Abstract
Purpose
For select men with low-risk prostate cancer, active surveillance (AS) is more often being considered a management strategy. In a multicenter retrospective study we evaluated the actuarial rates and predictors of remaining on AS, incidence of cancer progression, and pathologic findings of delayed radical prostatectomy.
Methods
A cohort of 262 men from four institutions met the following inclusion criteria: age ≤75, PSA ≤10 ng/ml, clinical stage T1-T2a, biopsy Gleason sum ≤6, ≤3 positive cores at diagnostic biopsy, a repeat biopsy before AS, and no treatment for six months following the repeat biopsy. AS started on the date of the second biopsy. Actuarial rates of remaining on AS were calculated and univariate Cox regression used to assess predictors of discontinuing AS.
Results
With a median follow-up of 29 months 43 patients ultimately received active treatment. The two and five-year probabilities of remaining on AS were 91% and 75%, respectively. Patients with cancer on the second biopsy (HR=2.23; 95% CI: 1.23–4.06; p=0.007) and a higher number of cancerous cores from the two biopsies combined (p=0.002) were more likely to undergo treatment. Age, PSA, clinical stage, prostate volume, and number of total biopsy cores sampled were not predictive of outcome. One patient developed skeletal metastases 38 months after starting AS. Of the 43 patients undergoing delayed treatment, 41 (95%) are without disease progression at a median of 23 months following treatment.
Conclusions
With a median follow-up of 29 months, AS for select patients appears to be safe and associated with a low risk of systemic progression. Cancer at restaging biopsy and a higher total number of cancerous cores are associated with a lower likelihood of remaining on AS. A restaging biopsy should be strongly considered to finalize eligibility for AS.
Keywords: prostate cancer, prostate biopsy, watchful waiting, active surveillance
Introduction
The introduction of PSA-based cancer screening, a tendency for lowering PSA thresholds to trigger a biopsy, and more numerous cores taken per biopsy session have contributed to 40% more men diagnosed annually with prostate cancer compared to 19851. Nearly 50% of these cancers have biologic characteristics associated with a low risk of cancer progression2, and among patients electing radical prostatectomy (RP) up to 30% harbor indolent features consistent with an exceedingly low risk of disease recurrence3.
Due to the stage migration of prostate cancer, the potential for patients to undergo unnecessary treatment, and the risk of treatment-related morbidity, there has been an increased interest in management strategies that offer the possibility of delaying,4 obviating5, or minimizing the impact of treatment6. One such strategy is active surveillance (AS) with selective delayed intervention. The management objectives are: 1) appropriate selection of patients to safely avoid radical treatment and its attendant potential for morbidity, 2) regular and rigorous monitoring of the cancer via physical examination, PSA, biopsies, and imaging, and 3) initiation of treatment with curative intent at any clinical, pathologic, or radiographic evidence of disease progression.
Multiple single-institution series suggest that a trial of active surveillance (AS) for select patients maintains urinary and sexual function and does not appreciably compromise disease-specific outcomes nor the ability for a delayed curative intervention4, 7–10. To better understand the durability and oncologic outcomes of AS, we compiled a multi-institutional cohort of patients who, based on age and a strict definition of low-risk cancer, were offered multiple management options but ultimately elected AS. By evaluating actuarial rates and predictors of remaining on AS, pathologic outcomes for patients undergoing delayed RP, and early disease-specific outcomes, our purpose was to better understand the role of AS.
Methods
Following Institutional Review Board approval, retrospective data were collected from four North American tertiary care academic medical centers (Cleveland Clinic Foundation, Memorial Sloan-Kettering Cancer Center, University of British Columbia, and University of Miami) offering AS for patients with low-risk prostate cancer. The pre-specified inclusion criteria at the time of diagnostic biopsy were selected to mirror patients that would otherwise be considered for surgery or radiation due to a life expectancy greater than 10 years: age ≤ 75, clinical stage T1-T2a, PSA ≤ 10 ng/ml, ≤ 3 positive cores at diagnostic biopsy, biopsy Gleason score ≤ 6, a restaging biopsy prior to commencing AS to confirm the pathologic findings, and no active treatment for a minimum of six months following the second biopsy. Based on our inclusion criteria, all patients had an estimated 5-year risk of biochemical recurrence following radical prostatectomy of <5%11.
AS was defined as commencing on the date of the second biopsy. Follow-up generally consisted of office visits, review of general health and urinary symptoms, digital rectal examination, and PSA every 6 – 12 months. Biopsies were routinely recommended within 18 months of starting AS and subsequently every 1 – 3 years or prompted by a change in clinical status, such as a significant and sustained PSA elevation, change in digital rectal examination, or new lower urinary tract symptoms concerning for disease progression. At individual centers, MRI imaging of the prostate was selectively used at diagnosis, every 1 – 3 years after starting AS, and in isolated cases prompted an earlier biopsy than scheduled.
Criteria for recommending treatment were non-standardized and physician-specific. Patients were discontinued from AS and underwent treatment for various reasons including change in patient preference, rising PSA, digital rectal examination suggestive of more advanced features, biopsy-evidence of increased tumor volume or higher grade, or new findings on MRI. We evaluated: 1) actuarial rates and predictors of remaining on AS, 2) pathologic findings of patients subsequently electing RP, 3) outcomes following treatment, and 4) overall incidence of disease progression or metastases.
Univariate Cox regression was used to assess predictors of discontinuing AS and the Kaplan-Meier method with log-rank tests to evaluate actuarial rates of remaining on AS. A p-value < 0.05 was considered statistically significant.
Results
Between 1991 and 2007, 262 patients meeting the inclusion criteria enrolled in AS at the four institutions (Table 1). Median (IQR) age was 64 (58, 69), median PSA was 4.9 ng/ml with 212 (81%) patients having an initial PSA ≤ 7 ng/ml, clinical stage ≤ T1c in 218 (83%), total positive cores combined prior to AS was ≤ 2 in 178 (78%), and AS commenced in 2001 or later for 197 (78%). The median (IQR) time between diagnostic biopsy and the second restaging biopsy was 6.0 months (3.7, 10.5). Median (IQR) follow-up was 29 months (15, 52) and 50 (19%) patients had more than 5 years of follow-up.
Table 1.
Patient characteristics
| UM | UBC | MSKCC | CCF | Total | |
|---|---|---|---|---|---|
|
| |||||
| Patients (No.) | 61 | 34 | 146 | 21 | 262 |
|
| |||||
| Years | 1992–2007 | 1994–2007 | 1991–2007 | 2000–2006 | |
|
| |||||
| Age (median, IQR) | 62 (56, 68) | 66 (63, 68) | 64 (58, 69) | 68 (64, 71) | 69 (58, 64) |
|
| |||||
| Family history | 13 (21%) | 4 (12%) | 27 (19%) | 2 (10%) | 46 (18%) |
|
| |||||
| PSA | |||||
| < 4 ng/ml | 18 (30%) | 12 (35%) | 54 (37%) | 4 (19%) | 88 (34%) |
| 4–7 ng/ml | 30 (49%) | 16 (47%) | 66 (45%) | 12 (57%) | 124 (47%) |
| 7.1–10 ng/ml | 13 (21%) | 6 (18%) | 26 (18%) | 5 (24%) | 50 (19%) |
|
| |||||
| Clinical stage | |||||
| T1a/b | 4 (7%) | 5 (15%) | 10 (7%) | 1 (5%) | 20 (8%) |
| T1c | 47 (77%) | 20 (59%) | 112 (77%) | 19 (90%) | 198 (75%) |
| T2a | 5 (8%) | 9 (26%) | 24 (16%) | 1 (5%) | 39 (15%) |
| N/A | 5 (8%) | 0 | 0 | 0 | 5 (2%) |
|
| |||||
| Biopsy Gleason score | |||||
| 2–5 | 10 (16%) | 16 (47%) | 3 (2%) | 1 (5%) | 30 (12%) |
| 6 | 51 (84%) | 18 (53%) | 143 (98%) | 20 (95%) | 232 (88%) |
|
| |||||
| Biopsy cores | |||||
| 12–20 | 25 (41%) | 27 (79%) | 35 (24%) | 0 | 87 (33%) |
| >20 | 12 (20%) | 1 (3%) | 66 (45%) | 20 (95%) | 99 (38%) |
| N/A | 24 (39%) | 6 (18%) | 45 (31%) | 1 (5%) | 76 (29%) |
|
| |||||
| Total positive cores | |||||
| 1–2 | 41 (67%) | 19 (56%) | 105 (72%) | 13 (62%) | 178 (68%) |
| 3–5 | 7 (11%) | 11 (32%) | 25 (17%) | 7 (33%) | 50 (19%) |
| N/A* | 13 (21%) | 4 (12%) | 16 (11%) | 1 (5%) | 34 (13%) |
|
| |||||
| Estimated prostate size (median, IQR), cc** | 40 (30, 54) | 50 (35, 62) | 49 (33, 66) | 36 (29, 56) | 45 (31, 61) |
|
| |||||
| Year of diagnosis | |||||
| prior to 2000 | 8 (13%) | 22 (66%) | 34 (23%) | 1 (5%) | 65 (25%) |
| 2001–2004 | 19 (31%) | 6 (18%) | 78 (54%) | 12 (57%) | 115 (44%) |
| 2005–2007 | 34 (56%) | 6 (18%) | 34 (23% | 8 (38%) | 82 (31%) |
|
| |||||
| Active treatment | |||||
| No. (%) | 3 (5%) | 5 (15%) | 29 (20%) | 6 (29%) | 43 (16%) |
|
| |||||
| Follow-up (months) | |||||
| Median (IQR) | 21 (13, 38) | 44 (19, 84) | 25 (13, 43) | 30 (15, 39) | 29 (15, 52) |
TURP (20 patients) or not available from repeat biopsy (14 patients)
Available for 216 patients (82%) via ultrasound or MRI
UM: University of Miami
UBC: University of British Colombia
MSKCC: Memorial Sloan-Kettering Cancer Center
CCF: Cleveland Clinic Foundation
After initiating AS, 157 (60%) patients had at least one further follow-up biopsy. Among all patients on AS for a minimum of 18 months (n=172; 65%), 56 (33%) had one follow-up biopsy, 38 (22%) had two, and 17 (10%) had at least three. Among patients entering AS since 2000 with at least 18 months follow-up (n=131; 50%), 100 (76%) had at least one follow-up biopsy.
A total of 11 patients had grade progression (Gleason ≥ 7) on surveillance biopsy representing 4% of all patients and 7% of those having a biopsy after starting AS. Seven underwent active treatment and 4 declined. Of those declining, they were followed for 10, 14, 22, and 25 months without evidence of metastases.
Patient and cancer characteristics associated with an increased chance of discontinuing AS included total number of positive cores from both pre-AS biopsies (p=0.002) and cancer identified on repeat biopsy prior to AS (HR=2.23; 95% CI: 1.23–4.06; p=0.007) (Table 2). Age, PSA, clinical stage, prostate volume, and total number of biopsy cores sampled were not associated with the likelihood of remaining on AS.
Table 2.
Predictors of Discontinuing Active Surveillance
| # patients (%) | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|
| PSA (log) (per ng/ml) | --- | 1.02 (0.90–1.15) | 0.7 |
|
| |||
| Age (per year) | --- | 0.99 (0.95–1.03) | 0.8 |
|
| |||
| Clinical stage | |||
| T1a/b | 20 (7%) | 0.91 (0.32–2.57) | 0.9 |
| T1c | 198 (76%) | REF | |
| T2a | 39 (15%) | 0.87 (0.36–2.07) | |
|
| |||
| Prostate volume (ultrasound or MRI) | |||
| <30 cc | 52 (24%) | REF | 0.6 |
| 30–50 cc | 74 (34%) | 0.59 (0.23–1.53) | |
| >50 cc | 90 (42%) | 0.75 (0.32–1.71) | |
|
| |||
| Repeat biopsy with cancer | |||
| No | 159 (61%) | REF | |
| Yes | 103 (39%) | 2.23 (1.23–4.06) | 0.007 |
|
| |||
| Total cores from both biopsies (per core) | --- | 1.02 (0.99–1.06) | 0.25 |
|
| |||
| Total positive cores from both biopsies | |||
| 1–2 | 178 | REF | 0.002 |
| 3–4 | 42 | 1.4 (1.11–1.94) | |
| 5–6 | 8 | 6.3 (1.72–15.36) | |
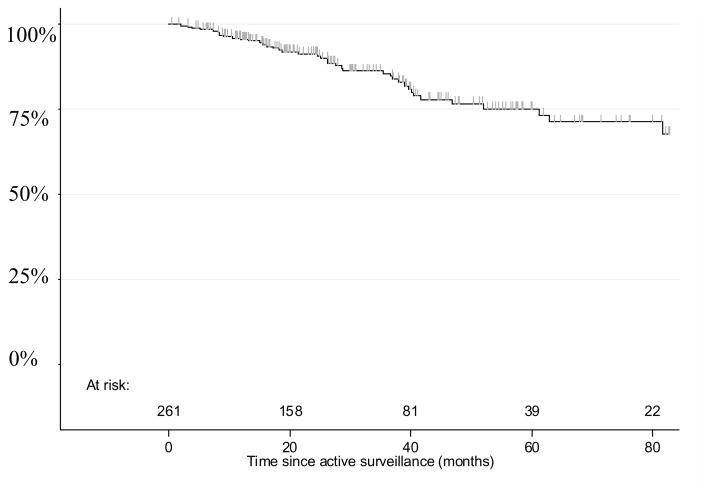
During the course of the study, 43 (16%) patients elected active treatment and the two and five-year probability of remaining on AS were 91% and 75%, respectively (Figure 1). The reasons for stopping active surveillance were most commonly upgrading (35%) or higher volume of cancer (16%) on surveillance biopsy or a change in patient preference (14%) (Table 3). The active treatment choices were RP for 26 (60.5%), radiation therapy for 13 (30%), cryotherapy for 1 (2.5%), and androgen deprivation for 3 (7%).
Figure 1.
Actuarial Estimate of Remaining on Active Surveillance
Table 3.
Reasons for Ceasing Active Surveillance and Electing Treatment (n=43)*
| Number (%) | |
|---|---|
| Gleason ≥ 7 on surveillance biopsy | 15 (35%) |
| Surveillance biopsy with > 3 cores or >50% in a single core | 7 (16%) |
| Change in patient preference | 6 (14%) |
| Rising PSA without worsening biopsy features | 2 (5%) |
| MRI findings | 2 (5%) |
| Voiding symptoms | 1 (2%) |
| Bone metastases | 1 (2%) |
| Upstaging via digital rectal examination | 1 (2%) |
| Unknown | 12 (28%) |
Greater than 100% as some patients had multiple reasons
For the 26 patients electing RP, Gleason 7 cancer, positive surgical margin, extracapsular extension, and lymph node involvement were present in 13 (50%), 2 (8%), 4 (15%), and 1 (4%), respectively. Of the 13 men with Gleason 7 prostate cancer identified at radical prostatectomy, 9 (69%) had a biopsy between the start of active surveillance and surgery. Of those 9 patients, 3 (33%) had evidence of Gleason 7 on a biopsy prior to surgery.
The patient with lymph node metastases had 1 of 28 nodes involved and is currently without biochemical recurrence at 24 months following RP. Following RP, 23 patients are free of biochemical recurrence at a median follow-up of 19 months since surgery, 2 had a biochemical recurrence following surgery but are without evidence of cancer progression following salvage radiation therapy, and 1 had a biochemical recurrence 60 months following surgery with his most recent PSA being 0.6 ng/ml. All three patients with a biochemical recurrence had a positive surgical margin. Following radiation therapy, all 13 patients are without biochemical recurrence (PSA nadir plus 2 ng/ml) at a median follow-up of 31 months. Following androgen deprivation, 2 of 3 patients maintain an undetectable PSA. The other patient receiving androgen deprivation was 73 years old at diagnosis with a PSA of 3.4 ng/ml, Gleason 6 cancer in one of 20 cores from two separate biopsy sessions, and staged as clinical T1c, but he never underwent a biopsy after initiating active surveillance and had a PSA of 6.5 ng/ml three years later (PSADT of 3.4 years). However, 38 months after starting active surveillance his PSA was 24 ng/ml and a bone scan revealed multifocal bone metastases in the thoracic and lumbar spine. Androgen deprivation was initiated and he has since been lost to follow-up.
Overall, of the 43 patients undergoing postponed treatment, 41 (95%) are currently without evidence of metastases at a median of 23 months following treatment. There were three deaths in the cohort but none from prostate cancer.
Discussion
Our study provides an overview of a multi-institutional retrospective experience with AS for men with low-risk prostate cancer at diagnosis who are also candidates for other management options such as radiation or surgery due to their estimated life expectancy. We provide further short-term evidence that for highly select patients AS appears to be safe, durable, and associated with a low but finite risk of disease progression.
The ultimate success of any AS program relies on accurate disease characterization at the time of diagnosis. By specifying strict clinical and pathologic inclusion criteria and requiring a restaging biopsy prior to commencing AS, we identified a cohort of men with a very low risk of disease progression with an estimated 5-year biochemical recurrence risk of less than 5% if they elected immediate prostatectomy. The rate of AS discontinuation in our study was approximately 5% per year, lower than similar series5, 9, 12, and reflects our more restrictive entry criteria, particularly a second prostate biopsy before starting AS.
The ability to predict relative disease indolence is imperfect, as evidenced by the modest accuracy of pre-treatment predictive tools intended for that purpose13, by the two patients diagnosed with metastases (bone metastases during AS and another with lymph node metastases at RP), and 67% of patients with Gleason 7 at RP showing lower grade cancer on biopsy. Therefore it is imperative that all men electing an AS program be counseled on the low but real risk of potentially life-threatening cancer progression. To minimize this risk, we feel strongly that a restaging biopsy prior to initiating AS is mandatory as it excludes up to 30% of patients considered for AS based on the initial diagnostic biopsy, minimizes the risk of a Gleason grade sampling error, and predicts the likelihood of remaining on AS. Berglund et al have shown that among 104 patients being considered for AS after a diagnostic biopsy with identical criteria as this study, 27% will be excluded from consideration of AS because of upstaging or upgrading at rebiopsy14. Additional support for the restaging biopsy is that Gleason upgrading on surveillance biopsy is the most common reason for discontinuing AS and is most likely a result of inaccurate assessment prior to AS rather than cancer progression15. When comparing our data to another well-defined cohort of 407 men undergoing AS15, median follow-up (29 versus 26 months), proportion of patients having at least one surveillance biopsy (60% versus 59%), and frequency of recommended surveillance biopsies were similar. However, the absolute proportion of patients with upgrading on surveillance biopsy was less (7% versus 19%), suggesting that our strategy of restaging biopsy prior to AS may be one way of minimizing progression while on AS. For these reasons, we feel the diagnostic biopsy may lead to a patient being an AS candidate but findings on the restaging biopsy are what ultimately determines and finalizes eligibility.
Multiple lines of overlapping evidence confirm the increased incidence of men being diagnosed with very low-risk prostate cancer, their highly unlikely progression to metastases, and increased interest in observational or minimally invasive management approaches. First, the lead-time bias afforded by intensive PSA screening leads to an increased detection rate of biologically indolent cancer and is estimated to be 3–12 years depending on different modeling techniques, screening intervals, and biopsy indications16, 17. Second, since the introduction and widespread use of PSA, rates of prostate cancer identified on autopsy have decreased, suggesting an increased detection and presumptive treatment of cancers not likely to have clinical manifestations18. Third, nearly 50% of men in the United States diagnosed with prostate cancer (CaPSURE) and 30% of European men (European Randomized Screening for Prostate Cancer trial) meet variable low-risk or indolent cancer criteria at the time of diagnosis2, 3, consistent with other estimated rates of overdiagnosis (23 – 50%). Fourth, many treated men are not likely to benefit from an intervention. A population-based assessment by Miller et al. estimates that up to 50% of men with low-risk prostate cancer (well-differentiated cancers in men of any age or moderately differentiated tumors in men over 70 years of age) are overtreated13. In the European Randomized Screening for Prostate Cancer trial 49% of men with clinical stage T1c-T2a, PSA < 20 ng/ml, Gleason 6 or less, <50% positive cores, and <20 mm of total cancer that underwent RP had pathologically indolent features (total tumor volume < 0.5 cc, confined to the prostate, and no Gleason grade 4 or 5)3. Despite the data suggesting lead-time bias, diagnosis of “autopsy-detected” cancers, overdiagnosis, and overtreatment, it is estimated that AS is utilized as a management strategy in only 10% of patients with newly diagnosed prostate cancer2. Our data argues for further consideration of AS in appropriately selected patients.
However, multiple limitations of our study should be considered. Based on the modest follow-up in our study (median 29 months) and others (median: 22 – 64 months)4, 5, 12, 19, caution should be exercised in extrapolating these findings to justify AS as a long-term management strategy. Extended follow-up is mandatory to answer this question, particularly since the Scandinavian Prostate Cancer Group20 analyzed men with untreated, localized, and primarily palpable prostate cancer, and found local progression and metastases frequently occur 10 – 20 years following the diagnosis and cancer-specific mortality rates 15 years after diagnosis are tripled compared to the first 15 years of follow-up20. Our data simply provides an observational experience which will continue to provide insights into the natural history of low-risk prostate cancer, generalized rates of delayed treatment given the variable practice patterns, overall cancer-specific success rates, and causes of death. Although one of the strengths of our study is the multi-institutional cohort, this has also led to variations in the intensity of follow-up, diagnostic and restaging biopsy strategies, frequency of surveillance biopsies, pathologic assessment, and indications for treatment. For these reasons, the generalizing our findings to other populations should be done with caution. While the most common reason for discontinuing AS in our series was results of a surveillance biopsy, in other AS series it was patient preference or a rising PSA alone, highlighting the variable nature of currently available series and the need for pre-specified study methodology. Ongoing prospective randomized trials such as START (Standard Treatment Against Restricted Treatment; National Cancer Institute of Canada) and ProtecT (Prostate Testing for Cancer and Treatment; National Health Service in United Kingdom) will provide larger-scale and sounder evidence regarding the role and limitations of AS. Over the course of our study, the best available clinical and pathologic data were used to establish inclusion criteria. It is hoped that more accurate predictors of tumor biology, such as advances in imaging (e.g MRI) or genetic assessment (e.g. ERG, TMPRSS2), may ultimately improve the patient selection process for AS. Lastly, the impact of lead-time bias should be considered when interpreting our findings. It is possible that patients meeting criteria for AS but undergoing active treatment within 6 months (excluded from our study) had higher-risk features and, if included, would have led to less favorable outcomes for the entire cohort.
Our view on AS is not disregard for men with low-risk cancer features but rather a strategy that encourages initial observation, frequent monitoring based on serial prostate biopsies, and if needed the implementation of active therapy while still at a highly curable stage. The short-term effectiveness of delayed treatment is exemplified by 42 of our 43 patients currently without evidence of disease following RP (n=26), radiation (n=13), or cryotherapy (n=1) or with an undetectable PSA after androgen deprivation (n=2). Similarly, Warlick et al found that among 38 patients electing radical prostatectomy 12 – 73 months after starting an AS program, the pathologic outcomes did not significantly differ from 150 men with similar cancer characteristics that chose immediate surgery at diagnosis4. These highlight many of the hallmark features and purported benefits of AS: most men will not require an intervention, those that do will have benefited from a period of time where their quality of life and cancer-related outcomes do not appear to be compromised.
Conclusions
Active surveillance with judicious monitoring for appropriately selected patients with low-risk prostate cancer appears to be safe, durable, and associated with a low risk of systemic progression. Restaging biopsy before considering active surveillance appears essential since cancer detected on restaging biopsy and a higher number of cores with cancer are associated with a lower likelihood of remaining on active surveillance. With selective inclusion criteria, 91% and 75% remain on active surveillance at 2 and 5 years, respectively, and short-term cancer control following active treatment is encouraging. Prospective randomized trials against primary treatment will ultimately determine the efficacy and role of active surveillance.
Acknowledgments
SEE is funded through a National Institute of Health Ruth Kirchstein National Research Service Award (T32-CA82088-06)
Glossary
- AS
active surveillance
- PSA
prostate specific antigen
- RP
radical prostatectomy
- CI
confidence interval
- IQR
interquartile range
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roemeling S, Roobol MJ, Kattan MW, van der Kwast TH, Steyerberg EW, Schroder FH. Nomogram use for the prediction of indolent prostate cancer: impact on screen-detected populations. Cancer. 2007;110:2218. doi: 10.1002/cncr.23029. [DOI] [PubMed] [Google Scholar]
- 4.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98:355. doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dall'Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 6.Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178:2260. doi: 10.1016/j.juro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klotz L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol. 2004;172:S48. doi: 10.1097/01.ju.0000141712.79986.77. [DOI] [PubMed] [Google Scholar]
- 9.Patel MI, DeConcini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 10.Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101:165. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie C, Parker C, Norman A, Eeles R, Horwich A, Huddart R, et al. Early outcomes of active surveillance for localized prostate cancer. BJU Int. 2005;95:956. doi: 10.1111/j.1464-410X.2005.05446.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 14.Berglund R, Masterson T, Vora K. Pathologic upgrading and upstaging with immediate repeat biopsy for patients eligible for active surveillance. Presented at the American Urologic Assocation; Orland, FL. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan TB, Carter HB, Wang W, Landis PB, Epstein JI. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J Urol. 2008;179:901. doi: 10.1016/j.juro.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schroder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Yamamoto T, Miyakubo M, Takechi H, Ohi M, Shibata Y, et al. Lead time of prostate cancer detected in population based screening for prostate cancer in Japan. J Urol. 2007;178:1258. doi: 10.1016/j.juro.2007.05.144. [DOI] [PubMed] [Google Scholar]
- 18.Konety BR, Bird VY, Deorah S, Dahmoush L. Comparison of the incidence of latent prostate cancer detected at autopsy before and after the prostate specific antigen era. J Urol. 2005;174:1785. doi: 10.1097/01.ju.0000177470.84735.55. [DOI] [PubMed] [Google Scholar]
- 19.van As NJ, Norman AR, Thomas K, Khoo VS, Thompson A, Huddart RA, et al. Predicting the Probability of Deferred Radical Treatment for Localised Prostate Cancer Managed by Active Surveillance. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]