Abstract
Human pluripotent stem cells (hPSCs) – including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) – are very promising candidates for cell therapies, tissue engineering, high throughput pharmacology screens, and toxicity testing. These applications require large numbers of high quality cells; however, scalable production of human pluripotent stem cells and their derivatives at a high density and under well-defined conditions has been a challenge. We recently reported a simple, efficient, fully defined, scalable, and good manufacturing practice (GMP) compatible 3D culture system based on a thermoreversible hydrogel for hPSC expansion and differentiation. Here, we describe additional design rationale and characterization of this system. For instance, we have determined that culturing hPSCs as a suspension in a liquid medium can exhibit lower volumetric yields due to cell agglomeration and possible shear force-induced cell loss. By contrast, using hydrogels as 3D scaffolds for culturing hPSCs reduces aggregation and may insulate from shear forces. Additionally, hydrogel-based 3D culture systems can support efficient hPSC expansion and differentiation at a high density if compatible with hPSC biology. Finally, there are considerable opportunities for future development to further enhance hydrogel-based 3D culture systems for producing hPSCs and their progeny.
Keywords: human embryonic stem cells, induced pluripotent stem cells, 3D culture system, thermoreversible hydrogel
Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs)35 and induced human pluripotent stem cells (hiPSCs)34, are being investigated for a broad range of biomedical applications because of their unique characteristics. Not only can they undergo effective long-term expansion in vitro to yield large quantities of cells, but they can also be differentiated into presumably all cell types in the adult body5. Thus, they are promising candidates in cell replacement therapies for various human degenerative diseases or injuries18,28, for generating engineered tissues or organs2, and for drug discovery and toxicity testing7,20.
All of these applications require a large number of cells2,7,20,28. In particular, the patient populations with degenerative diseases/injuries or organ failure are large, with for example ~8 million patients with myocardial infarction (MI), ~1–2.5 million with type I diabetes, and ~1 million with Parkinson’s disease (PD) in the US alone27,30. In addition, to treat an individual with MI, type I diabetes, or PD, approximately 109 surviving cardiomyocytes, 109 β cells, or 105 dopaminergic (DA) neurons are required, respectively30. Furthermore, due to the low survival of transplanted cells in vivo (e.g. ~ 6% DA neurons or 1% cardiomyocytes have survived several months after transplantation in rodents14,15), even more cells will be necessary in reality. In addition, tissue engineering endeavors would require ~109 hepatocytes or cardiomyocytes to create an engineered human liver or heart, respectively2. Finally, for drug discovery, ~1010 cells are necessary to screen a library with a million compounds7, and there are many large chemical, peptide, and nucleotide libraries that can be screened against many types of cells derived from hPSCs41. In summary, a substantial number of hPSCs are necessary for current and future research and development.
Current strategies for producing hPSCs or their derivatives at a large scale generally involve three steps30. First, a working cell bank containing many hPSC aliquots is established and cryopreserved. Second, an aliquot is grown into the desired number of cells through a series of expansions. Finally, these cells are then differentiated into the targeted cell types. An efficient and scalable bioprocess is required for both the expansion and differentiation30. In addition, if the cells are being produced for clinical application, the bioprocess must comply with good manufacturing practices (GMP)36. Currently, the most widely used systems involve the expansion and differentiation of hPSCs on 2D surfaces. Though significant advances have resulted in increasingly well-defined 2D culture systems (including a range of media and substrates), the production of cells on a large scale remains a challange30,38. For instance, at a typical density of ~5,000 DA neurons/cm2 or ~50,000 cardiomyocytes/cm2, ~0.5 km2 or 16 km2 of cell culture surfaces are necessary to contain sufficient numbers of DA neurons or cardiomyocytes to treat PD or MI populations in the US, not to mention the surface area required to expand the parent hPSCs.
Thus, it may be desirable, and even unavoidable, to move from 2D to 3D for the large-scale hPSC production19,30. A number of 3D suspension culture systems have been investigated for hPSC culture during the past decade. Single or small clumps of hPSCs have been suspended and cultured as cell aggregates in liquid medium under continuous stirring or shaking1,6,32,42. Alternatively, hPSCs have been first seeded onto polymeric microspheres coated with matrix proteins and then cultured as a microcarrier suspension in a liquid medium4,22. While these 3D systems have achieved some degrees of success, many challenges have also been reported17. In particular, considerable cell agglomeration, which can lead to cell death or uncontrolled differentiation, is frequently observed in suspension cultures4,17,22. Apoptosis induced by shear forces resulting from the medium flow is also common1,4,6,22,32,42. As a result of such constraints, suspension systems often use low initial seeding densities and result in relatively low cell expansion and volumetric cell yields 17,30. Encapsulating and culturing small clumps of hPSCs in a number of hydrogels have also been studied3,10,29,33. However, limited cell growth has been achieved to date, and uncontrolled differentiation can occur in such 3D culture systems 17,30. In short, cost-effective production of hPSCs or their derivatives on a large scale and under well-defined conditions is very challenging.
An efficient 3D culture system for large-scale hPSC production should exhibit a number of features. First, it should support a high density hPSC culture at a high cell growth rate. Culturing hPSCs at a high density can significantly reduce the space, labor, and material necessary for cell expansion, and is thus highly desirable for large-scale hPSC production. Likewise, the cell growth rate should be close or equal to the highest rate achieved on 2D surfaces. Second, the system should be well-defined such that production is reproducible and compatible with GMP. Third, the system should be simple, scalable, and easy to automate. Finally, it would be desirable to support single cell seeding to “synchronize” the environmental conditions that cells experience. While research has shown that cell dissociation promotes hPSC apoptosis24, single cell seeding offers the potential for more uniform and reproducible expansion and differentiation8,13,21.
We recently developed and reported a simple, well-defined, efficient, scalable 3D culture system, utilizing a thermoreversible hydrogel as a biomaterial scaffold, for both hPSC expansion and differentiation at high density17. In this paper, we describe additional characterization of the system as well as general design rationale for 3D systems that enable hPSC culture at a high density. Briefly, we found that under a typical set of conditions, we were unable to culture hPSCs as a suspension in a liquid medium with high volumetric yields. Substantial cell agglomeration was observed in these suspension cultures. The use of hydrogels as 3D scaffolds for hPSC culture was able to mitigate cell aggregation, and such scaffolds may also isolate cells from shear forces that accompany cell culture agitation and can lead to cytotoxicity1,4,6,22,32,42. Finally, the ability to adapt this system to also support cell differentiation – such as into neural lineages – is a useful feature, and future work may further enhance the ability of the system to promote economical cell expansion as well as differentiation into additional lineages.
Materials and Methods
Reagents
hESC lines H1 and H9 were obtained from WiCell Research Institute. iPS-MSC25 (derived from human mesenchymal stem cells) and iPS-Fib225 (derived from human dermal fibroblasts) were a kind gift from George Q. Daley at Children’s Hospital Boston. Essential 8 medium (E8)5, 0.5 mM EDTA, Accutase, ProLong® Antifade reagents, LIVE/DEAD® Cell Viability staining kit, Click-iT® EdU Alexa Fluor® 594 Imaging Kit and Alkaline Phosphatase Live Stain kit were obtained from Life Technologies. Small molecules Y-27632 (ROCK inhibitor, or RI), SB431542, and LDN193189 were from Selleckchem. Matrigel was obtained from BD Biosciences. PNIPAAm-PEG polymer (Mebiol Gel) was from Cosmo Bio, USA. SCID Beige mice were from Charles River Laboratory. Finally, the following antibodies and dilutions were used: Oct4 and Nanog (Santa Cruz Biotech, 1:100); FOXA2 or HNF3β (Santa Cruz Biotech, 1:200); Nestin (Millipore, 1:200); αSMA (Abcom, 1:200).
Culturing hPSCs on 2D surfaces
hPSCs were cultured on Matrigel-coated plates in E8 and passaged every 4 d with 0.5 mM EDTA. To return hPSCs to 2D surfaces after long term expansion in the 3D hydrogel, hPSC spheroids were incubated with Accutase at 37 °C for 10 min and mechanically dissociated into single cells that were cultured on Matrigel-coated plates in E8 with 10 μM Y-27632 for the first 24 hr.
Expanding hPSCs in suspension in liquid medium
hPSCs maintained on Matrigel-coated plates were incubated with Accutase at 37 °C for 5 min and dissociated into single cells. Single hPSCs were then suspended in mTeSR or E8 medium in low adhesion plates. For dynamic cell cultures, the plate was shaken at 70–90 rpm. To change medium, cells were spun down at 300 g for 3 min before replacing the old medium with fresh medium.
Expanding hPSCs in HA, agarose, or alginate hydrogels
Hyaluronic acids (60–90 kDa, Lifecore, #HA60K-1) were modified with acrylates and crosslinked via UV with 0.05% Irgacure 2959 and cells10,16. The agarose hydrogel was made from low melting temperature agarose (Lonza, #50080)33. The alginate hydrogel was prepared by extruding alginate (Sigma, #A2033) solution with cells into 100 mM CaCl23,29. Cells were encapsulated and cultured for 4 d in medium supplied with ROCK inhibitor for 24 hr or 4 d. On d 4, live/dead cell staining was performed.
Expanding hPSCs in thermoreversible hydrogels
Single cells were mixed with 10% ice-cold PNIPAAm-PEG solution that was then cast on tissue culture plates and incubated at 37 °C for 15 min to form hydrogels. To passage hPSCs, the medium was removed, and the gel was dissociated and dissolved with ice cold PBS for 2 min. Spheroids were collected, incubated with Accutase at 37 °C for 10 min, and dissociated into single cells. Cells were counted with the NucleoCounter NC-200 (Chemometec).
Staining and imaging
Cells on 2D surfaces were fixed with 4% paraformaldehyde (PFA) for 15 min, permeabilized with 0.25% Triton X-100 for 15 min, pre-blocked with 5% goat serum for 1 hr, then incubated with primary antibodies for 2 hr. After 3 washes with PBS, they were incubated with secondary antibodies in 2% BSA for 1 hr. After 3 washes with PBS, cells were imaged. hPSC spheroids were fixed with 4% PFA for 30 min, incubated with PBS + 0.25% Triton X-100 + 5% goat serum + primary antibodies overnight. After 3 washes with PBS, spheroids were incubated with secondary antibodies in 2% BSA for 4 hr. After 3 washes with PBS, cells were mounted with ProLong® Antifade reagent and imaged. All procedures were conducted at room temperature. Live/dead staining, EdU pulse labeling, and alkaline phosphatase staining were conducted with the LIVE/DEAD® Cell Viability staining kit, Click-iT® EdU Alexa Fluor® 594 Imaging Kit, and Alkaline Phosphatase Live Stain kit, respectively.
Embryoid body (EB) differentiation
Single hPSCs were suspended in liquid medium in low adhesion plates for 6 d to form EBs, which were then plated on gelatin-coated plate and cultured for another 6 d before fixation and staining. DMEM + 20% FBS + 10 μM β-mercaptoethanol were used for the EB differentiation.
Teratoma assay
The teratoma assay was approved by the Animal Care and Use Committee of the University of California, Berkeley. 5.0×106 hPSCs were suspended in 50 μl 50% Matrigel and injected subcutaneously in SCID Beige mice. Teratomas were harvested after 6–12 weeks; fixed with 4% PFA for 48 hr; dehydrated with 70%, 95%, 100% ethanol and xylene sequentially; and embedded in paraffin. 10 μm thick sections were cut and stained with hematoxylin and eosin. All procedures were conducted at room temperature.
Statistical analysis
Statistical analyses were conducted using the statistical package Instat (GraphPad Software, La Jolla, CA). For multiple comparisons, the means of triplicate samples were compared using the Tukey multiple comparisons analysis with the alpha level indicated in the figure legend.
Results and Discussion
3D Expansion of hPSCs in static and dynamic liquid cultures
The expansion and differentiation of hPSCs at high density offers the potential to provide uniform culture conditions for large numbers of cells, and to reduce production costs17. The 3D suspension cultures previously reported represent significant advances over prior practice, though they have typically utilized low initial seeding densities and resulted in limited final volumetric cell yields17,30. Some of these 3D systems reached relatively high cell yields, but had other problems, such as slow cell growth, using undefined medium, or uncontrolled differentiation 17,30. To assess final cell density in suspension cultures, single iPS-Fib2s (iPSCs derived from human dermal fibroblasts) were cultured as a suspension in broadly used mTeSR or in the well-defined Essential 8 medium (E8). Cell passaging and seeding as small clusters were previously described17. These experiments were performed in the absence and presence of ROCK inhibitor (RI), which was either added during the first 24 hr or during the entire culture period (4 d). In addition, three initial seeding densities – 2.5×105 (low), 1.0×106 (medium), and 2.5×106 cells/ml (high) – were tested. Finally, the culture was either maintained under static conditions (static suspension culture, Fig. 1) or shaken at a low speed (75–90 rpm, dynamic suspension culture, Fig. 2).
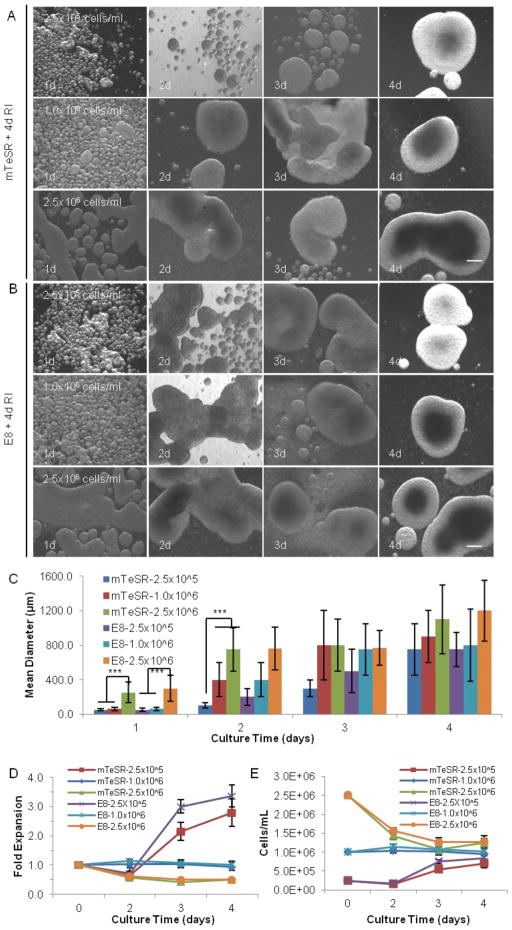
FIGURE 1.
3D static suspension culture. Single iPS-Fib2s were cultured for 4 d in static liquid culture with mTeSR or E8 medium and RI (present for the full 4 d) at low, medium, or high seeding density (2.5×105, 1.0×106, or 2.5×106 cells/ml, respectively). (A and B) Cell morphologies on d 1, 2, 3, and 4 are shown with phase contrast images. (C, D and E) Mean diameter of the hPSC aggregates, fold expansion and cell densities at different days within the 4 d culture period. *** indicates statistical significance at a level of p<0.001. Scale bar: 250 μm.
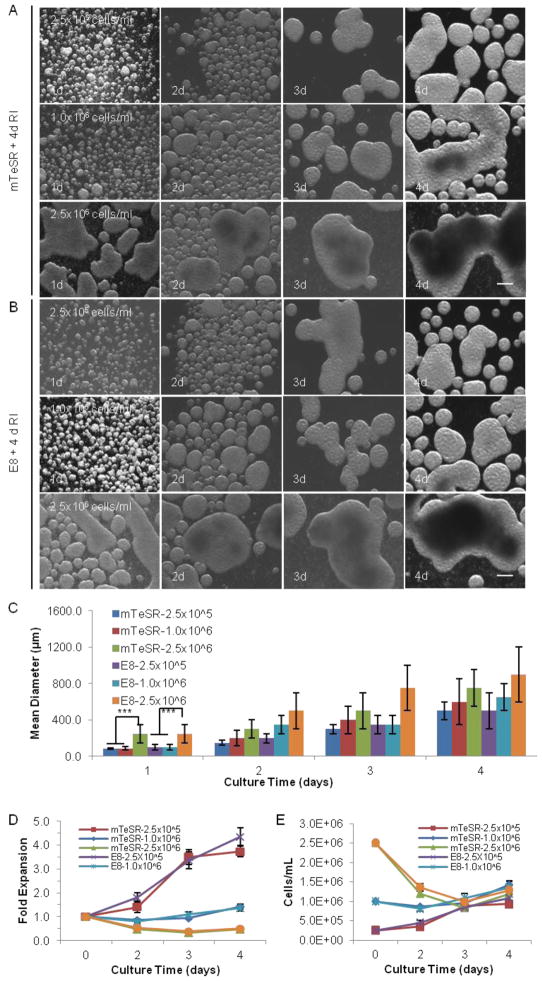
FIGURE 2.
3D dynamic suspension culture. Single iPS-Fib2s were cultured in liquid medium for 4 d in mTeSR or E8 with 4 d of RI at low, medium, or high seeding density (2.5×105, 1.0×106, or 2.5×106 cells/ml, respectively) under 75–90 rpm shaking. (A and B) Cell morphologies on d 1, 2, 3, and 4 are shown with phase contrast images. (C, D and E) Mean diameter of the hPSC aggregates, fold expansion and cell densities at intermediate times within the 4 d culture. *** indicates statistical significance at a level of p<0.001. Scale bar: 250 μm.
For both the static and dynamic liquid cultures, the addition of RI was crucial to the survival and expansion of hPSCs in suspension culture as demonstrated by the observation that no hPSCs survived after 4 d in the absence of RI (data now shown). No significant differences were observed in the final cell yields for the static or dynamic cultures grown in the presence of RI, either during the first 24 hr (data not shown) or during the entire 4 d, which indicates that RI is only important for the survival and expansion of the initial single hPSCs. It should also be noted that the hPSCs quickly associated to form small spherical aggregates in the suspension cultures.
Under static conditions at a low seeding density in liquid suspension culture, the cell number exhibited an increase on d 3 followed by a slower increase on d 4, resulting in a ~3.0-fold total expansion by d 4 with a final cell density at ~0.75×106 cells/ml (Fig. 1). After the first 2 d of such cultures, spheroids were relatively small and uniform. However, large aggregates were observed as the cell density approached 1.0×106 cells/ml on d 3 and 4 (Fig. 1A–C). Under static conditions at a medium seeding density, the cell number was constant during the 4 d, indicating that either no cell growth occurred or that cell growth was equal to cell death (Fig. 1D, E). Considerable cell agglomeration was observed from d 2 to 4 (Fig. 1A–C). Similarly, under static conditions at a high seeding density, excessive agglomeration and a progressive decrease in cell number were observed from d 1 to d 4, resulting in a final cell density of ~1.25×106 cells/ml (Fig. 1). In all cases for static conditions, there were no significant differences between the mTeSR and E8 media (Fig. 1).
The use of dynamic conditions significantly reduced the level of cell agglomeration, especially at the low or medium seeding densities (Fig. 2A–C), presumably due to shear forces either preventing aggregation or dissociating aggregates above a certain size13. Severe cell agglomeration was still observed for the culture with a high seeding density (Fig. 2A–C). Under dynamic conditions at a low seeding density, cell number increased linearly during the 4 d, resulting in a ~4.0-fold final expansion and a final density of ~1.0×106 cells/ml (Fig. 2D, E). In the case of the medium seeding density, a slight increase in the cell number was observed, resulting in a final density of ~1.25×106 cells/ml (Fig. 2D, E). Finally, at high seeding density, the cell density decreased to ~1.25×106 cells/ml at end of the 4 d period (Fig. 2D, E). Again, no significant differences were observed when mTeSR or E8 was used as the medium.
In summary, hPSCs tend to aggregate due to strong cell-cell interactions31. Culture agitation can reduce cell agglomeration but under the conditions explored here does not dramatically improve the cell yield. Regardless of the initial seeding density, the final volumetric yield for static or dynamic 3D suspension culture was ~1.0–1.5×106 cells/ml (Fig. 1, 2). Thus, complementary approaches should be explored to achieve high hPSC densities.
Expanding hPSCs in hydrogels
It is possible that encompassing hPSCs within a physical medium or barrier may prevent excessive cell agglomeration and isolate hPSCs from shear forces generated by medium flow. Based on prior literature that encapsulated hESCs within several materials, we investigated whether hydrogels could enable high density cultures or support single hPSCs in mTeSR or the well-defined E8 medium, and in the presence of RI. Chemically crosslinked hyarulonic acid (HA)10,16, physically associated agarose33, and ionically associated alginate hydrogels3,29 were prepared as described. In HA hydrogels, no cell expansion or spheroids were observed under the conditions and liquid medium used, and the majority of cells were nonviable on d 4 (Fig. 3). In agarose hydrogels, most of the cells remained viable, but no cell expansion or spheroid formation was observed here (Fig. 3). Finally, significant cell death was observed in alginate hydrogels when the RI was only present during the first 24 hr in either mTeSR or E8 media. However, while only sporadic spheroids were observed in the hydrogel with mTeSR, a significant number of spheroids formed in this hydrogel in the presence of the E8 medium when RI was supplied for 4 d. In short, these hydrogels successfully prevented cell agglomeration; however, cell growth in these gels under the defined conditions used was limited.
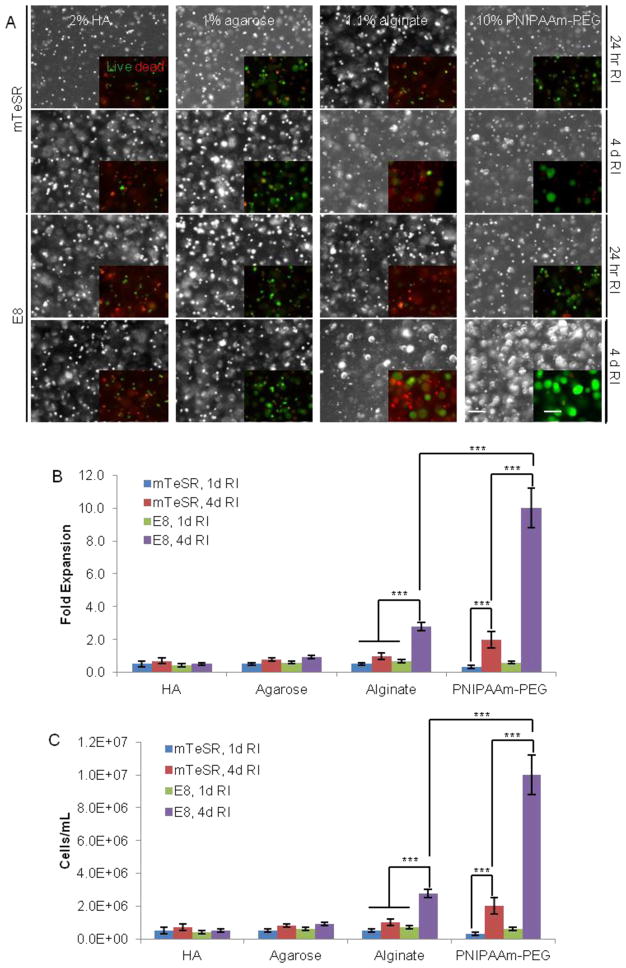
FIGURE 3.
Culturing iPS-Fib2s in various hydrogels with mTeSR or E8 and RI at medium seeding density. Cells were encapsulated and cultured for 4 d. Medium was supplied with RI for 24 hr or 4 d. On d 4, live/dead cell staining was conducted to evaluate the viability. (A) Phase contrast images and live dead cell staining showing the cell morphologies and viability. (B and C) Fold expansion and final cell density for hPSCs over d 4 in various hydrogels. *** indicates statistical significance at a level of p<0.001. Scale bar: 250 μm.
While the properties of the materials varied substantially, several suggestions may be gleaned from this experiment (Fig. 3). First, hPSCs grew better in the physically or ionically associated hydrogels that were tested here. The storage modulus for the HA, agarose, and alginate hydrogels was 3600, 3111, and 2100 Pa, respectively17. In addition to being slightly softer, the latter physically associated gels may also offer cells the capacity to forcibly rearrange the gel during cell and spheroid expansion. Second, compared with mTeSR, the well-defined E8 medium supports better hPSCs growth in hydrogels. Third, supplying RI for the first 24 hr is insufficient to support the survival and proliferation of single hPSCs in such materials. However, the fact that a ~3.0-fold expansion was achieved in the alginate hydrogel with the E8 medium and 4 d RI suggests that some hydrogels may be promising as scaffolds for hPSC culture (Fig. 3). It may thus be worthwhile to explore additional hydrogel materials.
Expanding hPSCs in thermoreversible hydrogels
We investigated the use of thermoreversible hydrogels as scaffolds for 3D hPSC culture. Thermoreversible hydrogels have the following features that make them attractive for the 3D hPSC culture: (A) they can be synthetically generated on a large scale to enhance reproducibility and reduce material costs; (B) they can be chemically well-defined and thus compatible with GMP; (C) they can be biocompatible and have low cytotoxicity; (D) they rapidly respond to temperature changes, which makes it possible to harvest or passage cells by simply changing the temperature from 4 to 37 °C; and (E) they can be processed into fibers or spheres through extrusion or emulsion for suspension culture in large bioreactors. We studied a number of “home-made” and commercially available thermoreversible hydrogels in a prior study17, and additional results reported here indicate that the 6–10% PNIPAAm-PEG hydrogels (Mebiol Gel) are able to support the survival, proliferation and pluripotency of single hPSCs.
6–10% aqueous PNIPAAm-PEG solutions exhibit low viscosity, allowing for facile and homogenous mixing with cells. In particular, they form soft and elastic hydrogels with G′ in the range of 300 to 1000 Pa upon heating to 22 °C within 5 min17, and they can be re-liquefied by cooling to 4 °C within 5 min in the culture conditions we used. As with the prior materials, we observed that hPSC growth is sensitive to the duration of RI presence and the type of medium. No cell growth was found when RI was added for only the first 24 hr. However, with the 4 d RI treatment, a 2- or 10-fold expansion was achieved in mTeSR or E8 medium, respectively (Fig. 3). After a systematic optimization with using a factorial designed experiment as we recently reported17, a system with a 8–10% PNIPAAm-PEG, well-defined E8, a 4 d RI treatment, and a medium seeding density were found to support approximately a 10.0- or 20.0-fold cell expansion in a single 4 d or 5 d passage, with doubling times (~27.7 hr) close to that on 2D surfaces (~27.0 hr).
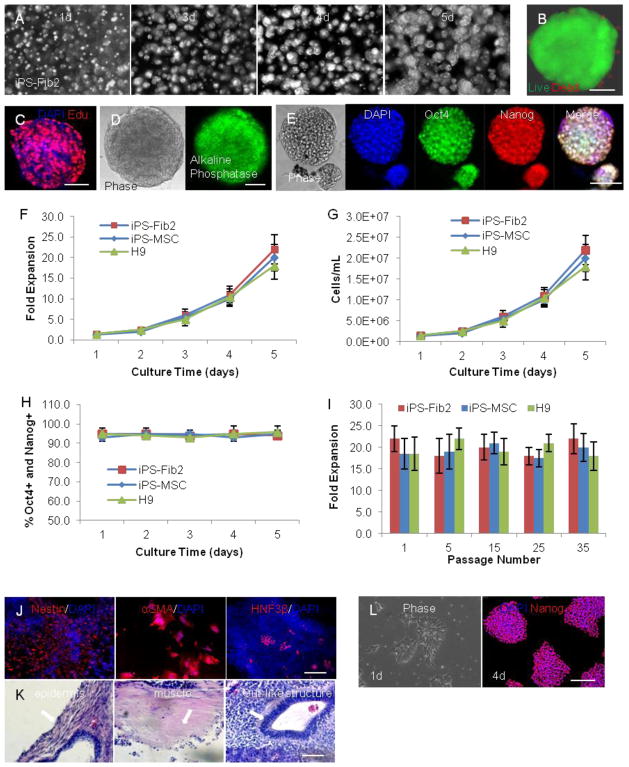
Using this optimized system, single hPSCs rapidly expanded and formed uniform spheroids without cell agglomeration (Fig. 4A). In addition, live/dead cell staining revealed that the majority of cells in the gel were viable (Fig. 4B). Also, uniform EdU pulse labeling was observed within spheroids, indicating efficient mass transport (e.g., nutrients, growth factors, and O2) within the hydrogel and the spheroids (Fig. 4C). Furthermore, the majority of cells in the gel expressed pluripotency markers, such as alkaline phosphatase, Oct4, and Nanog (Fig. 4D, E). We also found this system could support long-term culture for multiple hPSC lines, including iPS-Fib2s, iPS-MSCs, and H9s (H1 cells growth slightly slower17). Over a 5 d culture of such cells, the hPSCs exhibited a ~1.0, 2.5, 6.0, 10.0, and 20.0-fold expansion over d 1, 2, 3, 4, and 5, respectively (Fig. 4F), resulting a final density of ~2.0×107 cells/ml (Fig. 4G). Importantly, consistent ~20.0-fold expansions were achieved for each passage (5 d) during long-term serial expansion (Fig. 4I), and both Oct4 and Nanog expression was maintained at ~95% during the culture (Fig. 4H). Lastly, no significant differences in performance were observed between these 3 cell lines.
FIGURE 4.
Expanding hPSCs in the 3D thermoreversible hydrogel. Single hPSCs were encapsulated and cultured in 10% PNIPAAm-PEG hydrogel in E8 with RI for 5 d with daily medium change. (A) Phase contrast images showing the growing spheroids on d 1, 3, 4, and 5 within the hydrogel. (B) Live/dead cell staining revealed that the majority of cells were viable, and (C) uniform EdU pulse labeling was seen across the spheroid. Spheroids on d 5 are shown in (B, C). The majority of cells in the spheroids expressed the pluripotency markers alkaline phosphatase (D), as well as Oct4 and Nanog (E). Quantification of the expansion (F), cell density (G), and Oct4 and Nanog (H) expression during a 5 d culture. (I) Long term, serial expansions of iPS-Fib2, iPS-MSC, and H9 cells could be achieved in the 3D thermoreversible hydrogel. The fold expansion at passages 1, 5, 15, 25, and 35 are shown. Passaging was conducted every 5 d. (J) EB differentiation in vitro. iPS-Fib2s were expanded in the 3D hydrogel for 25 passages and harvested for EB differentiation. Expression of markers indicating differentiation into the three germ layers (e.g. ectoderm: Nestin; mesoderm: αSMA; and endoderm: HNF3β) were found. (K) Teratoma formation in vivo. iPS-Fib2s were expanded in the 3D hydrogel for 25 passages and harvested for teratoma analysis. Structures from all 3 germ layers (e.g. ectoderm: epidermis; mesoderm: muscle; and endoderm: gut-like-structure) (arrows) were found in tissue sections with hematoxylin and eosin staining. (L) iPS-Fib2s were cultured in the 3D gel for 10 passages and replated on 2D surfaces. Phase contrast image (1d) and Oct4 staining (4d) for iPS-Fib2s on 2D surfaces are shown. Scale bar: (B, C) 50 μm, (D, E, K) 100 μm and (J, L) 200 μm.
Finally, we assessed functional maintenance of pluripotency using in vitro EB differentiation and in vivo teratoma formation. Endodermal (HNF3β), mesodermal (αSMA), and ectodermal (Nestin) markers were observed in all EB differentiations from the three cell lines following 25 passages in the 3D hydrogel system (Fig. 4J). In addition, all hPSC lines formed teratomas in vivo, including epidermis from the ectoderm, muscle from the mesoderm, and gut-like structure from the endoderm (Fig. 4K). Moreover, karyotypes were normal for these hPSCs after long-term culture within the gel17. hPSCs cultured in the 3D hydrogel for long term could also be returned to conventional 2D surfaces (Fig. 4L). Finally, we the 3D culture system can efficiently support directed differentiation into multiple cell types17.
Conclusions
In summary, we developed a simple, well-defined and scalable 3D culture system for rapid hPSC expansion and efficient differentiation at high densities17. When cultured as a suspension in liquid medium under the reported conditions, there was a limit to the maximum volumetric cell yield that could be achieved due to substantial cell agglomeration and potential shear force induced cell apoptosis8,13,21. In contrast, the use of hydrogels as scaffolds for the hPSC culture can prevent cell agglomeration and may isolate cells from shear forces.
However, to achieve efficient hPSC expansion at a high density, a hydrogel-based 3D system (including the scaffold and the medium) must not only overcome engineering challenges, but also support certain aspects of hPSC biology17. For example, both hESCs and iPSCs are epiblast-like pluripotent stem cells whose survival and proliferation depends on multiple extracellular cues24,26. Soluble protein factors, such as FGF-2 and TGF-β, signal through a variety of pathways to support their survival, proliferation, and pluripotency12,37,39. Integrin-mediated cell-matrix interactions are also important40. Likewise, cadherin-mediated cell-cell contacts are key for hPSC survival and proliferation23,24. Dissociating hPSC colonies into single cells as a result leads to Abr-dependent RhoA and ROCK activation, followed by downstream actomyosin hyperactivation and apoptosis23,24. Other environmental stresses (e.g. mechanical forces11,30 and redox conditions5) can also impact their function.
To support cell viability and growth, a number of conditions were required. RI was necessary during the entire culture period to prevent the dissociation-induced cell death for single cultured hPSCs. In addition, physically associated and soft hydrogels may enable the force generated by the expanding hPSCs to deform the scaffold to create sufficient space for the growing cells. Furthermore, the hydrogel should be sufficiently porous to support efficient transport of nutrients, O2, and protein factors. Finally, the well-defined E8 medium may be favorable for expansion, as β-mercaptoethanol in mTeSR medium may negatively interact with 3D growth in the scaffold via unknown mechanisms, resulting in additional stresses that inhibit hPSC survival and proliferation17.
The reported system demonstrates promising results for the large-scale hPSC expansion and differentiation. In addition, future work will enable further enhancement of hPSC expansion and differentiation at various scales. First, characterization of the molecular mechanisms by which the 3D culture system enhances hPSC survival and rapid growth may enable additional improvement. Second, the system was shown to support the expansion and differentiation of 4 hPSC lines17, and additional work will extend its utility to further lines such as patient-specific iPSCs and potentially also adult stem cells. Also, numerous protocols are being developed for the directed differentiation of hPSCs into various cell types, and these can potentially be adapted to 3D culture. Third, enhancing cell culture economics will further benefit the system. hPSC expansion and differentiation media contain multiple, costly recombinant protein factors, and identifying small molecule substitutes or engineering systems for higher potency protein factor presentation can be pursued to reduce the cost. Fourth, processing thermoreversible hydrogels into formats that can be suspended in a liquid medium – such as spheres or fibers – will be necessary to incorporate these hydrogels into various types of existing or new bioreactors for the large-scale production of hPSCs or their derivatives, including automated bioreactors with hydrogels. Finally, hPSC biology is a rapidly evolving field, and this modular system (the material and the medium) can evolve in parallel to harness this new knowledge9. We thus anticipate that thermoreversible hydrogel systems can contribute to resolving significant challenges that currently limit the use of hPSCs in many biomedical applications.
Acknowledgments
This work was supported by California Institute of Regenerative Medicine grant RT2-02022, a California Institute for Regenerative Medicine training grant T1-00007 fellowship (to Y.L.), and NIH 1R01ES020903-01.
Footnotes
Conflicts of interest
Yuguo Lei, Daeun Jeong, Jifang Xiao and David V. Schaffer declare that they have no conflicts of interest.
Ethical standings
All animal studies were carried out in accordance with NIH and University of California, Berkeley guidelines, and approved by the Animal Care and Use Committee of the University of California, Berkeley. No human studies were carried out by the authors for this article.
References
- 1.Amit M, Laevsky I, Miropolsky Y, Shariki K, Peri M, Itskovitz-Eldor J. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat Protoc. 2011;6:572–9. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chayosumrit M, Tuch B, Sidhu K. Biomaterials Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials. 2010;31:505–514. doi: 10.1016/j.biomaterials.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 4.Chen AK, et al. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res. 2011;7:97–111. doi: 10.1016/j.scr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen VC, et al. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012;8:388–402. doi: 10.1016/j.scr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Desbordes SC, Studer L. Adapting human pluripotent stem cells to high-throughput and high-content screening. Nat Protoc. 2012;8:111–30. doi: 10.1038/nprot.2012.139. [DOI] [PubMed] [Google Scholar]
- 8.Fok EYL, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–42. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 9.Gafni O, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 10.Gerecht S, Burdick Ja, Ferreira LS, Townsend Sa, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh MH, Nguyen HT. Molecular mechanism of apoptosis induced by mechanical forces. Int Rev Cytol. 2005;245:45–90. doi: 10.1016/S0074-7696(05)45003-2. [DOI] [PubMed] [Google Scholar]
- 12.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–82. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 13.Kehoe DE, Jing D, Lock LT, Tzanakakis ES, DPh Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A. 2010;16 doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriks S, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–51. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laflamme Ma, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 16.Lei Y, Gojgini S, Lam J, Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci U S A. 2013:1–10. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 19.McDevitt TC, Palecek SP. Innovation in the culture and derivation of pluripotent human stem cells. Curr Opin Biotechnol. 2008;19:527–33. doi: 10.1016/j.copbio.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeish J. Embryonic stem cells in drug discovery. Nat Rev Drug Discov. 2004;3:70–80. doi: 10.1038/nrd1281. [DOI] [PubMed] [Google Scholar]
- 21.Mohamet L, Lea ML, Ward CM. Abrogation of E-cadherin-mediated cellular aggregation allows proliferation of pluripotent mouse embryonic stem cells in shake flask bioreactors. PLoS One. 2010;5:e12921. doi: 10.1371/journal.pone.0012921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie Y, Bergendahl V, Hei DJ, Jones JMPS. Scalable Culture and Cryopreservation of Human Embryonic Stem Cells on Microcarriers. Biotechnol Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohgushi M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–39. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21:274–82. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 26.Peerani R, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–55. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz TC, et al. A Scalable System for Production of Functional Pancreatic Progenitors from Human Embryonic Stem Cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serra M, et al. Microencapsulation technology: a powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS One. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–58. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Mok P, Balakrishnan T, Rahmat SNB, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165–79. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Steiner D, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol. 2010;28:361–4. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 33.Stenberg J, Elovsson M, Strehl R, Kilmare E, Hyllner J, Lindahl A. Sustained embryoid body formation and culture in a non-laborious three dimensional culture system for human embryonic stem cells. Cytotechnology. 2011;63:227–37. doi: 10.1007/s10616-011-9344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Thomson Ja. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17:R48–53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- 37.Vallier L, Alexander M, Pedersen Ra. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 38.Villa-Diaz LG, Ross aM, Lahann J, Krebsbach PH. The Evolution of Human Pluripotent Stem Cell Culture: From Feeder Cells to Synthetic Coatings. Stem Cells. 2012;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu RH, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–34. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zang R, Li D, Tang I, Wang J, Yang S. Cell-Based Assays in High-Throughput Screening for Drug Discovery. Int J Biotechnol. 2012;1:31–51. [Google Scholar]
- 42.Zweigerdt R, Olmer R, Singh H, Haverich A, Martin U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc. 2011;6:689–700. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]