Abstract
BACKGROUND
Increasing age is a significant risk factor for prostate cancer. The prostate is exposed to environmental and endogenous stress that may underlie this remarkable incidence. DNA methylation, genomic imprinting, and histone modifications are examples of epigenetic factors known to undergo change in the aging and cancerous prostate. In this review we examine the data linking epigenetic alterations in the prostate with aging to cancer development.
METHODS
An online search of current and past peer reviewed literature on epigenetic changes with cancer and aging was performed. Relevant articles were analyzed.
RESULTS
Epigenetic changes are responsible for modifying expression of oncogenes and tumor suppressors. Several of these changes may represent a field defect that predisposes to cancer development. Focal hypermethylation occurs at CpG islands in the promoters of certain genes including GSTP1, RARβ2, and RASSF1A with both age and cancer, while global hypomethylation is seen in prostate cancer and known to occur in the colon and other organs. A loss of genomic imprinting is responsible for biallelic expression of the well-known Insulin-like Growth Factor 2 (IGF2) gene. Loss of imprinting (LOI) at IGF2 has been documented in cancer and is also known to occur in benign aging prostate tissue marking the presence of cancer. Histone modifications have the ability to dictate chromatin structure and direct gene expression.
CONCLUSIONS
Epigenetic changes with aging represent molecular mechanisms to explain the increased susceptibly of the prostate to develop cancer in older men. These changes may provide an opportunity for diagnostic and chemopreventive strategies given the epigenome can be modified.
Keywords: aging, epigenetics, methylation, imprinting, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most common cancer among men in the United States and the second leading cause of cancer deaths [1]. Older age is a significant risk factor, with histologic PCa being found in 60% of men by the age of 70 years, and 80% having some form of the disease by 80 [2]. Interest has arisen in an epigenetic basis underlying this susceptibility of the prostate and other aging-related cancers including colon and breast. Monozygotic twins studies have been instrumental in demonstrating epigenetic changes occur during aging as a result of both stochastic processes and environmental exposures [3]. The accumulation of these alterations due to endogenous or exogenous (environmental) factors can promote fields of susceptibility to oncogenic triggers in aging organs that eventually give rise to cancer. This is one aspect of the “field effect” that is manifested in the known multifocality, consisting of 4 to 5 cancer foci, that has been noted in radical prostatectomy specimens [4]. A number of genetic and epigenetic changes throughout the histologically normal aging prostate have recently been identified.
Epigenetics refers to functionally relevant genomic information, other than the DNA sequence, that is, heritable during cell division [5]. There are several major types of epigenetic modifications including DNA methylation, genomic imprinting, and histone modifications. Changing epigenetic marks modulate chromatin structure, and alter gene expression without the requirement for changing DNA sequence [6]. These epigenetic modifications are crucial during embryonic development. More recently it has been recognized that aberrant reactivation can lead to altered gene expression associated with cancer development and progression [7].
An epigenetic field effect, or field defect, within the context of cancer is often thought of as resulting from the presence of a tumor. However, recent evidence suggests these changes may occur with age and potentially even precede the formation of cancer. We have termed these changes Age-Related Epigenetic Alterations inducing Susceptibility (AREAS). These age-related changes may be widespread throughout the prostate and can result in gene expression changes that induce or are selected for in the subsequent tumor (Table I).
TABLE I.
Identified Epigenetic Alterations Linked to Aging in Prostate Tissues
| Gene | Epigenetic change | Prostate tissuea | Function | Refs. |
|---|---|---|---|---|
| RARβ2 | Promoter hypermethylation | Normal | Mediates cell signaling in embryonic morphogensis, cell growth, and differentiation | [22,23] |
| RASSF1A | Promoter hypermethylation | Normal | Tumor suppressor; encoded protein similar to Ras effector proteins | [22] |
| GSTP1 | Promoter hypermethylation | Normal | Detoxification of electrophilic compounds | [22,25] |
| NKX2-5 | Promoter hypermethylation | Normal | Regulation of tissue specific gene expression for tissue differentiation | [80] |
| CDH1 | Gene body hypermethylation | Benign prostatic hyperplasia | Cell–cell adhesion glycoprotein, TSG | [81] |
| CDH13 | Gene body hypermethylation | Benign prostatic hyperplasia | Cadherin superfamily member | [81] |
| SPARC | Promoter hypermethylation | Normal | Cell growth regulation via interactions with extracellular matrix | [80] |
| TIMP3 | Promoter hypermethylation | Normal | Inactivation of metalloproteinases | [80] |
| EPHB2 | Promoter hypermethylation | Normal | Ephrin receptor, implicated in developmental processes | [82] |
| BRCA1 | Promoter hypermethylation | Normal | DNA double-strand break repair | [82] |
| IGF2 | Loss of imprinting | Normal and cancerous | Growth promoting hormone; structural similarity to insulin | [63] |
Type of prostate tissue analyzed for the age-related change, Normal = peripheral prostate tissue.
The purpose of this review is to examine the role of epigenetics in aging and as a susceptibility factor in the development of PCa. We will examine AREAS implicated in PCa development, specific changes in the aging prostate, and the recent finding of these changes associated with the field effect. One pitfall in screening for PCa is the inability to distinguish indolent versus aggressive disease. Further study of the epigenetic changes associated with the aging prostate may yield markers that allow differentiation between these variants and the earlier detection of, or predisposition for, disease. Furthermore, epigenetic changes are reversible and may provide an important target for chemoprevention efforts.
DNA METHYLATION
DNA methylation was the first epigenetic mark to be implicated in human cancer [5]. Alterations of DNA methylation in cancer and aging are typically characterized as global hypomethylation and regional hypermethylation. Methylation occurs at cytosine bases, typically CpG dinucleotides. CpG dinucleotides tend to cluster in regions called CpG islands (CGI), which can be defined as stretches of DNA (>200 bases) with a GC content greater than 50% and an observed/expected ratio of more than 0.6 [8]. CpG dinucleotides occur in only ~1% in the mammalian genome, however 60% of human promoters are associated with CGI’s in a mostly unmethylated state [9]. DNA hypermethylation refers to the gain of methylation at sites that are typically unmethylated and occurs mainly at CGIs. CGI hypermethylation is often associated with gene silencing, achieved by blocking DNA binding proteins, such as transcriptional regulators, that result in decreased transcription. Methylated DNA also act as a recruitment site for methyl-CpG-binding domain (MBD) proteins that elicit a chromatin silencing response when bound [10].
DNA methylation occurs at sites outside CGIs including island shores (up to 2 kb upstream of CGIs), gene bodies, and gene poor areas including repetitive sequences, retrotransposons, and intergenic regions. Human tumor cells often experience a global hypomethylation, despite hypermethylation at specific sites, because repetitive elements and CpG rich-satellites tend to be hypomethylated [11]. Hypomethylation in repetitive sequences and retrotransposons, found in gene poor areas, commonly occurs in PCa and may contribute to metastatic tumor heterogeneity [12]. Their properly methylated state protects genomic integrity by blocking reactivation of these endoparasitic sequences [10,11].
Global methylation levels have been reported in the prostate where analyses typically compare differing pathologic conditions. In general, more severe conditions experience greater hypomethylation. Bedford and van Helden [13] found 5-methylcytosine content of prostatic DNA significantly decreased from normal (4.01 ± 0.11), to benign prostatic hyperplasia (BPH) (3.62 ± 0.14), to metastatic PCa (3.29 ± 0.24). Other studies have found more extensive methylation loss in high grade and metastatic cancers [12,14].
DNA hypomethylation is found in prostatic intra-epithelial neoplasia (PIN) when compared to normal prostate tissue suggesting hypomethylation may precede frank cancer [14,15].
An analysis of global hypomethylation in the aging peripheral prostate has not yet been performed. However, given the clinical similarities between the colon and prostate, including the prevalence with aging and multifocality, colon research may be informative. In the colon, global demethylation with aging correlates with increased genomic damage, and precedes cancer development [16]. Furthermore, patients with more extensive hypomethylation in normal colon tissue are predisposed to the development of multiple tumors [17]. Some have hypothesized hypomethylation is more important to the progression of PCa and not as relevant to the aging prostate [12]. Research examining hypomethylation in the aging human prostate is required to definitively test this.
Genome-wide methylation studies and DNA methylation microarray sets are useful tools for the characterization of methylation changes and have been used to document hypermethylation in the prostate [18]. One of the first hypermethylation events demonstrated in human aging involved a linear increase in the estrogen receptor in normal colon tissue that was selected for in the colon cancers studied [19]. Other hypermethylation events in the colon with aging include N33, MYOD, P16(INK4A), MLH1, and DAPK [20,21]. MYOD, P16(INK4A), and MLH1 have all been implicated as tumor-suppresor genes, while MLH1 has additionally been implicated in DNA mismatch repair. Silencing by promoter hypermethylation events may have functional relevance in tumorigenesis, or alternatively may represent bystander alterations associated with altered chromatin structure.
One of the few papers to exclusively examine DNA methylation in aging prostate tissues was performed by Kwabi-Addo et al. [22]. They assessed nine genes known to be hypermethylated in PCa in the peripheral prostate tissues of men without cancer and found increased promoter methylation with aging in 5 (RARβ2, RASSF1A, GSTP1, NKX2-5, and ESR1). This frequent finding of hypermethylation with aging in loci altered with cancer (56%) is striking and suggests AREAS may occur more commonly than anticipated. In another study, Henrique et al. [23] found APC and RARβ2 were more frequently hypermethylated with aging in normal prostate samples from patients assessed across a narrow age range 50–72 years. Frequent RARβ2 silencing by epigenetic events may desensitize prostate cells to retinoic acid (RA) treatment. However, RA treatment coupled with epigenetic modulators shows promise as a viable treatment [24]. Epigenetic modulators may be required to sensitize tumors to therapeutic intervention.
In prostate tissues taken from men with cancer, several genes undergo increased methylation with aging in the normal appearing tissue. Some genes analyzed in these studies were previously found to precede disease including NKX2-5, and GSTP1 [22,25]. Others that demonstrate a positive association with age include TIG1, CDH12, EGFR5, MCAM, SLIT2 [25], ESR1 [25,26], and DLC-1 [27]. Vasiljevi et al. [25] noted a moderate effect of age common to increasing methylation of all genes analyzed in their study. The functional consequence and mechanism of these methylation changes involved with aging and cancer have not been investigated to date.
In an effort to compare aging histologically normal tissue in men with and without cancer, we performed a genome-wide analysis of DNA methylation changes in normal peripheral prostate tissue at 385,000 loci based on the ENCODE18 genomic sequence [28]. There were 615 significantly differentially methylated probes in tumor associated tissues found. The majority (87%) of loci demonstrated hypomethylation and the remainder hypermethylation. Notably, these arrays were not focused on promoter CGIs and reflected more global changes in methylation. This pattern of methylation fits the idea that global decreases in methylation parallel hypermethylation at CpG islands. A closer look at some of the more significantly altered probes associated with CGIs indicate many of these changes occurred at the edges or periphery of the islands consistent with data that DNA methylation spreads from the edges of CGIs inward [29].
This study also examined whether the DNA methylation defect was found adjacent to tumors suggesting a peritumor effect, or at a distance (>1 cm) raising the possibility of a more widespread field defect encompassing the peripheral prostate. The loci used for mapping the extent of this field defect did not degrade with increased distance to the main tumor focus suggesting a spatially widespread field of cancer susceptibility consistent with an age-related phenomenon. Methylation changes, both hyper and hypomethylation, have been found in the colon with aging that are postulated to increase cancer risk [19,30,31]. Further data will determine whether a similar aging-dependent field of susceptibility also occurs in the prostate. Interestingly, not all genes altered in the prostate were enriched for in the associated tumors. This may suggest that some changes (e.g., FGF-1) are important in the initiation of PCa that are not selected for as the tumor develops. This study also identified several changes associated only with the development of high-grade cancer. This suggests a unique biologic background may give rise to these more aggressive variants. These changes may be important in identifying not only the disease at an earlier timepoint, but also the genesis of high grade cancer.
Recently, the epigenetic and cancer fields have seen a growing interest into 5-hydroxymethylcytosine (5hmC). 5-methylcytosine (5mC) found in CpG dinucleotides can be converted to 5hmC by the Ten-eleven translocation 1-3 (TET1-3) enzymes [32]. In general, 5hmC presence correlates with tissue differentiation, with terminally differentiated cells containing higher 5hmC content than tissue stem/progenitor cells [33]. The conversion of 5mC to 5hmC by TET enzymes is thought to be part of a stepwise mechanism in the demethylation of the target cytosine [32,34]. A study classifying 5hmC levels in various tissues found that carcinoma of the prostate, breast, and colon all had a significant reduction in 5hmC levels by immunohistochemistry [33]. Of interest small low grade lesions also contained significant reduction in 5hmC levels suggesting it may be an early step in prostate tumorigenesis [33]. TET activity may be downregulated by environmental stressors, providing a link between 5hmC, aging and cancer development [35].
ENVIRONMENTAL EFFECTS ON DNA METHYLATION
DNA methylation patterns in cells are sensitive to modulation by the external environment. Oxidative stressors, environmental toxins, hormonal influences and nutrition pose constant challenges to the epigenetic environment with aging [36]. Inflammation and histologic lesions such as post-inflammatory atrophy (PIA) occur with increased frequency in the aging prostate [37]. Oxidative stress results in the formation of reactive oxygen species (ROS), which can convert deoxyguanine in CpGs to 8-hydroxy-2′ deoxyguanosine (8-OHdG). Oxidative damage is manifest by an accumulation of nuclear 8-OHdG and this adduct is found with increasing frequency in aging prostate tissues [38]. This DNA adduct inhibits methylation at neighboring 5′ cytosines by blocking methyltransferase activity resulting in DNA hypomethylation in vitro [39,40]. In vivo, we analyzed mice containing inactivating mutations in superoxide dismutase (SOD), a gene responsible for handling superoxide radicals [41]. These animals experience an accelerated loss of DNA methylation in the prostate with aging. This occurs, both globally and at specific loci including the Igf2-H19 differentially methylated region. This suggests that oxidative stress may mediate some of the age-related epigenetic events that occur in the aging prostate and elucidation of this connection may provide a target for chemoprevention.
Nutritional influences also impact DNA methylation patterns. Folate and methionine deficiencies occur with frequency in the aging human, and are associated with an increased risk of prostate and colon cancer [42,43]. Folate and other dietary methyl donors provide methyl groups that cycle to maintain adequate levels of S-adenosylmethionine (SAM) used in cellular methylation reactions including DNA and histone methylation. Methyl-deficient diets in rats result in DNA hypomethylation accompanied by gene expression changes in known oncogenes, including c-myc, c-fos, and c-Ha-ras [44]. These diets primarily alter the liver which is particularly susceptible to neoplastic formation. A previous study in our laboratory analyzed the epigenetic effects on the prostate of a choline-methionine deficient (CMD) diet [45]. The data showed CMD diets resulted in a reversible increase in Igf2 and H19 expression. Underlying these expression changes was a derepression of histone methylation permissive to gene expression at these gene promoters.
One striking discovery was the impact that altered nutrition has on DNA methylation of the offspring and their transgenerational effects. Exposure during gestation appears to be a particularly susceptible period. A classic study involved the Agouti mouse in which folate supplementation of the pregnant dam alters DNA methylation of a retroposon resulting in altered coat color in the offspring [46]. This presents an intriguing implication that folate supplementation during pregnancy may have unrecognized epigenetic and phenotypic consequences in humans.
Recently, research interest into other epigenetically active compounds has increased in the prostate. Ho et al. reported that neonatal exposure in rats to the environmentally active estrogen bisphenol A increased the development of high-grade PIN and PCa. This induced DNA methylation changes at several genes in the adult prostate including Pde4d4 [47]. Periods of rapid prostate growth, specifically, in utero development and puberty, present windows of susceptibility for epigenetic alterations that may play a role in adult-onset disease progression.
Neonatal exposure to other potential teratogens may also alter the epigenetics of subsequent generations. Gestational exposure to Vinclozolin, an agricultural antifungal, resulted in epigenetic transgenerational inheritance of low sperm count in the immediate offspring and future generations [48]. Endocrine disruptors may also induce transgenerational diseases including obesity and reproductive disease [49,50]. This raises the distinct possibility that epigenetic insults may, in some situations, give rise to the known familial association of PCa. This hypothesis has yet to be examined.
GENOMIC IMPRINTING
Genomic imprinting is an epigenetic mechanism in which individual alleles are silenced based on the parent of origin. The imprinted allele is established with blastocyst formation, and disruption results in a lethal phenotype [51,52]. Expression occurs in a developmental and tissue-specific manner. Imprinted genes generally reside in clusters and roughly 100 human genes have been identified thus far [51]. It is postulated that this grouping allows coordinated control by imprinting centers also known as imprint control regions or ICRs [53]. Imprinting deregulation was initially recognized in childhood syndromes including Prader–Willi, Angelman, Beckwith–Wiedermann (BWS), and Silver–Russell [51]. In BWS a LOI at the Igf2/H19 and KCNQ1-KCNQ10T1 domains leads to fetal and postnatal overgrowth along with a much higher incidence of cancer [54]. These diseases provide evidence that the proper regulation of imprinting is required for normal growth and maturation.
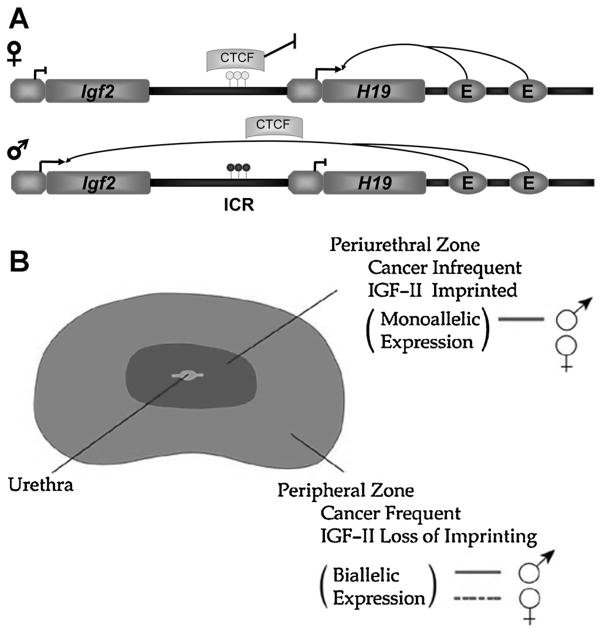
The insulin-like growth factor 2 (Igf2) gene and its 3′ neighbor H19 on 11p15 comprise one of the most widely studied ICRs. The role of DNA methylation and the insulator gene CTCF have been characterized in establishing the imprinted allele (Fig. 1). Igf2 is a potent growth factor and loss of Igf2 imprinting in normal tissues has been casually associated with an increased cancer incidence in the colon [55,56]. Igf2 is known to act upon epithelial cells predominantly in a paracrine fashion due to its higher expression in stromal cells. However, some evidence for autocrine loops in cultured human prostate cells has been presented [57]. LOI of Igf2 has also been found in other types of adult cancers including gastric [58], ovarian [59], and esophogeal [60]. The spatially distinct relationship of disease in the prostate presents a unique scenario. Initially, biallelic expression of the Igf2 gene was shown in both cancerous and associated histologically normal tissue in the peripheral prostate, but not in BPH which is centrally located and embryologically distinct [61]. We have found that this LOI marks a widespread field effect throughout the peripheral prostate that may contribute to the multifocality of the disease [62]. In mice and in humans, a clear erosion of the Igf2 imprint can be seen with aging in the prostate [63]. In the mouse this change is not seen in kidney, liver or the adjacent seminal vesicle. These data provide evidence that AREAS may underlie the predisposition of the prostate for cancer formation.
Fig. 1.
Igf2 imprinting. A: Schematic of Igf2-H19 imprint regulation. The imprinted Igf2/H19 locus contains an intergenic imprint control region (ICR) along with shared enhancers that collectively coordinate gene expression. Normal imprinting in this region is characterized by CpG methylation on the paternal allele (lower) within the ICR. This CpG methylation blocks CTCF, a chromatin insulator, binding to Igf2 and facilitates enhancer binding to the Igf2 promoter leading to expression of the gene. CTCF binds hypomethylated CpGs on the maternal allele (upper) at the ICR blocking enhancer binding to the Igf2 promoter and silencing of gene expression. Aging is associated with decreased CTCF levels and altered methylation at the ICR. B: Model of Igf2 imprinting in the human prostate. The perhipheral zone demonstrates the regional loss of imprinting associated with prostate disease that is age-dependent. Cancers commonly form in this region and also demonstrate a loss of imprinting. In the central zone, benign prostatic hyperplasia (BPH) is found. Igf2 imprinting is maintained in this region.
The H19/Igf2 locus demonstrates both DNA methylation and insulator genes, such as CTCF, are both required to maintain imprinting status. Our studies indicate CTCF binding decreases at the Igf2/H19 ICR in the aging mouse prostate [63]. As a genomic insulator, CTCF binds widely throughout the genome and is implicated in epigenetic homeostasis [64]. This involvement with both imprinting and epigenetic processes makes CTCF an important candidate for the dysregulation of genes seen in aging and PCa.
LOSS OF IMPRINTING AND CARCINOGENESIS
These studies and many others establish a link between LOI and carcinogenesis. The understanding of when and how these changes occur is still evolving. Mutations in imprinted genes are rare and would only explain a focal cancer development. Environmental stressors, or inherent susceptibility, or a combination of the two may better explain the widespread relaxation of an imprint within a tissue. Environmental exposures may induce epigenetic changes or elicit signaling anomalies leading to widespread reprogramming at imprinted regions.
Similar to DNA methylation, imprinting marks, such as the differentially methylated Igf2/H19 imprint control region, can be altered by oxidative stress and diet. A mouse model of oxidative stress generated by deletion of CuSOD shows a decrease in DNA methylation at the Igf2 promoter and CTCF binding site 3 in the ICR [41]. These decreases correlated with a twofold decrease in Igf2 expression at 12 months. Changes in Igf2 expression are consistent with other studies and may be due to the sensitivity of the Igf2/H19 region to de novo methylation [65]. Other known factors that modulate the Igf2 imprint, such as CTCF, also represent a potential mechanism underlying changes with oxidative and other stress.
Igf2 is a well-studied imprinted locus, however other imprinted genes are susceptible to changes in methylation. Holm et al. used a transient demethylation model to generate mice lacking imprinting at all loci. Chimeric adult mice derived from this model demonstrate widespread LOI that resulted in increased tumorigenesis in the liver and intestine [66]. A study on mouse mammary carcinogenesis demonstrated imprinted genes may play a role in AREAS development in breast cancer. The imprinted gene Decorin decreased with age and was associated with spontaneous tumor development [67]. Changes in imprinting may also demonstrate transgenerational effects. Cui et al. [55] found an association between LOI in colon tissues and a positive family or personal history of colorectal neoplasia suggesting altered imprinting may be involved in disease susceptibility.
Novel techniques are opening new doors in the research of imprinted genes. The use of transcriptome sequencing was used to probe imprinted genes throughout the genome [52]. This early study identified six novel imprinted genes in mouse tissues. More recently, an interest in allele-specific methylation (ASM) has arisen in the development of disease [68]. Differential methylation of alleles may occur during PCa progression and is more widespread than previously thought occurring at greater than 30% of CpGs in hypermethylated or hypomethylated CGIs in one study [69]. These new interests may aid in our understanding of complex disease genetics and provide new insights into aging and cancer.
HISTONE MODIFICATIONS
Histones are fundamental building blocks for the tightly regulated chromatin packaging within the genome. The nucleosome consists of two of each of the core histones (H2A, H2B, H3, and H4), with 147 base pairs of DNA wrapped around the octamer [70]. Histones have amino-terminal tails that are subject to post-translational modifications resulting in the histone code. Acetylation and methylation are the most widely studied modifications in aging and have been implicated in transcriptional activation or repression. Histone phosphorylation and ubiquitination are a subject of significant research in cancer. Acetylation is generally associated with euchromatin and an active transcription profile. Methylation of histone tails presents a more complex situation, where activation or repression is linked to the position of the residue within the histone [71]. Each of these modifications affects chromatin in a different way, by either altering molecular associations of chromatin or recruiting other proteins to the chromatin.
Studies have suggested that alterations in histone modifications are important in PCa [72]. Immunohistochemical analysis of primary prostatectomy tissue samples revealed an association of H3K18Ac, H3K4Me2, H4K12Ac, and H3K9Ac with increasing tumor grade [73]. Remarkably, by analyzing the percentile staining of just two modifications, H3K4Me2 and H3K18Ac, patients could be grouped into lower and higher risk recurrence risk groups. In normal prostate cells active gene promoters are associated with H3 lysine 4 methylation (H3K4me2 and H3K4me3) and H3 lysine 9 acetylation (H3K9acetyl). These are replaced by repressive marks (H3K9me2, H3K9me3, and H3K27me3) in transcriptionally silenced genes [72]. H4K20me1 specifically identifies CRPC, while H4K20me2 distinguishes different stages of PCa [72].
To date, no analyses have been performed on the state of histone modifications during aging. Decreases of H3K4me2 occur with aging human fibroblasts in vitro [74]. DNA methyltranferases regulate aging in stem cells via EZH2, an important histone methylase in PCa progression [75]. Studies of monozygotic twins reveal acetylation of histones H3 and H4 patterns diverge between twins with increasing age in various tissues, including lymphocytes and sketetal muscle [3]. Recent evidence suggests that modulating histones may impact prostate function. In a study involving men on long-term antiepileptics (e.g., valproic acid, carbamazepine, oxcarbazepine, lamotrigine, and leve-tiracetam) drugs known to inhibit histone deacetylases (HDAC), treatment resulted in lower PSA levels than age-matched counterparts [76]. This effect was more marked in older men. As HDAC inhibitors, these drugs may impact the androgen receptor with aging resulting in lower PSA levels. This study provides intriguing data suggesting a role for histone modifiers in cancer risk.
These epigenetic states pose questions about whether these marks have a casual or bystander relationship with cancer development [77]. Furthermore, to date there have been no demethylases identified or other clear mechanisms that underlie the significant reduction of H4K20me1/2 or other histone methylation. The aging studies clearly indicate histone modifications may be altered by environmental factors and as such represent an area of research with regard to epigenetic cancer susceptibility.
CONCLUSIONS
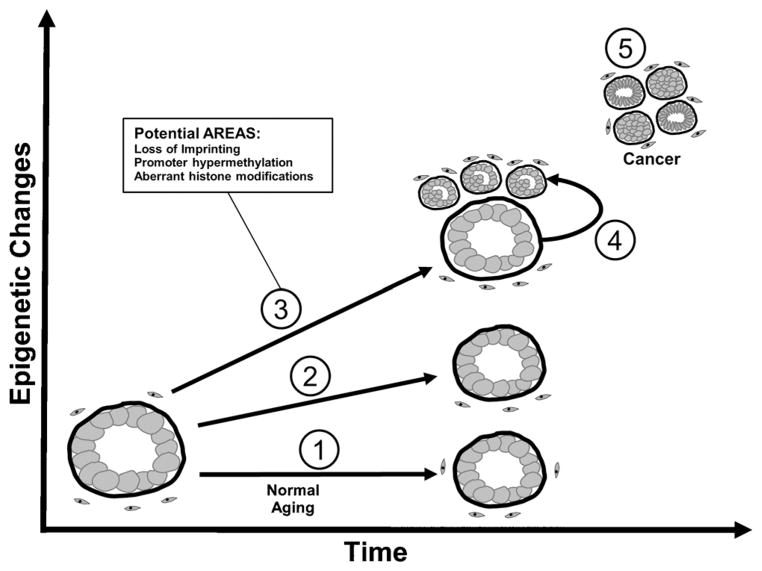
During aging, alterations in the epigenetic landscape results from endogenous and exogenous exposures. These changes affect tightly regulated imprinted regions, CpG methylation at islands, intergenic DNA methylation, and histone modifications. Epigenetic changes over a lifetime may accumulate in the prostate and result in the marked prevalence of the disease with aging. These accumulated changes that increase cancer incidence can be referred to as AREAS (Fig. 2). They involve regions of the susceptible prostate that give rise to cancer. One view of the field defect is that alterations are induced in normal tissues adjacent to tumors. In contrast, AREAS are widespread and give rise to subsequent neoplasms.
Fig. 2.
Age related epigenetic alterations inducing susceptibility (AREAS) in the prostate. Specific epigenetic alterations, including loss of imprinting, gene methylation, and histone modifications, are either stable (1) or erode (2) over time in the epithelium (or stroma) of the prostate that does not develop cancer. Alternately, in the prostate gland that develops cancer epigenetic alterations occurring during aging result in gene expression changes that confer a selective advantage to early neoplastic changes (3). These changes represent a field of susceptibility and may be accelerated by environmental insults. These epigenetic changes that occur in aging tissues are frequently selected for in tumors (4). Other cancer specific epigenetic alterations arise within the tumor and are age-independent (5).
Recent data on IGF2 imprinting supports a role for AREAS in the development of neoplastic lesions [62]. AREAS may be selected for in the subsequent tumor or may have an inductive effect that is not necessarily carried into the tumor cells. One interesting observation in both prostate and colon is an increase in epigenetic alterations found in tumors that arise from older individuals compared to tumors from younger patients [16]. This finding of increased epigenetic changes in the tumor with aging suggests a biologic selection for these increasingly altered epigenetic changes in aging mammals.
Further study of AREAS may provide insight into the progression from a normal prostate to a diseased state with aging. There are examples of modulation of a normal epigenetic state during gestation (parental folate status), puberty (altered hormonal exposure), and in the aging adult prostate (environmental toxins). Studies show that induced epigenetic changes are not simply silent passengers. They result in gene expression changes that provide selection advantages, such as those in growth factors like IGF2 or inactivation of tumor suppressors like p57, which mediate disease progression.
Further characterization of AREAS generates a number of interesting questions in the study of prostatic disease. First, can these epigenetic changes be prevented or corrected? Epigenetic modulators are already being used in cancer therapy. Epigenetic maintenance may become an important factor in age-related disease prevention. In the study of aging, epigenetic maintenance has received attention and includes the study of compounds found in red wine, green teas, and curcumins, so called “Nutriepigenomics.” It is of interest that many compounds being studied in longevity, such as resveratrol, also have chemoprevention properties [78]. Researchers are already focusing on many of these compounds in the context of prostate health and disease.
Can AREAS be used to identify individuals at increased risk for cancer, in addition to those with cancer? Studies have already shown how certain epigenetic marks (histone modifications, DNA methylation) correlate with disease outcome. The altered epigenome with aging that predisposes and is associated with cancer provides a potential mechanism to identify individuals at risk or early in their disease course. Recent work in our laboratory evaluated DNA methylation markers in a series of biopsies containing histologically normal tissue associated with or without the presence of cancer [79]. EVX1 and FGF1 in combination were able to strongly distinguish the presence of cancer (AUC 0.79 and a negative predictive value of 0.91). In conclusion, the aging prostate represents an understudied area that offers important clues to both the development of the disease and its cure.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH); Grant number: 5R01CA097131.
Footnotes
No financial connection between any of the authors and the subject matter.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male-patients. J Urol. 1993;150(2):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 3.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein JI, Carmichael MJ, Partin AW, Walsh PC. Small high grade adenocarcinoma of the prostate in radical prostatectomy specimens performed for nonpalpable disease: Pathogenetic and clinical implications. J Urol. 1994;151(6):1587–1592. doi: 10.1016/s0022-5347(17)35309-0. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 6.Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339(6127):1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdasco M, Esteller M. Hot topics in epigenetic mechanisms of aging: 2011. Aging Cell. 2012;11(2):181–186. doi: 10.1111/j.1474-9726.2012.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Leung FC. An evaluation of new criteria for CpG islands in the human genome as gene markers. Bioinformatics. 2004;20(7):1170–1177. doi: 10.1093/bioinformatics/bth059. [DOI] [PubMed] [Google Scholar]
- 9.Straussman R, Nejman D, Roberts D, Steinfeld I, Blum B, Benvenisty N, Simon I, Yakhini Z, Cedar H. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16(5):564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Serra L, Esteller M. Proteins that bind methylated DNA and human cancer: Reading the wrong words. Br J Cancer. 2008;98(12):1881–1885. doi: 10.1038/sj.bjc.6604374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 12.Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese TL, Isaacs WB, Bova GS, De Marzo AM, Nelson WG. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68(21):8954–8967. doi: 10.1158/0008-5472.CAN-07-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedford MT, van Helden PD. Hypomethylation of DNA in pathological conditions of the human prostate. Cancer Res. 1987;47(20):5274–5276. [PubMed] [Google Scholar]
- 14.Yang B, Sun H, Lin W, Hou W, Li H, Zhang L, Li F, Gu Y, Song Y, Li Q, Zhang F. Evaluation of global DNA hypomethylation in human prostate cancer and prostatic intraepithelial neoplasm tissues by immunohistochemistry. Urol Oncol. 2011;31(5):628–634. doi: 10.1016/j.urolonc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Cho NY, Kim JH, Moon KC, Kang GH. Genomic hypomethylation and CpG island hypermethylation in prostatic intraepithelial neoplasm. Virchows Arch. 2009;454(1):17–23. doi: 10.1007/s00428-008-0706-6. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Suzuki I, Leodolter A, Alonso S, Horiuchi S, Yamashita K, Perucho M. Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell. 2006;9(3):199–207. doi: 10.1016/j.ccr.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Kamiyama H, Suzuki K, Maeda T, Koizumi K, Miyaki Y, Okada S, Kawamura YJ, Samuelsson JK, Alonso S, Konishi F, Perucho M. DNA demethylation in normal colon tissue predicts predisposition to multiple cancers. Oncogene. 2012;31(48):5029–5037. doi: 10.1038/onc.2011.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Absher DM, Gulzar ZG, Young SR, McKenney JK, Peehl DM, Brooks JD, Myers RM, Sherlock G. DNA methylation profiling reveals novel biomarkers and important roles for DNA methyltransferases in prostate cancer. Genome Res. 2011;21(7):1017–1027. doi: 10.1101/gr.119487.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7 (4):536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 20.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58 (23):5489–5494. [PubMed] [Google Scholar]
- 21.Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Grieu F, Watanabe G, Iacopetta B. DNA hypermethylation in the normal colonic mucosa of patients with colorectal cancer. Br J Cancer. 2006;94(4):593–598. doi: 10.1038/sj.bjc.6602940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwabi-Addo B, Chung W, Shen L, Ittmann M, Wheeler T, Jelinek J, Issa JP. Age-related DNA methylation changes in normal human prostate tissues. Clin Cancer Res. 2007;13(13):3796–3802. doi: 10.1158/1078-0432.CCR-07-0085. [DOI] [PubMed] [Google Scholar]
- 23.Henrique R, Jerónimo C, Teixeira MR, Hoque MO, Carvalho AL, Pais I, Ribeiro FR, Oliveira J, Lopes C, Sidransky D. Epigenetic heterogeneity of high-grade prostatic intraepithelial neoplasia: Clues for clonal progression in prostate carcinogenesis. Mol Cancer Res. 2006;4(1):1–8. doi: 10.1158/1541-7786.MCR-05-0113. [DOI] [PubMed] [Google Scholar]
- 24.Pili R, Salumbides B, Zhao M, Altiok S, Qian D, Zwiebel J, Carducci MA, Rudek MA. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer. 2012;106 (1):77–84. doi: 10.1038/bjc.2011.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasiljevi N, Wu K, Brentnall AR, Kim DC, Thorat MA, Kudahetti SC, Mao X, Xue L, Yu Y, Shaw GL, Beltran L, Lu YJ, Berney DM, Cuzick J, Lorincz AT. Absolute quantitation of DNA methylation of 28 candidate genes in prostate cancer using pyrosequencing. Dis Markers. 2011;30(4):151–161. doi: 10.3233/DMA-2011-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li LC, Shiina H, Deguchi M, Zhao H, Okino ST, Kane CJ, Carroll PR, Igawa M, Dahiya R. Age-dependent methylation of ESR1 gene in prostate cancer. Biochem Biophys Res Commun. 2004;321 (2):455–461. doi: 10.1016/j.bbrc.2004.06.164. [DOI] [PubMed] [Google Scholar]
- 27.Guan M, Zhou X, Soulitzis N, Spandidos DA, Popescu NC. Aberrant methylation and deacetylation of deleted in liver cancer-1 gene in prostate cancer: Potential clinical applications. Clin Cancer Res. 2006;12(5):1412–1419. doi: 10.1158/1078-0432.CCR-05-1906. [DOI] [PubMed] [Google Scholar]
- 28.Yang B, Bhusari S, Kueck J, Weeratunga P, Wagner J, Leverson G, Huang W, Jarrard DF. Methylation profiling defines an extensive field defect in histologically normal prostate tissues associated with prostate cancer. Neoplasia. 2013;15(4):399–408. doi: 10.1593/neo.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 30.Horii J, Hiraoka S, Kato J, Harada K, Kuwaki K, Fujita H, Toyooka S, Yamamoto K. Age-related methylation in normal colon mucosa differs between the proximal and distal colon in patients who underwent colonoscopy. Clin Biochem. 2008;41 (18):1440–1448. doi: 10.1016/j.clinbiochem.2008.08.089. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka S, Kato J, Horii J, Saito S, Harada K, Fujita H, Kuriyama M, Takemoto K, Uraoka T, Yamamoto K. Methylation status of normal background mucosa is correlated with occurrence and development of neoplasia in the distal colon. Hum Pathol. 2010;41(1):38–47. doi: 10.1016/j.humpath.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haffner MC, Chaux A, Meeker AK, Esopi DM, Gerber J, Pellakuru LG, Toubaji A, Argani P, Iacobuzio-Donahue C, Nelson WG, Netto GJ, De Marzo AM, Yegnasubramanian S. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget. 2011;2(8):627–637. doi: 10.18632/oncotarget.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2012;13(1):7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 35.Chia N, Wang L, Lu X, Senut MC, Brenner C, Ruden DM. Hypothesis: Environmental regulation of 5-hydroxymethylcyto-sine by oxidative stress. Epigenetics. 2011;6(7):853–856. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- 36.Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, Haile RW, Laird PW. Environmental epigenetics: Prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012;131(10):1565–1589. doi: 10.1007/s00439-012-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: The evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61(16):6025–6028. [PubMed] [Google Scholar]
- 39.Turk PW, Laayoun A, Smith SS, Weitzman SA. DNA adduct 8-hydroxyl-2′-deoxyguanosine (8-hydroxyguanine) affects function of human DNA methyltransferase. Carcinogenesis. 1995;16 (5):1253–1255. doi: 10.1093/carcin/16.5.1253. [DOI] [PubMed] [Google Scholar]
- 40.Weitzman SA, Turk PW, Milkowski DH, Kozlowski K. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci USA. 1994;91(4):1261–1264. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhusari SS, Dobosy JR, Fu V, Almassi N, Oberley T, Jarrard DF. Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics. 2010;5 (5):402–409. doi: 10.4161/epi.5.5.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: A review. J Nutr. 2002;132(8 Suppl):2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 43.Shannon J, Phoutrides E, Palma A, Farris P, Peters L, Forester A, Tillotson CJ, Garzotto M. Folate intake and prostate cancer risk: A case–control study. Nutr Cancer. 2009;61(5):617–628. doi: 10.1080/01635580902846593. [DOI] [PubMed] [Google Scholar]
- 44.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: Effects on DNA methylation and gene expression. Cancer Res. 1992;52(7 Suppl):2071s–2077s. [PubMed] [Google Scholar]
- 45.Dobosy JR, Fu VX, Desotelle JA, Srinivasan R, Kenowski ML, Almassi N, Weindruch R, Svaren J, Jarrard DF. A methyl-deficient diet modifies histone methylation and alters Igf2 and H19 repression in the prostate. Prostate. 2008;68(11):1187–1195. doi: 10.1002/pros.20782. [DOI] [PubMed] [Google Scholar]
- 46.Waterland RA, Jirtle RL. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66 (11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE. 2013;8(1):e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34(4):694–707. doi: 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horsthemke B. Mechanisms of imprint dysregulation. Am J Med Genet C Semin Med Genet. 2010;154C(3):321–328. doi: 10.1002/ajmg.c.30269. [DOI] [PubMed] [Google Scholar]
- 52.Babak T, Deveale B, Armour C, Raymond C, Cleary MA, van der Kooy D, Johnson JM, Lim LP. Global survey of genomic imprinting by transcriptome sequencing. Curr Biol. 2008;18 (22):1735–1741. doi: 10.1016/j.cub.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 53.Reik W, Walter J. Genomic imprinting: Parental influence on the genome. Nat Rev Genet. 2001;2(1):21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 54.McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37 (1):179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 55.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: A potential marker of colorectal cancer risk. Science. 2003;299(5613):1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 56.Woodson K, Flood A, Green L, Tangrea JA, Hanson J, Cash B, Schatzkin A, Schoenfeld P. Loss of insulin-like growth factor-II imprinting and the presence of screen-detected colorectal adenomas in women. J Natl Cancer Inst. 2004;96(5):407–410. doi: 10.1093/jnci/djh042. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa JA, Lee AV, Jackson JG, Yee D. Proliferation of cultured human prostate cancer cells is inhibited by insulin-like growth factor (IGF) binding protein-1: Evidence for an IGF-II autocrine growth loop. J Clin Endocrinol Metab. 1995;80(12):3476–3482. doi: 10.1210/jcem.80.12.8530586. [DOI] [PubMed] [Google Scholar]
- 58.Zuo QS, Yan R, Feng DX, Zhao R, Chen C, Jiang YM, Cruz-Correa M, Casson AG, Kang XD, Han F, Chen T. Loss of imprinting and abnormal expression of the insulin-like growth factor 2 gene in gastric cancer. Mol Carcinog. 2011;50(5):390–396. doi: 10.1002/mc.20731. [DOI] [PubMed] [Google Scholar]
- 59.Hiura H, Okae H, Kobayash H, Miyauchi N, Sato F, Sato A, Suzuki F, Nagase S, Sugawara J, Nakai K, Yaegashi N, Arima T. High-throughput detection of aberrant imprint methylation in the ovarian cancer by the bisulphite PCR-Luminex method. BMC Med Genomics. 2012;5:8. doi: 10.1186/1755-8794-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao R, DeCoteau JF, Geyer CR, Gao M, Cui H, Casson AG. Loss of imprinting of the insulin-like growth factor II (IGF2) gene in esophageal normal and adenocarcinoma tissues. Carcinogenesis. 2009;30(12):2117–2122. doi: 10.1093/carcin/bgp254. [DOI] [PubMed] [Google Scholar]
- 61.Jarrard DF, Bussemakers MJ, Bova GS, Isaacs WB. Regional loss of imprinting of the insulin-like growth factor II gene occurs in human prostate tissues. Clin Cancer Res. 1995;1(12):1471–1478. [PubMed] [Google Scholar]
- 62.Bhusari S, Yang B, Kueck J, Huang W, Jarrard DF. Insulin-like growth factor-2 (IGF2) loss of imprinting marks a field defect within human prostates containing cancer. Prostate. 2011;71 (15):1621–1630. doi: 10.1002/pros.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, Srinivasan R, Berres M, Svaren J, Weindruch R, Jarrard DF. Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res. 2008;68(16):6797–6802. doi: 10.1158/0008-5472.CAN-08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herold M, Bartkuhn M, Renkawitz R. CTCF: Insights into insulator function during development. Development. 2012;139 (6):1045–1057. doi: 10.1242/dev.065268. [DOI] [PubMed] [Google Scholar]
- 65.Biniszkiewicz D, Gribnau J, Ramsahoye B, Gaudet F, Eggan K, Humpherys D, Mastrangelo MA, Jun Z, Walter J, Jaenisch R. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22 (7):2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holm TM, Jackson-Grusby L, Brambrink T, Yamada Y, Rideout WM, Jaenisch R. Global loss of imprinting leads to widespread tumorigenesis in adult mice. Cancer Cell. 2005;8(4):275–285. doi: 10.1016/j.ccr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Gu Y, Zhang S, Wu Q, Xu S, Cui Y, Yang Z, Zhao X, Sun B. Differential expression of decorin, EGFR and cyclin D1 during mammary gland carcinogenesis in TA2 mice with spontaneous breast cancer. J Exp Clin Cancer Res. 2010;29:6. doi: 10.1186/1756-9966-29-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aryee MJ, Liu W, Engelmann JC, Nuhn P, Gurel M, Haffner MC, Esopi D, Irizarry RA, Getzenberg RH, Nelson WG, Luo J, Xu J, Isaacs WB, Bova GS, Yegnasubramanian S. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci Transl Med. 2013;5(169):169ra110. doi: 10.1126/scitranslmed.3005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin PC, Giannopoulou EG, Park K, Mosquera JM, Sboner A, Tewari AK, Garraway LA, Beltran H, Rubin MA, Elemento O. Epigenomic alterations in localized and advanced prostate cancer. Neoplasia. 2013;15(4):373–383. doi: 10.1593/neo.122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Travers AA, Vaillant C, Arneodo A, Muskhelishvili G. DNA structure, nucleosome placement and chromatin remodelling: A perspective. Biochem Soc Trans. 2012;40(2):335–340. doi: 10.1042/BST20110757. [DOI] [PubMed] [Google Scholar]
- 71.Kouzarides T. SnapShot: Histone-modifying enzymes. Cell. 2007;128(4):802. doi: 10.1016/j.cell.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Behbahani TE, Kahl P, von der Gathen J, Heukamp LC, Baumann C, Gütgemann I, Walter B, Hofstädter F, Bastian PJ, von Ruecker A, Müller SC, Rogenhofer S, Ellinger J. Alterations of global histone H4K20 methylation during prostate carcinogenesis. BMC Urol. 2012;12:5. doi: 10.1186/1471-2490-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435(7046):1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 74.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17(10):1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.So AY, Jung JW, Lee S, Kim HS, Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS ONE. 2011;6(5):e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stettner M, Krämer G, Strauss A, Kvitkina T, Ohle S, Kieseier BC, Thelen P. Long-term antiepileptic treatment with histone deacetylase inhibitors may reduce the risk of prostate cancer. Eur J Cancer Prev. 2012;21(1):55–64. doi: 10.1097/CEJ.0b013e32834a7e6f. [DOI] [PubMed] [Google Scholar]
- 77.Balakrishnan L, Milavetz B. Decoding the histone H4 lysine 20 methylation mark. Crit Rev Biochem Mol Biol. 2010;45(5):440–452. doi: 10.3109/10409238.2010.504700. [DOI] [PubMed] [Google Scholar]
- 78.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Truong M, Yang B, Livermore A, Wagner J, Weeratunga P, Huang W, Dhir R, Nelson J, Lin DW, Jarrard DF. Using the epigenetic field defect to detect prostate cancer in biopsy negative patients. J Urol. 2012;189(6):2335–2341. doi: 10.1016/j.juro.2012.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwabi-Addo B, Wang S, Chung W, Jelinek J, Patierno SR, Wang BD, Andrawis R, Lee NH, Apprey V, Issa JP, Ittmann M. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin Cancer Res. 2010;16(14):3539–3547. doi: 10.1158/1078-0432.CCR-09-3342. [DOI] [PubMed] [Google Scholar]
- 81.Maruyama R, Toyooka S, Toyooka KO, Virmani AK, Zöchbauer-Müller S, Farinas AJ, Minna JD, McConnell J, Frenkel EP, Gazdar AF. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin Cancer Res. 2002;8(2):514–519. [PubMed] [Google Scholar]
- 82.Rabiau N, Thiam MO, Satih S, Guy L, Kemeny JL, Boiteux JP, Fontana L, Bignon YJ, Bernard-Gallon D. Methylation analysis of BRCA1, RASSF1, GSTP1 and EPHB2 promoters in prostate biopsies according to different degrees of malignancy. In Vivo. 2009;23(3):387–391. [PubMed] [Google Scholar]