Abstract
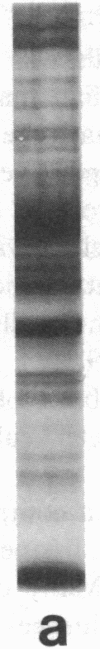
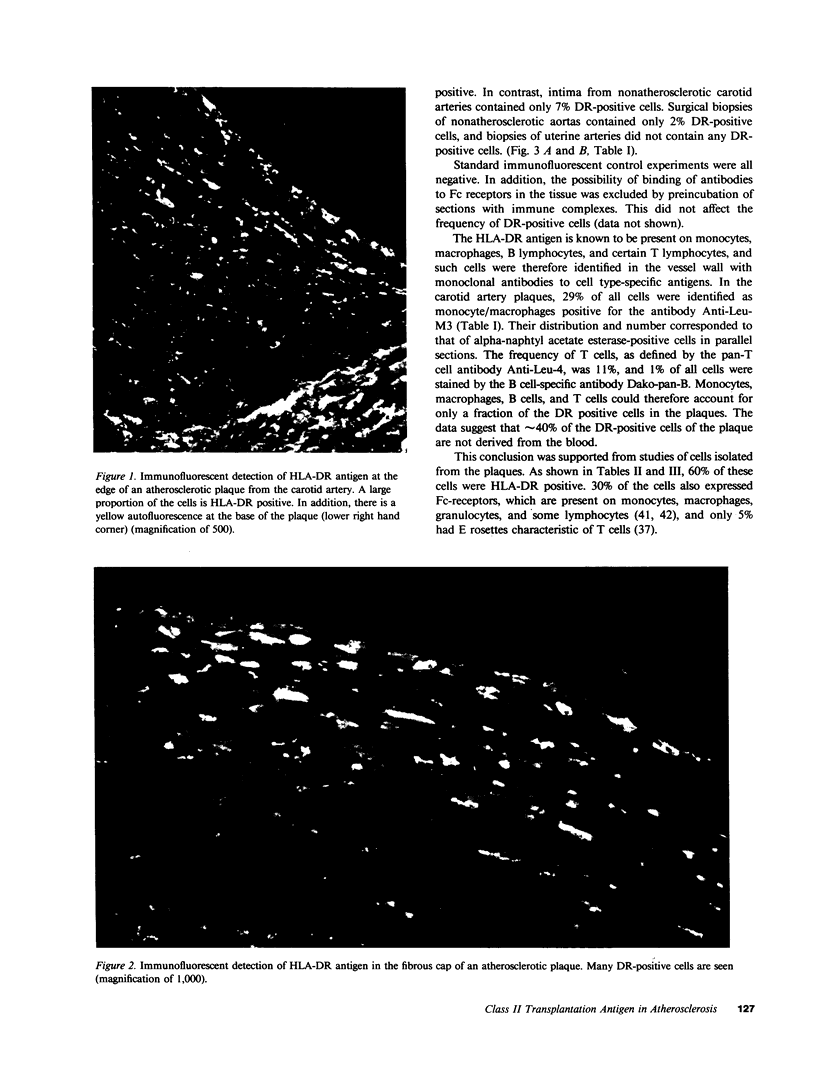
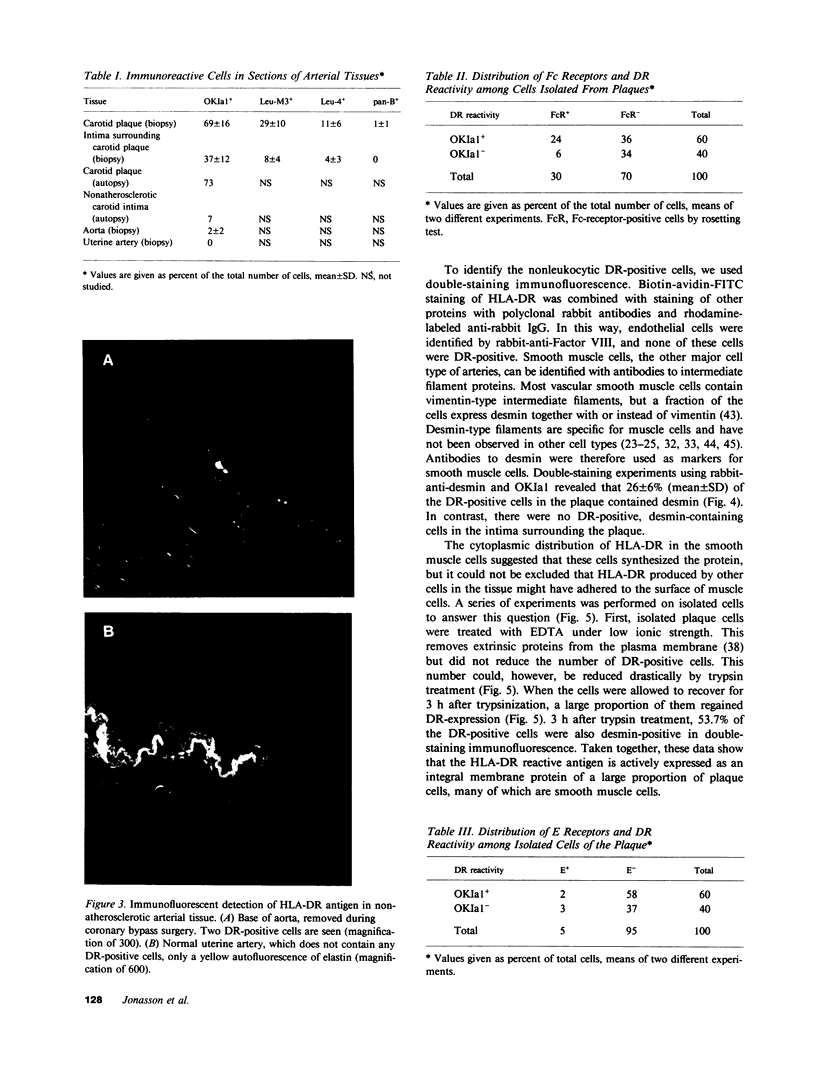
A large proportion of the cells of the human atherosclerotic plaque is assumed to be derived from medial smooth muscle cells. In contrast to these, the cells of the plaque have the capacity to accumulate lipid, and they also proliferate at a higher rate than medial cells. It has therefore been suggested that smooth muscle cells undergo a change of phenotype during atherogenesis, but there has been no evidence for such a change on the molecular level. We have now analyzed carotid artery plaques using a battery of antibodies against cell surface and cytoskeletal antigens, and found that most of the cells express the class II transplantation antigen (Ia antigen) HLA-DR. Also, the beta chain of HLA-DR was detected by immunoblotting of plaque extracts with the OKIa1 monoclonal antibody. HLA-DR is normally present on cells of the immune system, but only 60% of the DR-positive cells of the plaque reacted with monoclonal antibodies specific for macrophages and lymphocytes. Many of the remaining DR-positive cells contained the muscle-specific intermediate filament protein, desmin. This indicates that smooth muscle cells of atherosclerotic plaques express DR antigen. In contrast, very few DR-positive cells were found in normal human arteries. This suggests that expression of class II antigen is part of a phenotypic change in smooth muscle cells in atherosclerosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Graft rejection and Ia antigens--paradox resolved? Nature. 1983 Jun 2;303(5916):382–383. doi: 10.1038/303382b0. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Richardson N. E., McConnell I., Feinstein A. Distinct populations of class II molecules identified by monoclonal antibodies. Clin Exp Immunol. 1983 Oct;54(1):117–126. [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Popadynec L., Nestel P. J., Campbell G. R. Lipid accumulation in arterial smooth muscle cells. Influence of phenotype. Atherosclerosis. 1983 Jun;47(3):279–295. doi: 10.1016/0021-9150(83)90059-x. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Forsum U., Klareskog L., Peterson P. A. Distribution of Ia-antigen-like molecules on non-lymphoid tissues. Scand J Immunol. 1979;9(4):343–349. doi: 10.1111/j.1365-3083.1979.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Gabbiani G., Rungger-Brändle E., de Chastonay C., Franke W. W. Vimentin-containing smooth muscle cells in aortic intimal thickening after endothelial injury. Lab Invest. 1982 Sep;47(3):265–269. [PubMed] [Google Scholar]
- Haley N. J., Shio H., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. I. Resolution of aortic cell populations by metrizamide density gradient centrifugation. Lab Invest. 1977 Sep;37(3):287–296. [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Björnheden T., Bylock A., Bondjers G. Fc-dependent binding of monocytes to areas with endothelial injury in the rabbit aorta. Exp Mol Pathol. 1981 Jun;34(3):264–280. doi: 10.1016/0014-4800(81)90044-7. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Starkebaum G. A., Benditt E. P., Schwartz S. M. Fc-mediated binding of IgG to vimentin-type intermediate filaments in vascular endothelial cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3103–3107. doi: 10.1073/pnas.81.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Braathen L. R., Thorsby E. Antigen presentation by vascular endothelial cells and epidermal Langerhans cells: the role of HLA-DR. Immunol Rev. 1982;66:57–77. doi: 10.1111/j.1600-065x.1982.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Hjelm E., Forsum U., Klareskog L. Anti-Ia-reactive cells in the urinary tract of man, guinea-pig, rat and mouse. Scand J Immunol. 1982 Dec;16(6):531–538. doi: 10.1111/j.1365-3083.1982.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Peterson P. A. Hormonal regulation of the expression of Ia antigens on mammary gland epithelium. Eur J Immunol. 1980 Dec;10(12):958–963. doi: 10.1002/eji.1830101212. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Tjernlund U. M., Rask L., Peterson P. A. Expression of Ia antigen-like molecules on cells in the corneal epithelium. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):310–313. [PubMed] [Google Scholar]
- Klareskog L., Tjernlund U., Forsum U., Peterson P. A. Epidermal Langerhans cells express Ia antigens. Nature. 1977 Jul 21;268(5617):248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Trägårdh L., Rask L., Peterson P. A. Isolation and characterization of detergent-solubilized human HLA-DR transplantation antigens. Biochemistry. 1979 Apr 17;18(8):1481–1489. doi: 10.1021/bi00575a015. [DOI] [PubMed] [Google Scholar]
- Klein J., Hauptfeld V. Ia antigens: their serology, molecular relationships, and their role in allograft reactions. Transplant Rev. 1976;30:83–100. [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Koyama K., Fukunishi T., Barcos M., Tanigaki N., Pressman D. Human Ia-like antigens in non-lymphoid organs. Immunology. 1979 Oct;38(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Niederhuber J. E. The role of I region gene products in macrophage - T lymphocyte interaction. Immunol Rev. 1978;40:28–52. doi: 10.1111/j.1600-065x.1978.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Palacios R. Mechanism of T cell activation: role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Parish C. R. Separation and functional analysis of subpopulations of lymphocytes bearing complement and Fc receptors. Transplant Rev. 1975;25:98–120. doi: 10.1111/j.1600-065x.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr Expression of Ia-like antigens by human vascular endothelial cells is inducible in vitro: demonstration by monoclonal antibody binding and immunoprecipitation. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6641–6645. doi: 10.1073/pnas.79.21.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Thorud E. Is heterogeneous expression of HLA-dr antigens and CEA along with DNA-profile variations evidence of phenotypic instability and clonal proliferation in human large bowel carcinomas? Br J Cancer. 1983 Oct;48(4):543–551. doi: 10.1038/bjc.1983.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Osborn M., Rungger-Brändle E., Gabbiani G., Weber K., Franke W. W. Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Exp Cell Res. 1982 Feb;137(2):329–340. doi: 10.1016/0014-4827(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. DR (Ia-like) antigens on human melanoma cells. Serological detection and immunochemical characterization. J Exp Med. 1979 Mar 1;149(3):658–668. doi: 10.1084/jem.149.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]