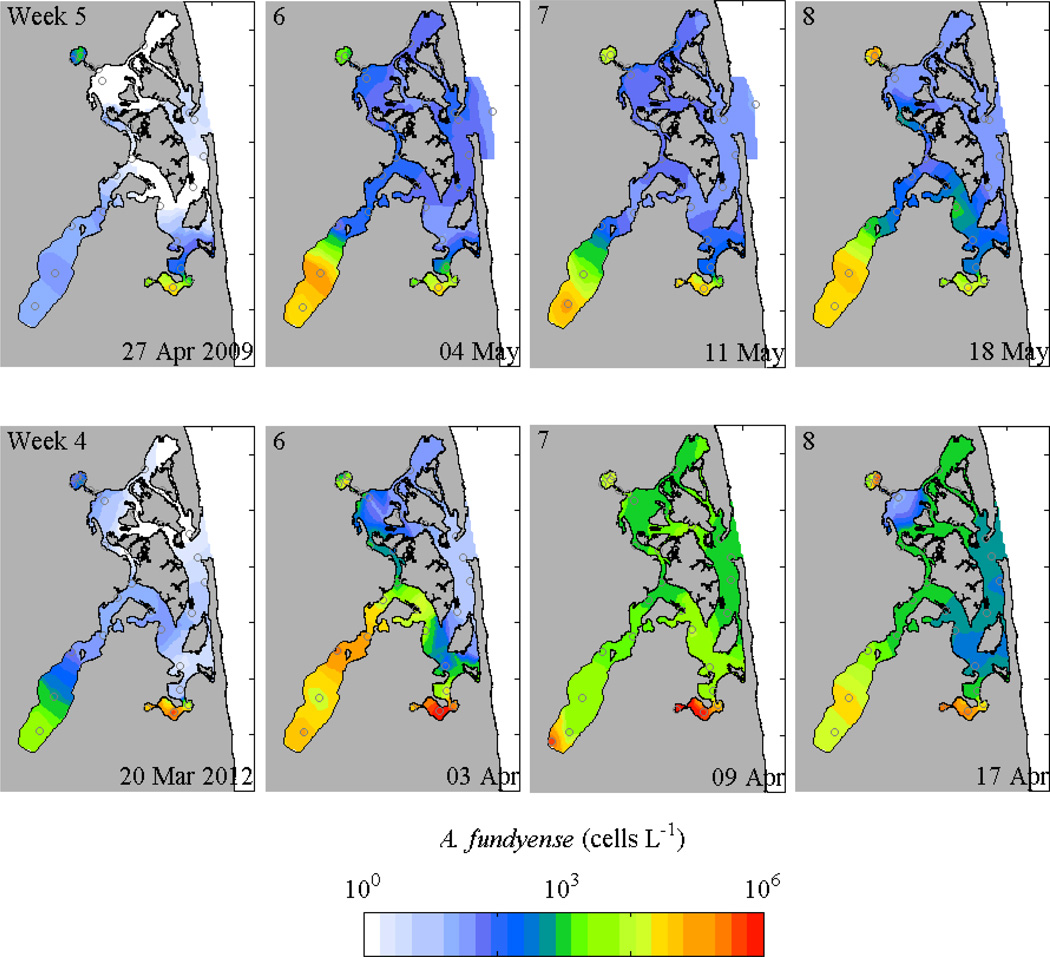
Data from large-scale surveys in 2009, 2011, and 2012 were used to assess variability in the spatial and temporal structure of the A. fundyense bloom in Nauset, and to examine how interannual variability in the estuarine conditions may have contributed to the observed patterns. Cell concentration distributions from large-scale surveys in 2009 and 2012 were largely consistent with the 2011 observations (Fig. 5). In surveys from around the peak of the bloom each year, cell concentrations were orders of magnitude greater in the ponds than in the central estuary. The succession of bloom peaks within the ponds was also consistent among years, with concentrations increasing first in Mill Pond, then Town Cove, and then Salt Pond. Maximum concentrations varied moderately among the years, ranging for example from 7 × 105 to 2 × 106 cells L−1 in Mill Pond and 1 × 105 to 1 × 106 cells L−1 in Town Cove, but in all three years A. fundyense concentrations were much greater than typically observed during blooms in the Gulf of Maine (Townsend et al. 2001) and were sufficiently high to instigate shellfishing closures in Nauset. More notably, the timing of the bloom varied from year to year, with for example, similar cell concentrations during the 2012 bloom occurring more than a month earlier than in 2009.
Figure 5.
Contours of maximum A. fundyense cell concentrations around the peaks of blooms in 2009 and 2012 from weekly surveys (log scale); station locations are marked.
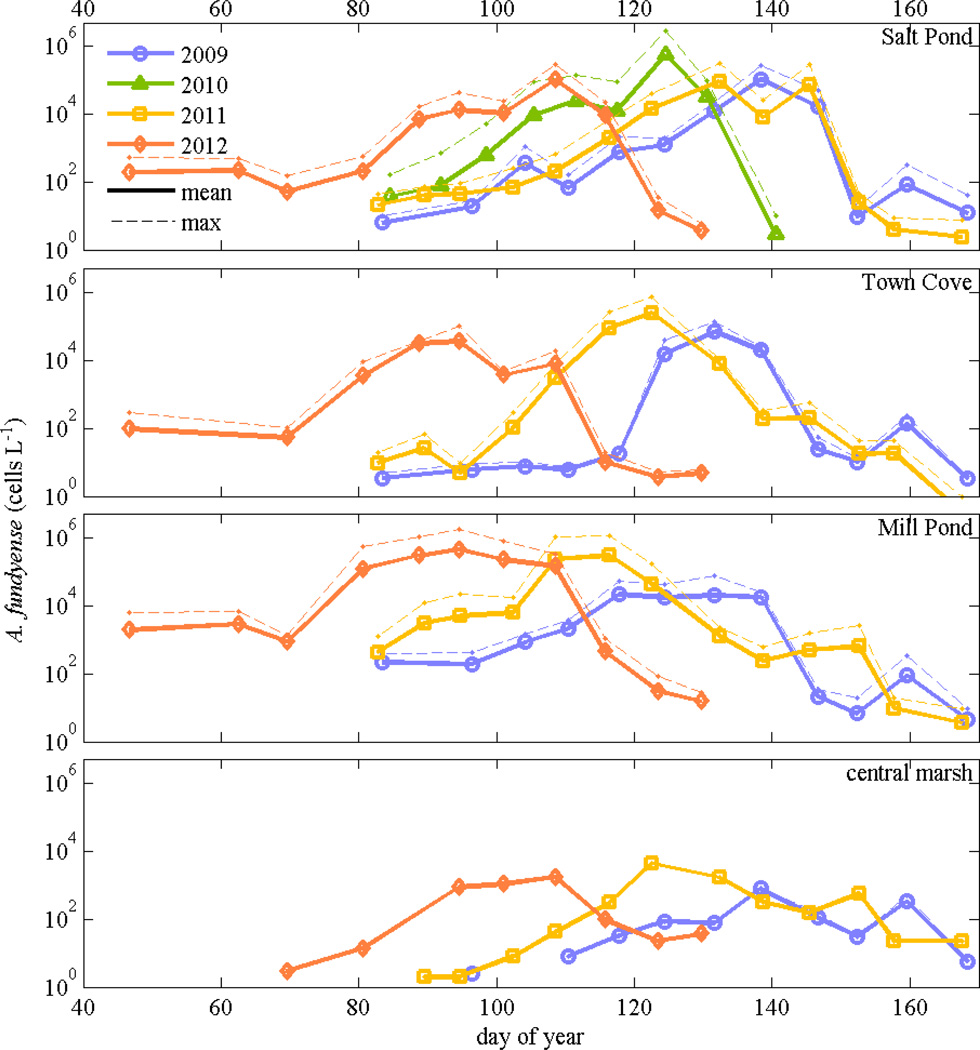
Time series from stations in the center of each of the ponds, plus one station representative of conditions in the central marsh, were compared directly to evaluate the temporal shifts in the bloom development among the ponds and years (Fig. 6). Interannually, the timing of the maximum cell concentrations in a given pond varied by up to 40 days, with the earliest bloom occurring in 2012 and the latest in 2009. Bloom development in Mill Pond tended to lead the bloom in Salt Pond by two to three weeks. Concentrations at the central marsh station were substantially lower than in the ponds, with the maximum concentrations typically occurring between the Town Cove and Salt Pond peaks, consistent with a mixing of exported cells from these two populations.
Figure 6.
Maximum (max) A. fundyense concentration from weekly surveys at stations in Salt Pond, Town Cove, Mill Pond, and the central marsh (Hemenway) in 2009 through 2012; only Salt Pond had observations in 2010.
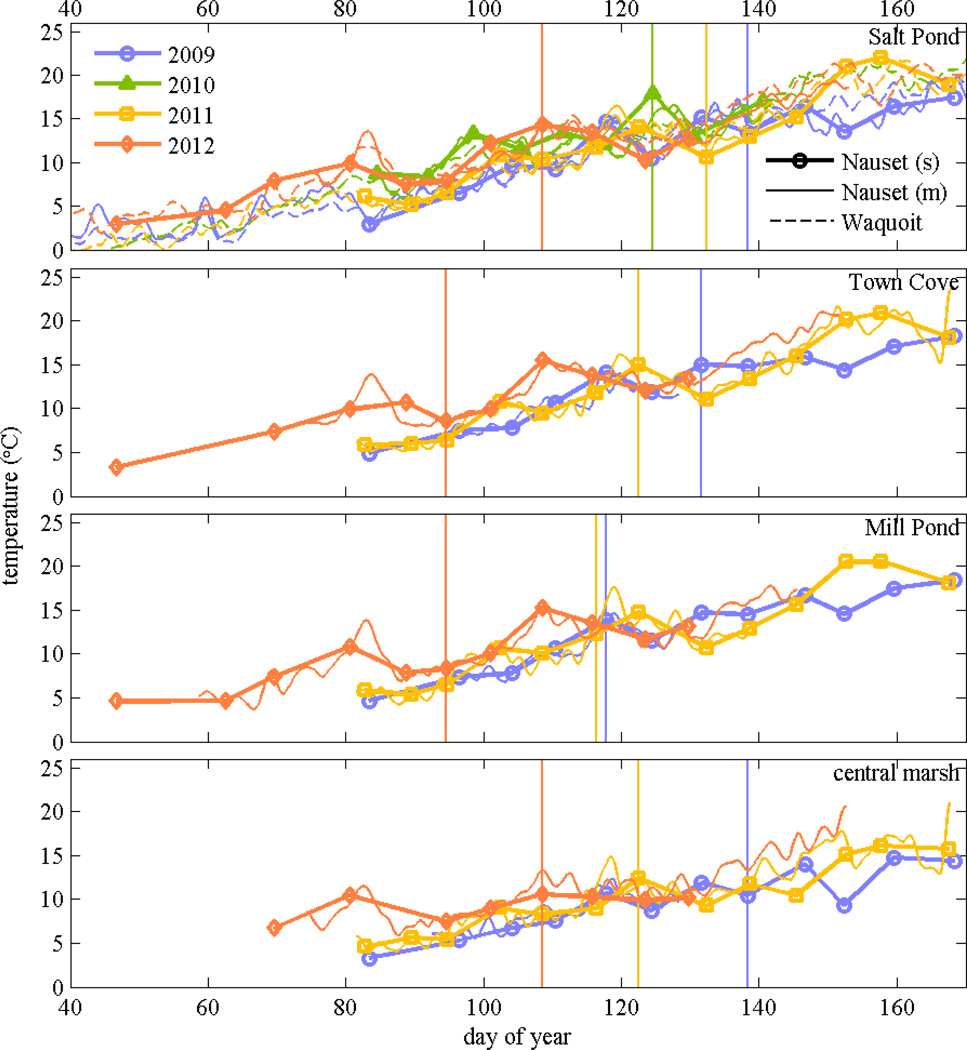
A. fundyense growth rates depend on numerous factors, including temperature, salinity, nutrients, and irradiance (Watras et al. 1982; Flynn et al. 1996; Etheridge and Roesler 2005), so considering variance in these factors among years and ponds may help explain the differences in bloom development. Temperature data from the weekly surveys of the ponds and central marsh are plotted along with continuous temperature time series from Waquoit Bay (Fig. 7). Water temperatures during the early phases of bloom development in 2012 were 2–3°C higher than in previous years, but this difference disappeared by mid-April. Temperatures were slightly warmer in the ponds in 2011 than in 2009, but the differences were smaller and more variable. Generally, the interannual temperature differences found in the weekly Nauset surveys also were observed in Waquoit Bay.
Figure 7.
Near-surface water temperature from stations in Salt Pond, Town Cove, Mill Pond, and the central marsh (Hemenway) from weekly surveys in 2009 through 2012 (s); only Salt Pond had observations in 2010. Also shown are continuous time series from moored temperature sensors near each location (m). In the top panel, dashed lines are moored temperature time series from Waquoit Bay in the same years. Vertical lines denote when maximum cell concentrations were observed at each location.
The temperature differences between ponds were more subtle than the interannual variability in a given pond. Upper Mill Pond had the warmest temperatures based on the CTD profiles during the weekly, full-estuary survey. On average, temperatures of near surface water (< 4 m) in Upper Mill Pond were about 1.0°C higher than in central Mill Pond and Town Cove, and about 2.0°C higher than in Salt Pond. All of the ponds were significantly warmer than the central marsh, which was on average about 1.0°C cooler than Salt Pond due to the greater influence of water from the coastal ocean. The spatial patterns were consistent during the three years that had full estuary surveys, and were statistically significant (p < 0.001) considering either the full bloom period or just the growth phase.
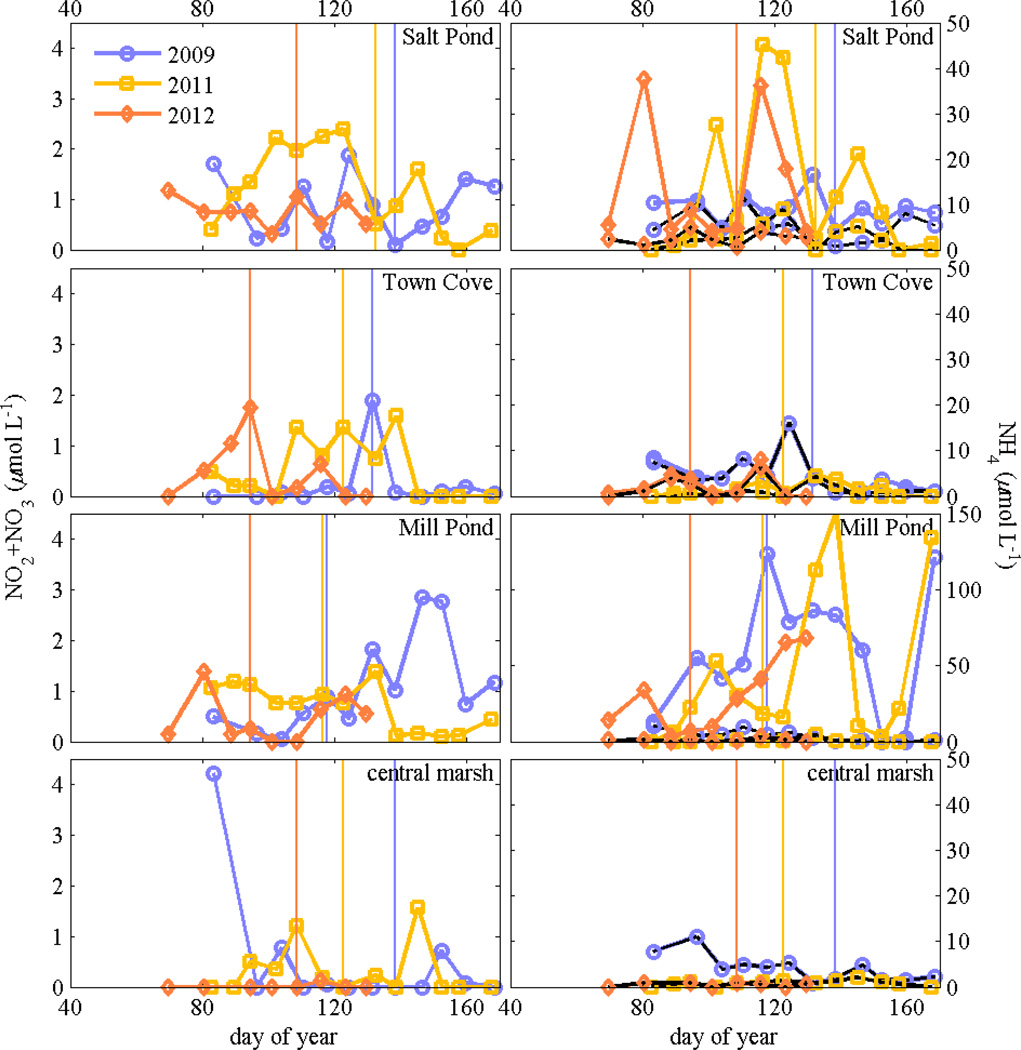
As noted in the full-estuary concentration maps from 2011, spatial and temporal variability in nutrient concentrations did not have any clear correlation with bloom development. Nitrate concentrations were moderately elevated but highly variable among the ponds, and were generally lower in the central marsh (Fig. 8). Ammonium concentrations were much greater, particularly in Salt and Mill Ponds, but also were highly variable and did not correspond with cell concentrations. Variability in surface salinity among ponds and among years was small (~1), and thus did not have a clear relationship with the A. fundyense population.
Figure 8.
Maximum nitrate plus nitrite concentrations (left panels) and ammonium concentrations (right) at stations in Salt Pond, Town Cove, Mill Pond, and the central marsh (Hemenway) from weekly surveys in 2009, 2011, and 2012. Note the scale is different for ammonium in Mill Pond. Minimum ammonium concentrations are also shown with black lines. Vertical lines denote when maximum cell concentrations were observed at each location.
Growth and temperature
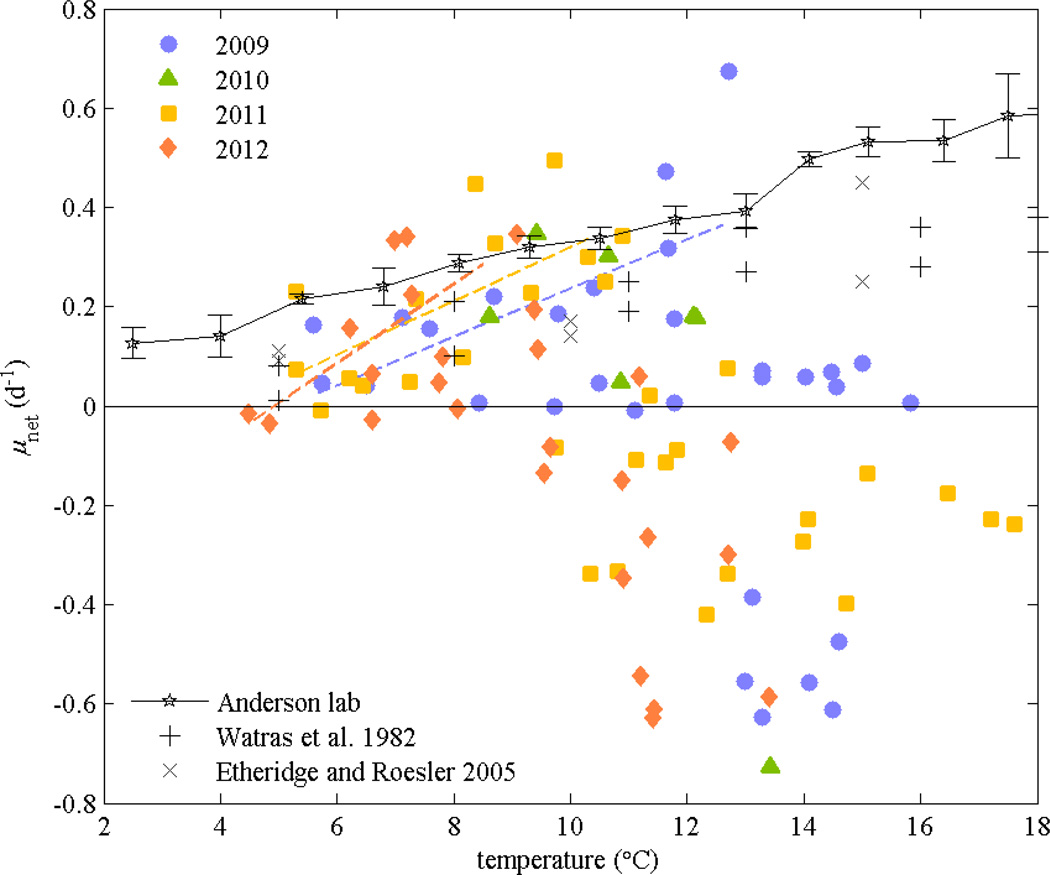
Net growth rates calculated from differences in cell abundance between weekly surveys varied with temperature (the average for the 3-week moving window), increasing approximately linearly up to about 10–13°C, depending on the year (Fig. 9). For comparison, results from previous lab studies of A. fundyense growth rates are also plotted, including isolates from Mill Pond (Watras et al. 1982) and the Gulf of Maine (Etheridge and Roesler 2005; D.M. Anderson unpubl. data). A stepwise linear regression of net growth rates combining the 4 years of data was calculated for the growth phase of the bloom (prior to the maximum cell concentrations). Potential predictor variables that were tested included temperature, salinity, nitrate, ammonium, phosphate, and day of year, as a proxy for irradiance. Of the variables tested, only temperature had a significant relationship (p < 0.05) with net growth rate. The correlation of net growth with temperature using all 4years of data had r2 = 0.30 (p < 0.001). Alternatively, the net growth rates in individual years also had significant linear dependence on temperature, with r2 = 0.38 in 2009 (p = 0.02), 0.46 in 2011 (p = 0.006), and 0.46 in 2012 (p < 0.001). In 2010, the sample size was smaller with data only from Salt Pond, and the correlation with temperature was not significant. The inferred growth rates from the field surveys then are largely consistent with the linear dependence of growth rate in the lab experiments over the range of temperatures in Nauset, indicating that other factors affecting growth such as nutrients, light intensity, or loss due to mortality or advection are small compared with the temperature dependence, or are similar from year to year.
Figure 9.
Net growth rates from observed A. fundyense concentrations vs. temperature in Salt Pond, Town Cove, and Mill Pond from weekly surveys, based on a 3 week moving window. Trend lines are shown from linear regressions for data prior to the bloom peaks when grazing and encystment losses are expected to be minor. For comparison, A. fundyense growth rates from laboratory studies are also shown (Watras et al. 1982; Etheridge and Roesler 2005; D.M. Anderson unpubl. data).
Key similarities and differences among the years were evident in the relationship between temperature and net growth rate. At lower temperatures, the years were essentially indistinguishable, with net growth rates increasing with temperature. The growth rates were notably high, in the range of 0.2 to 0.4 d−1 and similar to the maximum growth rates derived from laboratory studies under ideal conditions that did not include losses due to advection, grazing, or other mortality. Variability among the years was more apparent in the decline of the population, or when μnet was negative, as the timing of the crash did not depend directly on temperature. In 2012 when early season temperatures were warmer and the bloom developed earlier, the transition to negative net growth occurred around 10°C, while the cooler conditions and later bloom during the spring of 2009 had net positive population growth until about 14°C. The temperatures at which net growth became negative in the other two years fell in between these cases.
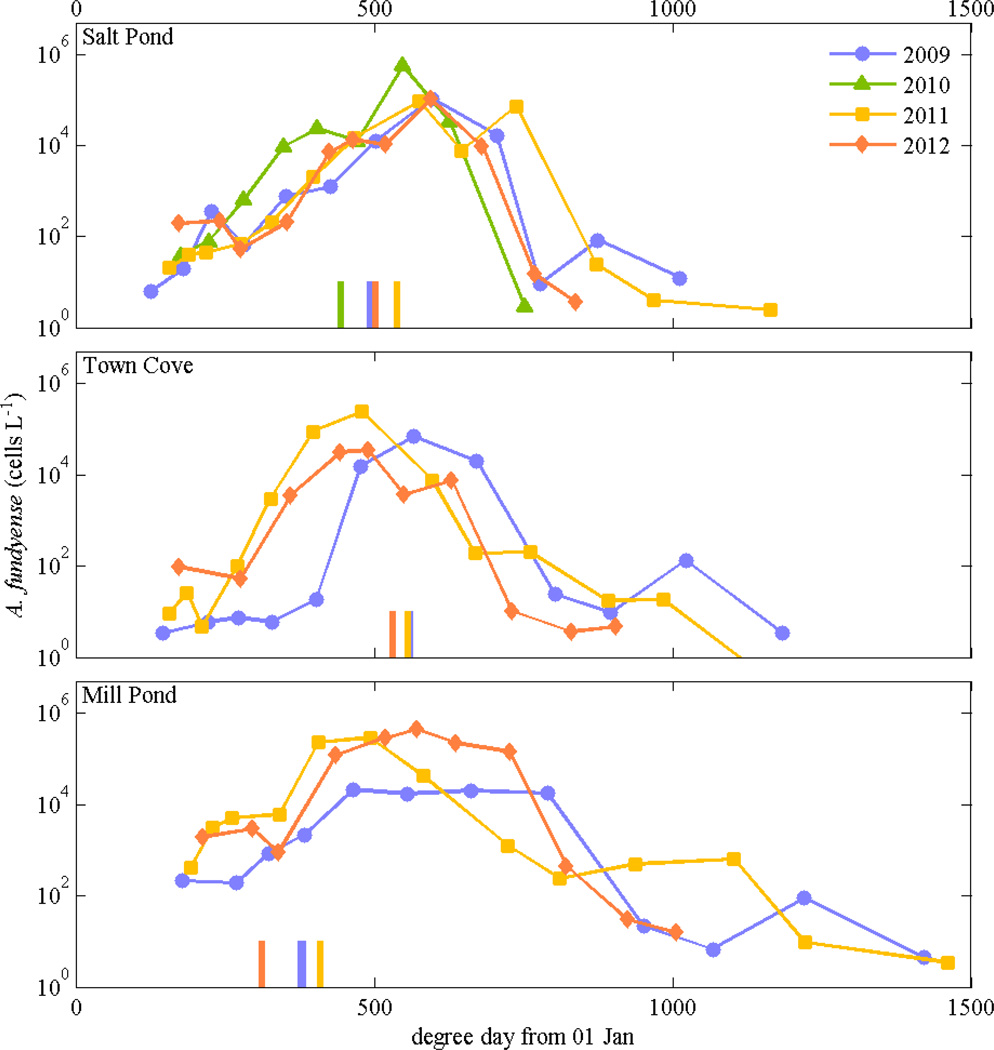
Using the degree day approach, much of the interannual variability in the timing of the A. fundyense bloom in each pond disappears (Fig. 10). Degree days account for the change in the population due both to the duration of the bloom and rate of growth, as a direct comparison of cell concentration vs. temperature alone does not explain the interannual variation. For example, a relatively warm January and February 2012 produced earlier blooms, but the water temperatures at the peaks of the blooms that year were much lower than in previous years (Figs. 5, 6). The ~30 day variation in the timing of the bloom peaks contrasts with similar degree day development times. Considering data from the ponds from all 4 years, degree days in Nauset are linearly correlated with the log of cell concentrations up to the bloom peaks, with r2 = 0.40 (p < 0.001). Alternatively, degree days calculated using only temperatures measured in Waquoit Bay also have a linear correlation with the log of cell concentrations in Nauset, with slightly lower r2 = 0.36 (p < 0.001). A correlation between cell abundance and year day would be expected if the growth rate were constant, rather than linearly dependent on temperature, but in Nauset the correlation with year day (r2 = 0.07, p = 0.049) was much weaker than with degree day. Environmental factors such as nutrients and salinity were not significantly correlated with cell abundance over multiple years individually or in a stepwise linear regression. The degree day metric did not correlate well with A. fundyense abundance in the central marsh because concentrations there depend on advection from the ponds and the coastal ocean rather than local retention and division.
Figure 10.
A. fundyense concentrations in Salt Pond, Town Cove, and Mill Pond vs. degree day, assuming Tbase = 0°C and starting the integration 01 January each year. Degree day was calculated from moored instruments in each pond, plus using temperature data from Waquoit Bay prior to instrument deployment in Nauset. Cell concentrations were from weekly surveys in 2009 through 2012; only Salt Pond had observations in 2010. Vertical bars at the bottom of each plot indicate degree day on the date that toxicity sampling at each location exceeded the regulatory threshold of 80 µg toxin per 100 g mussel tissue (MA Division of Marine Fisheries).
Plotting cell concentration as a function of degree day substantially reduces the differences in phasing of the bloom among the ponds. Concentrations increased first in Mill Pond each year, as temperatures were warmer there than in the other ponds. In particular, the restricted tidal exchange to the Upper Mill Pond subembayment made it typically the warmest and freshest part of the entire estuary (Figs. 1, 3). Upper Mill Pond also had extremely high concentrations of A. fundyense (up to 5 × 106 cells L−1), at times higher than other stations in Mill Pond. In contrast, Salt Pond warmed more slowly and the bloom occurred later than in the other ponds, typically by a couple of weeks. Placing the cell concentrations in degree day space makes the phasing of the blooms similar among the three ponds, albeit with some remaining variability that can be attributed to low sampling frequency and consequently coarse resolution of the blooms’ development phases.
Discussion
Observations from four consecutive years indicate that the salt ponds within the Nauset estuary provide excellent incubators for A. fundyense populations. The bathymetry of deep salt ponds connected through shallow tidal channels and marsh permits limited tidal exchange with the coastal ocean and with each other. Groundwater creates stratification in the ponds and delivers abundant nutrients for growth. Solar heating of the stratified surface layer warms the ponds before the central marsh or coastal ocean, resulting in faster growth rates, and the limited tidal exchange reduces advective losses from the ponds, with population retention further enhanced by the vertical swimming behavior of the cells. Consequently, net growth rates based on the rate of change of the populations in the ponds were high, comparable to growth rates derived from laboratory studies (Fig. 9). Net growth rates increased with temperature as in lab studies, but only up to a point, and most years the population crashed before temperatures reached the levels at which cells began to grow poorly in laboratory studies (typically in excess of 20°C). Furthermore, bloom termination commenced at different temperatures each year.
During the growth phase of the bloom, the temperature dependence of net growth rate was similar among years and among ponds. Consequently, a time series of growing degree days was used to explore the effects of interannual variability and spatial gradients in water temperature on population development (Fig. 10). The results suggest that in the Nauset system, other factors such as nutrients, salinity, and various loss terms are not major sources of interannual variability in the growth of the bloom. This approach collapses the high level of variability in the timing of bloom onset, development, and termination observed within individual ponds across years, and between ponds in the same year (Fig. 10), suggesting that this relatively simple metric for integrated growth could be used as an early-warning indicator or predictor for A. fundyense blooms and shellfish toxicity in Nauset and similar estuaries.
For example, the bloom began in Nauset about 1 month earlier in 2012 than in previous years, so the monitoring program for shellfish toxicity had not begun that year. A rapid response by state officials to sample and subsequently close parts of the estuary to shellfishing occurred based on cell concentrations found in our first large-scale survey that year, but without that population sampling, the early onset might have been missed by routine monitoring. The bloom might have been anticipated more readily with continuous monitoring of water temperature, which is easy, inexpensive, and can readily be automated and communicated to shore.
In the weekly monitoring, shellfish toxicity typically crossed the regulatory threshold of 80 µg saxitoxin per 100 g mussel tissue at a similar number of degree days – around 500 degree days at the Salt Pond and Town Cove monitoring stations, and around 350 degree days in Mill Pond (Fig. 10). Difference between the stations may be due to the exposure of the sampling locations to the spatially heterogeneous cell populations in the ponds. The utility of degree days for shellfish monitoring depends on a correlation between cell abundance and toxicity, which may not always be as direct as in Nauset, so multiple years of physical and biological observations in similarly affected estuaries would be necessary to evaluate whether this approach could be generally applicable. Bloom development in other estuaries may depend on additional environmental factors, as in a lagoon in the western Mediterranean where both water temperature and wind patterns had to be favorable for A. catenella blooms to occur (Laanaia et al. 2013). The degree day approach is most likely to be applicable to blooms where the organism net growth rate increases linearly with temperature, where nutrients and light are sufficiently abundant as to not constrain growth, and where the loss rates due to advective or biological processes are small.
Another noteworthy observation from the net growth rate calculations is that the declining phase of the bloom was even more rapid than the growth phase (Fig. 9). The explanation for the magnitude and timing of the crash remains unclear. Using the degree day calculation described above, the peak of the blooms occurred around degree day 500 each year, and populations declined rapidly thereafter. Numerical models of A. fundyense in the Gulf of Maine have used a temperature-dependent mortality to represent the end of the widespread coastal bloom that occurs there (He et al. 2008). In these Nauset observations however, temperature does not appear to be the primary controlling variable for mortality (Fig. 9). The degree day approach does reasonably scale both the growth and decline phases of the bloom across multiple years (Fig. 10), but these results are not yet linked directly to specific processes. Possible factors for the loss of vegetative cells include predation, parasitism, or encystment. Nutrient limitation does not appear to be a significant factor based on the lack of correlations between nutrient concentrations and cell abundance. The timing of the decline may depend on cell concentrations, which could control rates of parasitism or encystment, or it may be a function of the duration of the bloom. Understanding the processes controlling bloom termination is critical to developing robust predictive models for A. fundyense both in estuaries and in the coastal ocean.
Climate change is anticipated to lead to warmer temperatures during the spring bloom period, such that future A. fundyense blooms in Nauset may look more like 2012 than the previous years. A. fundyense growth began significantly earlier in 2012, but the duration of the bloom and maximum cell concentrations were similar to the other years. Similarly, an analysis of records from 1993 to 2007 in Puget Sound found no increase in the frequency, magnitude, duration, or extent of paralytic shellfish toxin events, but that events were occurring significantly earlier in the year over that period (Moore et al. 2009). The Nauset observations were consistent with these results, in that warmer water earlier in the year did not extend the growing period for the bloom, but rather shifted the point at which the bloom crashed to lower temperatures. Climate change may also alter freshwater inputs to the estuary, which could affect stratification and the retention of cells in the salt ponds. An additional anthropogenic effect on growth rates in Nauset is the introduction of excess nitrogen from the watershed (Giblin and Gaines 1990; Colman and Masterson 2008). Nutrient loading to the estuary has increased in the past few decades with development, and this in combination with bathymetry that efficiently incubates growing cells may be key factors behind the increased occurrence of toxic blooms in Nauset. The results here suggest that monitoring temperature may be a useful means of predicting when in the year those blooms occur.
Acknowledgments
We are grateful to K. Norton and many other members of the Anderson lab for their contributions to the Nauset surveys, as well as to H. Lind and M. O’Connor from the Town of Eastham, D. Farber from the Town of Orleans, and S. Fox, S. Hall, and K. Lee from Cape Cod National Seashore. This work was supported by National Science Foundation Division of Ocean Sciences (OCE) grants OCE-0430724 and OCE-0911031 to DKR and DMA through the Woods Hole Center for Oceans and Human Health, National Park Service Cooperative Agreement H238015504 with DKR and DMA, and by an Environmental Protection Agency Science to Achieve Results graduate fellowship (FP-91688601) to MLB. We thank two anonymous reviewers for thoughtful comments that improved the manuscript.
References
- Anderson DM. Toxic algal blooms and red tides: A global perspective. In: Okaichi D, Anderson DM, Nemoto T, editors. Red tides: Biology, environmental science and toxicology. Elsevier; 1989. pp. 11–16. [Google Scholar]
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern US. Limnology and Oceanography. 1997;42:1009–1022. [Google Scholar]
- Anderson DM, Cembella AD, Hallegraeff M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science. 2012;4:143–176. doi: 10.1146/annurev-marine-120308-081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Chisholm SW, Watras CJ. Importance of life cycle events in the population dynamics of Gonyaulax tamarensis. Marine Biology. 1983;76:179–189. [Google Scholar]
- Anderson DM, Kulis DM, Binder BJ. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: Cyst yield in batch cultures. Journal of Phycology. 1984;20:418–425. [Google Scholar]
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Research II. 2005a;52:2467–2490. [Google Scholar]
- Anderson DM, McGillicuddy DJ, DeGrasse SL, Sellner KG, Bricelj VM, Turner JT, Townsend DW. Harmful algae in the Gulf of Maine: Oceanography, population dynamics, and toxin transfer in the food web. Deep Sea Research II. in press. [Google Scholar]
- Anderson DM, McGillicuddy D, Jr, Townsend D, Turner J. The ecology and oceanography of toxic Alexandrium fundyense blooms in the Gulf of Maine. Deep Sea Research II. 2005b;52:2365–2368. [Google Scholar]
- Anderson DM, Stolzenbach KD. Selective retention of two dinoflagellates in a well-mixed estuarine embayment: The importance of diel vertical migration and surface avoidance. Marine Ecology Progress Series. 1985;25:39–50. [Google Scholar]
- Aubrey DG, Speer PE. A study of non-linear tidal propagation in shallow inlet/estuarine systems Part I: Observations. Estuarine, Coastal and Shelf Science. 1985;21:185–205. [Google Scholar]
- Colman JA, Masterson JP. Transient simulations of nitrogen load for a coastal aquifer and embayment, Cape Cod, MA. Environ. Sci. Technol. 2008;42:207–213. doi: 10.1021/es070638b. [DOI] [PubMed] [Google Scholar]
- Crespo BG, Keafer BA, Ralston DK, Lind H, Farber D, Anderson DM. Dynamics of Alexandrium fundyense blooms and shellfish toxicity in the Nauset Marsh System of Cape Cod (Massachusetts, USA) Harmful Algae. 2011;12:26–38. doi: 10.1016/j.hal.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppley RW. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972;70:1063–1085. [Google Scholar]
- Etheridge SM, Roesler CS. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Research II. 2005;52:2491–2500. [Google Scholar]
- Flynn K, Jones KJ, Flynn KJ. Comparisons among species of Alexandrium (Dinophyceae) grown in nitrogen- or phosphorus-limiting batch culture. Mar. Biol. 1996;126:9–18. [Google Scholar]
- Franks PJS, Anderson DM. Alongshore transport of a toxic phytoplankton bloom in a buoyancy current: Alexandrium tamarense in the Gulf of Maine. Marine Biology. 1992;112:153–164. [Google Scholar]
- Giblin A, Gaines A. Nitrogen inputs to a marine embayment: the importance of groundwater. Biogeochemistry. 1990;10:309–328. [Google Scholar]
- Gillooly JF. Effect of body size and temperature on generation time in zooplankton. J. Plankton Res. 2000;22:241–251. [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Hallegraeff GM. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. Journal of Phycology. 2010;46:220–235. [Google Scholar]
- He R, McGillicuddy DJ, Anderson DM, Keafer BA. Historic 2005 toxic bloom of Alexandrium fundyense in the western Gulf of Maine: 2. Coupled biophysical numerical modeling. J. Geophys. Res. 2008;113:C07040. doi: 10.1029/2007JC004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanaia N, Vaquer A, Fiandrino A, Genovesi B, Pastoureaud A, Cecchi P, Collos Y. Wind and temperature controls on Alexandrium blooms (2000–2007) in Thau lagoon (Western Mediterranean) Harmful Algae. 2013;28:31–36. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Jr, Anderson DM, Keafer BA. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: In-situ observations and numerical modeling. Continental Shelf Research. 2009;29:2069–2082. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly EL, Halanych KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) Journal of Phycology. 2007;43:1329–1338. [Google Scholar]
- Mackas DL, Goldblatt R, Lewis AG. Interdecadal variation in developmental timing of Neocalanus plumchrus populations at Ocean Station P in the subarctic North Pacific. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1878–1893. [Google Scholar]
- McGillicuddy DJ, Anderson DM, Lynch DR, Townsend DW. Mechanisms regulating large-scale seasonal fluctuations in Alexandrium fundyense populations in the Gulf of Maine: Results from a physical–biological model. Deep Sea Research II. 2005;52:2698–2714. [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, Anderson DM. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnology and Oceanography. 2011;56:2411–2426. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster GS, Wilhelm WW. Growing degree-days: one equation, two interpretations. Agricultural and Forest Meteorology. 1997;87:291–300. [Google Scholar]
- Moore SK, Mantua NJ, Hickey BM, Trainer VL. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae. 2009;8:463–477. [Google Scholar]
- Neuheimer AB, Taggart CT. The growing degree-day and fish size-at-age: The overlooked metric. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:375–385. [Google Scholar]
- Portnoy JW, Nowicki BL, Roman CT, Urish DW. The discharge of nitrate-contaminated groundwater from developed shoreline to marsh-fringed estuary. Water Resour. Res. 1998;34:3095–3104. [Google Scholar]
- Rose JM, Caron DA. Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnology and Oceanography. 2007;52:886–895. [Google Scholar]
- Townsend DW, Pettigrew NR, Thomas AC. Offshore blooms of the red tide dinoflagellate Alexandrium sp., in the Gulf of Maine. Continental Shelf Research. 2001;21:347–369. [Google Scholar]
- Wang JY. A critique of the heat unit approach to plant response studies. Ecology. 1960;41:785–790. [Google Scholar]
- Watras CJ, Chisholm SW, Anderson DM. Regulation of growth in an estuarine clone of Gonyaulax tamarensis Lebour: Salinity-dependent temperature responses. Journal of Experimental Marine Biology and Ecology. 1982;62:25–37. [Google Scholar]