Abstract
Advances in the treatment of myocardial infarction (MI) have improved survival after ischemic cardiac injury. Post-infarct structural and functional remodeling results in electrophysiologic substrates at risk for monomorphic ventricular tachycardia (MMVT). Characterization of this substrate using a variety of clinical and investigative tools has improved our understanding of MMVT circuits, and has accelerated the development of device and catheter-based therapies aimed at identification and elimination of this arrhythmia.
This review will discuss the central role of the ischemic heart disease substrate in the development MMVT. Electrophysiologic characterization of the post-infarct myocardium using bipolar electrogram amplitudes to delineate scar border zones will be reviewed. Functional electrogram determinants of reentrant circuits such as isolated late potentials will be discussed. Strategies for catheter ablation of reentrant ventricular tachycardia, including structural and functional targets will also be examined, as will the role of the epicardial mapping and ablation in the management of recurrent MMVT.
Keywords: Ventricular tachycardia, Ischemic cardiomyopathy, Catheter ablation, Mapping
1. Introduction
Significant improvements in the prevention, diagnosis, and treatment of coronary artery disease have resulted in higher rates of survival following acute myocardial infarctions (MIs).1 As a result of rapid reperfusion, increased implementation of hemodynamic support devices, and adherence to antiplatelet and neurohormonal therapies, an increasing number of patients with healed myocardial infarcts require electrophysiologic management for the treatment and prevention of ventricular arrhythmias.2
This review will concentrate on the structural and functional features of ischemic myocardial substrates in the genesis of ventricular arrhythmias after healed MI. Polymorphic ventricular tachycardia and ventricular fibrillation, which are more prevalent in the acute to sub-acute ischemic period, will not be discussed. Components of the ischemic cardiomyopathic substrate, which facilitate the reentrant pathways characteristic of monomorphic ventricular tachycardia (MMVT) will be discussed, along with implications for catheter-based management of these arrhythmias. Automatic ventricular tachycardia, which may appear monomorphic, will not be discussed in this review.
2. Post-infarct myocardial remodeling
2.1. Structural remodeling
The healing phase of infarcted myocardium is characterized by infiltration of the infarcted myocardial tissue by inflammatory cells. Necrotic myocytes are cleared by macrophages, and replacement fibrous tissue, consisting of collagen, is deposited by fibroblasts over the next days, weeks, and months. The ischemic wavefront of necrosis proceeds from the subendocardium to epicardium during myocardial ischemia,3 and scar deposition parallels this sequence. Critical elements arise during this process, which permit the reentrant circuits that characterize MMVT. The heterogeneous process of myocyte resorption and collagen deposition results in islands of surviving myocardial cells within healed infarct scars as shown in Fig. 1.4 These preserved myocardial bundles exist as a “labyrinth” in three dimensions, involving the endocardium, mid-myocardium, and epicardium. The functional electrophysiologic characteristics of these channels will be discussed in detail later in this review.
Fig. 1.
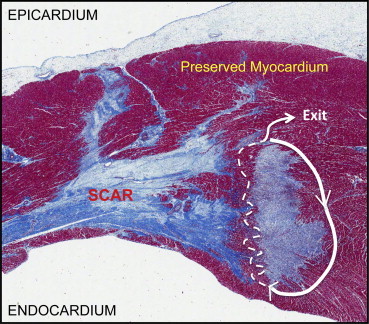
Histologic characterization of ischemic heart disease substrates. Masson trichrome stain of infarcted myocardium with “islands” of preserved myocardium surrounded by scar tissue (blue). A schematized representation of a possible ventricular tachycardia circuit is depicted with the while lines. Slowed “zig-zag” conduction through the “isthmus” of the circuit is represented by the broken white lines.
Adapted from Tung R, Boyle NG, Shivkumar K. Circulation. 2011; 123(20):2284–2288.
Channels that facilitate or sustain MMVT, consist of an entry site, a protected isthmus, and a breakthrough exit site that activates the ventricles.5 Functional or fixed conduction slowing and block in a channel can result in an excitable gap for reentry. These localized regions of slow conduction within scar exhibit “zig-zag” conduction during normal sinus rhythm through heterogeneously connected myocytes traversing dense unexcitable scar6 (Fig. 1). Post-infarct structural remodeling may also involve deposition of adipose tissue in the infarcted bed. A recent report showed that electrophysiologic consequences of post-infarct myocardial fatty replacement in ovine MI included decreased conduction velocity, and reduced bipolar electrogram amplitude.7 The presence of inducible VT in MI animals was associated with greater adipose content and slower conduction velocity in the border zones of infarcts.
These structural changes alter local “source–sink” mismatch (in favor of the “source”), given the diminution in electrotonic forces that minimize membrane voltage deviations in one (or a few) myocytes exerted via gap junctions. Reductions in source-sink mismatch facilitate the genesis of premature ventricular depolarizations, which trigger VTs.
2.2. Functional cellular remodeling
Remodeling of other important elements within and beyond the infarcted myocardial bed also occur, and add further complexity to the post-MI substrate. These include remodeling of myocyte ionic currents and gap junctions, direction of activation propagation, and intra-myocardial nerve fibers. Although human data are lacking, studies in animal models have provided insight into how ventricular myocyte action potential (AP) remodel in cardiomyocytes adjacent to a healed MI. These include reduction in the duration, upstroke amplitude, and velocity of border zone myocyte AP.8 Reductions have also been reported in peak calcium currents in surviving border zone cells. In addition, refractoriness in the infarcted animal heart is known to be more nonuniform compared to normal hearts, with varying degrees of AP prolongation exhibited by individual surviving myocytes adjacent to and remote from the infarcted myocardium. In the border zones of infarcts, this worsens heterogeneity in refractorinesss, and facilitates the development of unidirectional block, critical to genesis and perpetuation of VT.
The distribution of connexin 43 gap junctions in the border zone of the infarcted human heart is also aberrant. Fewer gap junctions are organized into transverse (side-to-side) connections, rather the gap junctions are redistributed in a longitudinal fashion.9 Detailed animal studies support light microscopy findings in humans, with altered anisotropic conduction in the healed infarct heart. In addition, interstitial fibrosis results in displacement of myocytes from each other, and a significant decrease in the syncytial connection of myocytes in the infarct border zone.10
Another component of post-MI functional change is neural remodeling. Nerve endings in the infarcted bed are resorbed, however, since the neuronal cell body from which these nerves originate is removed from the infarcted territory, plastic growth is driven at the most distal intact endings adjacent to the infarcted tissue. This nerve sprouting in the border zone is heterogeneous, and is unable to penetrate scar tissue. The presence of these sprouts has been associated with ventricular arrhythmias and sudden death in humans,11 likely related to local heterogeneity in myocyte electrophysiological properties. We have demonstrated that the border zone of infarcts exhibits variable responses to autonomic stress (Fig. 2).12 Remodeling within stellate ganglia, which partly control myocardial excitability, has also been reported, and likely contributes to enhanced sympathetic tone and electrical heterogeneity seen after MI.13
Fig. 2.
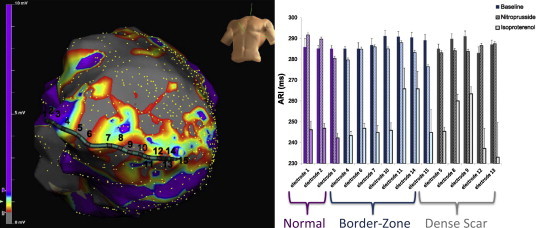
Heterogeneous responses to direct and reflex sympathetic nerve stimulation in scar, border zone, and preserved myocardium. Image on the left depicts an electroanatomic map of a patient with a large anterior wall infarction (with dense scar in gray, preserved myocardium in purple, and border zone in other colors) and the location of a multi-electrode catheter. The image on the right shows the activation recovery interval (ARI) at baseline and in response to reflex (nitroprusside) and direct (isoproterenol) sympathetic stimulation. In preserved myocardium, direct activation via adrenergic receptors is fairly uniform, however in the border zone and scar regions, this response is heterogeneous, reflecting varying degree of adrenergic receptor responses in adjacent myocardium. In response to reflex sympathetic stimulation induced by cardiac sympathetic nerves, significant heterogeneity is seen across the entire spectrum of scar, border zone, and even “preserved” myocardium.
Adapted from Vaseghi M, Lux RL, Mahajan A et al. Am J Physiol Heart Circ Physiol 2012, 302(9):H1838–46.
In summary, the healed infarct heart exhibits changes in the structural and functional characteristics of the myocardial substrate. These alterations facilitate the conditions required for initiation and maintenance of sustained reentrant ventricular arrhythmias.
3. Characterization of ischemic heart disease substrates
3.1. Role of imaging
Myocardial imaging has an increasing role in the diagnosis and treatment of VT in the ischemic heart disease substrate. Contrast enhanced-magnetic resonance imaging (MRI) appears to provide the most valuable information on cardiac structure, owing to its superior resolution (Fig. 3A) over positron emission tomography (PET) and computed tomography (CT).14
Fig. 3.
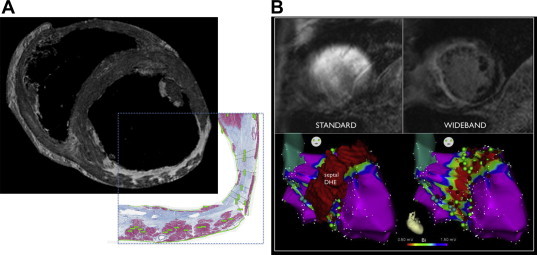
Characterization of ischemic heart disease substrates – magnetic resonance imaging. A An ex vivo magnetic resonance image (MRI) of an infarcted porcine heart is shown (top image), with the corresponding Masson's trichrome stain of the same region shown in the inset. The high resolution of this MRI image relative to the histologic image can be readily recognized. It should be noted that artifacts created by cardiac motion and presence of thoracic structures are not present in ex vivo MR images. B The top left and right images depict an MR image before and after application of the WIDEBAND method of device artifact attenuation. The bottom left and right images depict the corresponding voltage map (right anterior oblique projection of left ventricular endocardium) showing consistency between the WIDEBAND (left) and electroanatomic (right) location of scar.
Adapted from Stevens SM, Tung R, Rashid S et al. Heart Rhythm 2013 in press.
Pre-procedurally, imaging is used to identify the location and transmurality of scar. Patchy scars (which may support VT) and border zones of scar (where VT exit sites are typically located) can be identified prior to the procedure to guide mapping. Scar transmurality has been correlated to sites of VT termination, as are sites with late potentials.15,16 A recent study using CT showed consistent findings with MRI data, with regards to post infarct wall thinning.17 Regions of heterogeneity in contrast enhanced MRI, also known as “gray zones”, have been related to MMVT inducibility.
The resolution of in vivo MRI still has limitations, due to partial volume effects from cardiac motion, making determinations of transmurality suboptimal. The presence of ICDs in most patients undergoing VT ablation raises concerns about performing MRIs. At our center we have routinely performed MR imaging in this patient population (except when abandoned leads are present, or when the patient is pacing-dependent) without adverse outcomes. Image artifacts created by the device can also be minimized (Fig. 3B) to improve the quality and usefulness of images obtained in patients with devices.18 Intra-procedural imaging in contemporary VT ablation can be achieved with ICE,19 although its utility extends beyond ablation of the ischemic substrate. Intra-procedural MRI remains under development, however its role in real-time characterization of lesion formation, and ischemic substrate homogenization remains promising.20
3.2. Electrophysiologic characterization
3.2.1. Development and validation of electrogram amplitude
The characteristics of bipolar electrograms recorded from normal myocardium and scar are distinct. This difference is the basis of voltage mapping. Electrical signals are recorded from cardiac tissue using catheters fitted with bipolar (or multipolar) electrodes. These signals are filtered at 30–40 Hz to 400–500 Hz, and bipolar electrograms demonstrating sharp bi or tri-phasic waveforms with amplitudes greater than 3 mV and durations under 70 ms are consistent with normal myocardium. Bipolar electrograms with amplitudes less than 0.5 mV, showing durations >133 ms, and showing fractionations are consistent with recordings from regions of myocardial scar.21 Current mapping cut-offs utilize bipolar electrogram amplitudes of >1.5 mV and <0.5 mV22 to delineate normal myocardium and dense scar respectively, with regions demonstrating intermediate amplitudes designated as border-zone areas (Fig. 4). These voltage thresholds have been validated in multiple studies. In a porcine healed infarct model, Callans et al showed that bipolar electrograms <1 mV in amplitude correlated with regions of infarction.23 Wrobleski et al24 similarly correlated such areas in chronic infarcted porcine heart with bipolar electrograms <1.5 mV and under ≥50 ms in duration. More detailed validation of electroanatomic data recorded in humans has been performed using imaging modalities such as MRI, PET,25 and CT.17,26
Fig. 4.
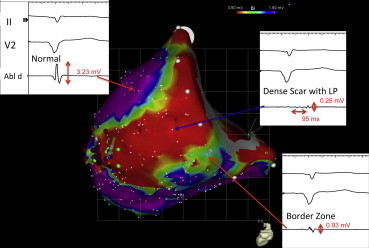
Bipolar electrogram morphology in ischemic heart disease substrates. Representative examples of bipolar electrograms (and amplitudes) recorded from regions of “normal”, dense scar, and border zone myocardium are shown superimposed onto a voltage map (0.5–1.5 mV). A significant portion of the electrograms recorded in the dense car region are “late” (i.e. occur well after the surface QRS) and are very low in amplitude (<0.5 mV).
3.2.2. Electroanatomic mapping
Electroanatomic mapping systems are capable of creating 3-D myocardial geometry based on tissue contact, and superimpose color-coded characterization of normal, border zone, and dense scar myocardial regions (Fig. 4). Myocardium is sampled point-by-point, with the corresponding bipolar voltage amplitude binned into scar (<0.5 mV), border zone (0.5–1.5 mV), or normal (>1.5 mV) tissue. Intra-procedural electrical characterization of the myocardial substrate using one of the two available platforms (magnetic-based CARTO, Biosense Webster, Diamond Bar, CA and electrofield-based Ensite NaVX, St Jude Medical, Minneapolis, MN) is the standard in contemporary VT ablation.
The correlation between EAM data and CE-MRI delineation of myocardial substrates remains imperfect. In a study by Wijnmaalen et al, although there was excellent correlation between infarcted regions on CE-MRI and electrical infarct localization (bipolar electrogram <1.5 mV), scar areas on MRI were significantly larger than scar areas delineated by EAM.27 In this study, approximately 20% of points taken in MRI-designated regions of dense transmural scar recorded “normal” bipolar electrograms (>1.5 mV). The converse may also account for poor correlation between EAM and other imaging modalities or histopathology i.e. bipolar electrograms <1.5 mV recorded from areas with normal tissue. The latter issue may arise due to poor catheter tissue contact or stability, changes in the volume loaded state of the patient (increased during EAM relative to imaging), inadequate delineation on CE-MRI.
4. Treatment of ventricular tachycardia in ischemic substrates
4.1. Pharmacologic therapy
Despite decades of intense research and development, few effective drugs with minimal side effects are available for the treatment of recurrent MMVT. Beta-adrenergic receptor blockers have been studied in a variety of post-myocardial infarction trials, demonstrating substantial reduction in the risk of sudden death and recurrent VT28,29. The efficacy of beta blockers in stable MMVT is however limited. Sotalol demonstrates greater efficacy compared to beta blockers, however, the risk of prolonged QT and PMVT restricts its use. The proarrhythmic risk of anti-arrhythmics drugs in the post-infarct setting have long been recognized,30,31 with amiodarone demonstrating a safer risk profile.32–34,23 Given the long-term risk of side effects, amiodarone is a less favorable choice for long-term management of VTs in ICM patients. Newer agents such as ranolazine have shown some promise in reducing VT and ICD shocks in ICM patients without significantly increased risk profile.35
4.2. Defibrillator therapy
Implantable cardioverter-defibrillators have been demonstrated in multiple trials to reduce the risk of sudden death in patients with ischemic heart disease substrates.36 Although defibrillators save lives, they do not prevent the initiation of VT, and patients frequently present to medical attention with repetitive ICD shocks. Defibrillator shocks are associated with significant medical and psychiatric morbidity. Post hoc analysis of ICD studies demonstrate that patients who receive appropriate ICD shocks have clinical outcomes that are less favorable than those patients without VT and who do not receive ICD shocks.37 While the underlying reason for this phenomenon is not clear, it emphasizes that although defibrillators have a role in preventing sudden death from ventricular arrhythmias, adjunctive therapies are required to mitigate arrhythmogenesis.
4.3. Surgical treatment
In contemporary management of recurrent MMVT, surgical therapy has a limited role. Although intraoperative mapping studies provided substantial insights into the mechanisms underlying scar-related VT,38,39 it has fallen out of favor due to the morbidity of surgical exposure of the heart for mapping and ablation.40 This is especially true in the era of minimally based access to the endocardium and epicardium, and mini-thoracotomy for epicardial access, which is increasingly implemented.41,42 However, it is important to note that the advent of border zone ablation was an attempt to mimic encircling ventriculotomy and subendocardial resection.43,44
4.4. Catheter ablation
Catheter-based management of MMVT is an increasingly adopted strategy, with a number of studies supporting its role over antiarrhythmic drugs.45–48 It requires careful patient selection, pre-procedural planning and imaging, procedural substrate characterization, induction and mapping of tolerable MMVTs, ablation of electrophysiologic and substrate targets, and careful post-procedural care, while minimizing risks of complications. Catheter ablation is often reserved for patients with ischemic heart disease who suffer ICD shocks or recurrent arrhythmias (including electrical storm), although a growing trend is the early introduction of catheter ablation of VT.
4.4.1. Identification of clinical VT
Patients may be inducible for multiple VT morphologies, however, only one, or a few, may be responsible for the patient's presentation. Identification of clinically relevant VT by 12-lead electrocardiogram, if possible, is important, as it provides a target, and meaningful endpoint for the procedure. The ventricular site of origin of the clinical VT deduced from a 12-lead ECG may also guide procedural planning and approach. If the 12-lead ECG suggests an epicardial exit, preparatory measures for epicardial access can be performed before the procedure.49,50
For some patients, only ICD recordings of the clinical VT may be available. The VT cycle length may be a useful clue to the clinically relevant VT. Patients with devices can also undergo a noninvasive programmed stimulation (NIPS) in the day(s) prior to VT ablation, or at the start of the procedure. The morphologies of inducible VTs can be noted on the electrophysiologic recording systems, and used as templates during the procedure, when the patient is typically under deeper sedation. The morphologies of induced VTs can also be compared to ICD recordings in terms of morphology and CL for VTs that were monitored, or for which therapies were delivered.51
4.4.2. Procedural strategy
The major determinant of mapping strategy is whether the VT is hemodynamically tolerated. An approach, used at our center, is detailed in the chart shown in Fig. 5.52 For hemodynamically tolerated VTs, activation mapping is preferable, as the origin of the VT can be mapped in detail for exit sites and critical isthmuses showing diastolic potentials (Fig. 6). Entrainment mapping during VT can be performed to confirm critical isthmuses or exit sites, and ablation performed. Attention can then be focused on addressing the substrate facilitating the clinical VT.
Fig. 5.
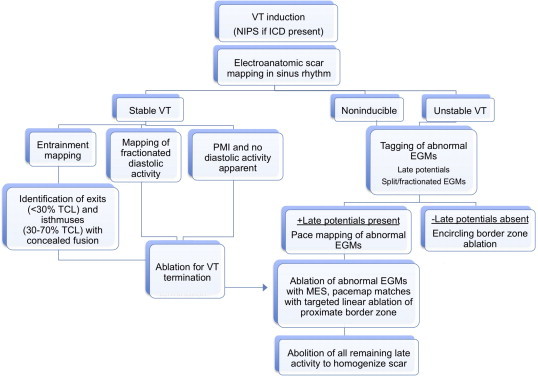
Approach to catheter mapping and ablation of ventricular tachycardia (VT). EGM: electrogram. ICD: implantable cardioverter-defibrillator; NIPS: noninvasive programmed stimulation; TCL: tachycardia cycle length.
Adapted from Tung R, Mathuria N, Michowitz Y et al. Circ Arrhythmia Electrophysiol 2012;5:264–272.
Fig. 6.
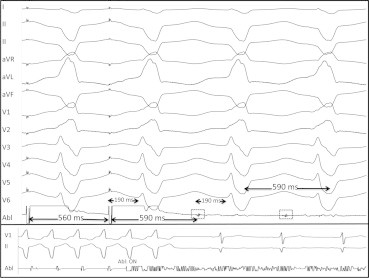
Entrainment and ablation of monomorphic ventricular tachycardia (MMVT). Top panel: an MMVT with a cycle length of 590 ms is entrained at 560 ms. The post-pacing interval (PPI) is 590 ms, and exactly matches the TCL. The stimulus to QRS (Stim-QRS) interval shown on the last paced complex is 190 ms, which also exactly matches the electrogram to QRS (EGM-QRS) interval at 190 ms. The entrainment demonstrated here is also concealed (paced QRS morphology is identical to MMVT QRS morphology, i.e. no evidence of QRS fusion) These conditions (PPI = TCL, Stim-QRS = EGM-QRS, and concealed entrainment) confirm that the site is a protected and critical isthmus for this MMVT. Lower panel: Ablation at the site with entrainment characteristics demonstrated above terminates MMVT immediately.
Adapted from Tung R, Boyle NG, Shivkumar K. Circulation. 2011;123(20):2284–2288.
As most VTs are poorly tolerated hemodynamically, a substrate mapping approach is more commonly used to guide ablation. Strategies include anatomic and electrogram-based approaches.
4.4.3. Substrate mapping – anatomic strategies
4.4.3.1. Border zone ablation & scar homogenization
Border zone tissue has been shown to harbor both isthmus and exit sites for MMVT.53,54 The strategy of border zone ablation relies upon adequate mapping and electrophysiologic characterization of the substrate, which can be facilitated by multipolar catheters. Strategies to achieve border zone homogenization include creating linear lesions,45 transecting the scar with a “T” shaped lesion set, in addition to encircling.46 Substrate homogenization involves eradicating regions of preserved voltage within dense scar regions, which may serve as isthmuses or exit sites, and can be guided by abnormal electrograms.55
4.4.3.2. Identification and ablation of channels
As described previously, channels bordered by electrically unexcitable scar tissue form the basis for the majority of MMVTs. Adjusting voltage settings during mapping may identify macroscopic channels. Arenal et al demonstrated 23 channels in 20 patients using a tiered voltage threshold of 0.1–0.5 mV. Twenty of such channels were related to a VT morphology. In another study utilizing a tiered scar display, a mean voltage threshold of 0.33 ± 0.15 mV was found to be optimal for identifying channels, with an average observed channel length of 32 ± 21 mm (range 12–87 mm).54
Channels have been alternatively identified by electroanatomic mapping during hemodynamically tolerated MMVT. Channel characteristics identified include a mean length and width of 31 ± 7 mm and 16 ± 8 mm respectively. In addition, the location of the circuit was associated with channel orientation, with perimitral circuits demonstrating channels parallel to the mitral annulus, while all other channels were oriented perpendicular in 21 studied patients. Confirming the concept, ablation lesions transecting the narrowest portion of a common channel terminated VT in 97% of cases. In contrast to these studies, others have failed to find strong associations between channels and critical isthmuses of VTs. Mountantonakis and colleagues demonstrated that 88% of scar maps contained channels using tiered voltage settings, however, only 30% were shown by entrainment to contain the isthmus.56
As noted earlier, these channels exist as a labyrinth in 3-D, and may be interconnected. Our group assessed the connectivity between channels, such that ablation of a critical LP reflecting the common isthmus of these channels, causes eradication of multiple channels (Fig. 7A).57
Fig. 7.
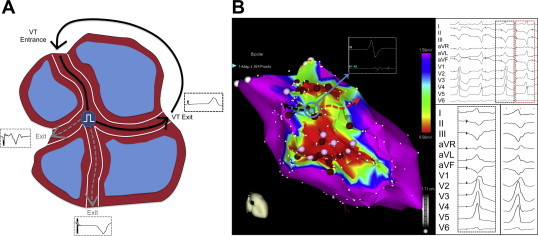
Multiple exit sites and channels. A Schematic representation of interconnected channels with a common isthmus, and three spatially separate exits, each producing a distinct QRS morphology. B The image on the left depicts the electroanatomic map from a patient with inferior wall low voltage areas (0.5–1.5 mV). The electrogram recorded at an isthmus site (inset, indicated by blue circle and arrow) with two exits is shown. Pacing at this site produced two distinct QRS morphologies and two stimulus to QRS intervals (black and red box in top right tracing). One of the paced QRS morphologies (black box) matches the clinical VT (shown in right lower tracing).
Adapted from Tung R, Mathuria N, Michowitz Y et al. Circ Arrhythmia Electrophysiol 2012;5:264–272.
4.4.3.3. Multiple exit sites
Akin to multiple interconnected channels, complex scars may also contain protected isthmuses that are provided spatially distinct exits. This phenomenon results in ≥2 distinct QRS morphologies from a single stable pacing site, indicating multiple exits sites (MES) are available to this common isthmus (Fig. 7A and B). In a recent report,52 our group defined an MES site as an abnormal EGM site that produces ≥2 distinct QRS complexes with at least three leads showing distinct QRS complexes qualitatively (Fig. 7B). When MES sites are identified and ablated, a greater freedom from recurrent VT was seen, suggesting that these are surrogates for critical isthmuses.52
4.4.4. Functional strategies
4.4.4.1. Late and abnormal potentials
Bipolar recording over dense scar typically demonstrates late or absent activation, owing to slower or absent conduction in this tissue. Local low amplitude fractionated bipolar electrograms that have a distinct onset after the end of the QRS complex are classified as isolated late potentials (Fig. 8) in sinus or paced rhythm or diastolic potentials in VT. These signals identify sites that are activated later than the bulk of the ventricular tissue and have been shown to have specificity as sites critical for reentry. Ablation strategies specifically targeted at LPs have been demonstrated to be effective in eradicating and preventing recurrent VT.58,59
Fig. 8.
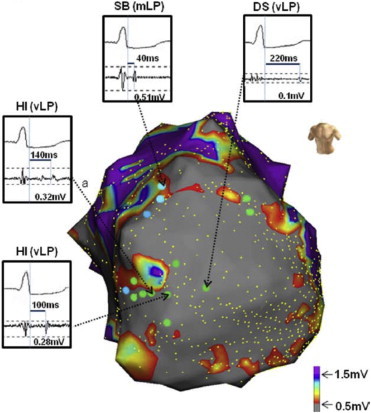
Distribution of late potentials in ischemic heart disease substrates. An electroanatomic map is shown in antero-posterior view depicting an anterior myocardial infarction, with heterogeneous islets (HIs, regions of preserved voltage within dense scar) shown. Very late potentials (VLPs, green tags) were recorded around HIs or dense scar (DS) areas. Mid-late potentials (MLPs, blue tags) were recorded at scar border SB between DS and normal tissue. Yellow dots reflect electrogram sampling points.
Adapted from Nakahara S, Tung R, Ramirez RJ et al. Heart Rhythm 2010;7:1817–1824.
In the ischemic substrate, sites with LPs are encountered more frequently than in the non-ischemic substrate. Nakahara et al,60 showed that “very” late potentials (occurring greater than 100 ms after the surface QRS complex) were three and two-fold more common in the endocardium and epicardium respectively, of ischemic cardiomyopathy patients relative to non-ischemic patients. Interestingly, when high-density electroanatomic mapping was performed in patients with ICM, heterogeneous islets (HI) (Fig. 8) with relatively preserved voltage amplitudes were observed deep within dense infarct scar.61 Fifty seven percent of sites demonstrating vLPs were encountered within or adjacent to an HI. This electrophysiologic finding is consistent with histologic characterization of the healed infarct heart, as islands of surviving myocardium are frequently encountered.
4.4.4.2. Pace map induction
Induction of VT during pacemapping at a site of abnormal electrograms has been termed pace map induction (PMI) (Fig. 9). The significance of this finding is the identification of a functionally important site for MMVT. The requisite for VT induction from this site is unidirectional block, supporting unique characteristics of a critical isthmus. Other features that may be observed during PMI are concealed entrainment and longer stimulus to QRS interval, further indications of MMVT isthmus sites. In our report, ablation at a PMI site successfully terminated VT in 93% of cases.52 Multiple distinct QRS morphologies may also be observed during PMI, and PMI may identify more relevant VTs. Entrainment maneuvers can be performed after PMI to confirm that a critical isthmus has been identified.5,62 Ablation can be initiated rapidly, with the goal of terminating the VT during energy delivery (Fig. 9, lower panel).
Fig. 9.
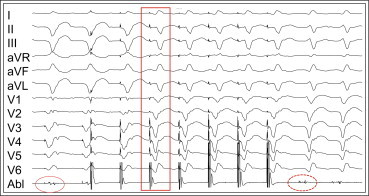
Pace map induction (PMI). A representative tracing showing PMI. Low amplitude electrograms with split potentials (solid red circle on ablation catheter, Abl) recorded at a site from which pacing induced a monomorphic ventricular tachycardia (MMVT). The red box indicates the first beat of MMVT. The split potentials become diastolic potentials during MMVT (broken red circle).
An often sought or important endpoint during ablation is termination of VT early during RF delivery. Whether or not this has any relationship to VT inducibility after RF delivery is unclear. As most clinical MMVTs are poorly tolerated, mechanical hemodynamic support to permit mapping and ablation during VT has been advocated by some. These include intra-aortic balloon pump (IABP), TandemHeart & Impella ventricular assist devices, and extra-corporeal membrane oxygenation (ECMO). Whether clinical outcomes are significantly improved in patients with poorly tolerated VT who undergo substrate-based ablation compared to ventricular assist devices is unclear.
4.4.5. Epicardial mapping and ablation
In patients with ischemic heart disease substrates, an epicardial approach is often favored after previous failed endocardial approach. This is in contrast to other substrates such as Chagasic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC), or idiopathic dilated cardiomyopathies where epicardial VT substrates are encountered more frequently. The pericardial space is typically accessed via subxiphoid puncture using a Tuohy needle as shown in Fig. 10, with an anterior or posterior entrance into the pericardial space selected based on whether the posterior or anterior myocardium respectively, is the most likely origin of VT. In most cases, use of angled sheaths and deflectable catheters enables access to all aspects of the epicardium. In patients who have had prior cardiac surgery, prior epicardial access, or prior pericarditis, focal or global adhesions may be present, restricting access to the pericardial space. Limited surgical exposure can be utilized to gain epicardial access in these cases.
Fig. 10.
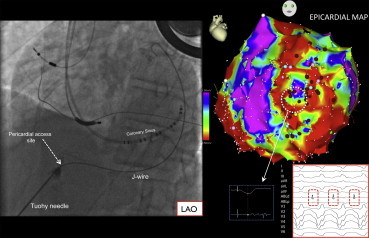
Epicardial access and mapping for ventricular tachycardia (VT). The left panel depicts an image of epicardial access in the left anterior oblique (LAO) projection. The Tuohy needed is seen entering the pericardium as indicated, small amounts of contrast are injected during the course of the needle to identify structures passed in its trajectory. A J-tipped wire passed through the needle into pericardial space outlines the lateral heart border in LAO. The right panel image depicts an epicardial voltage map in the LAO projection. A site with late potentials during baseline paced rhythm is shown (broken white circle and inset), as well as the diastolic potentials recorded during VT.
The true incidence of epicardial VTs in ICM remains unknown. As circuits may be transmural, termination of VT on a given surface is an imperfect gold standard. Additionally, 12-lead ECG localization only assists in determining the exit site, which can be spatially remote from the critical isthmus. Martinek et al demonstrated that traditional 12 lead criteria lacked specificity in ICM substrates.63
Additionally, selection and referral bias are present in reports from experienced tertiary centers. In patients referred after failed endocardial ablation, epicardial substrate was found in 71%. In our experience, where 81% of ICM patients had failed a prior ablation, the long-term freedom from VT was improved with combined endocardial-epicardial ablation strategy.64 Di Biase et al55 demonstrated that a combined endo-epicardial approach was better in ICM patients with electrical storm, although a homogenization strategy was also employed in this study, making it unclear whether critical sites were on the endocardium or epicardium. The role of combined endocardial–epicardial ablation as an initial strategy requires further prospective investigation.
Risks of epicardial access include cardiac perforation and/or tamponade, phrenic nerve palsy, hepatic laceration, bowel perforation, pericarditis, and epicardial coronary artery injury. At our center, of 95 patients who underwent epicardial access, complications were seen in 8.8% of cases (eight patients), including six (6.7%) cases of epicardial bleeding (one confirmed RV puncture), and two patients with phrenic nerve palsy. These complication rates are in line with multicenter experience detailing the safety of epicardial mapping and ablation of VT.65
5. Conclusion
After infarction, the myocardial substrate undergoes significant structural and functional alterations, from a molecular to macroscopic level, resulting in substrates capable of facilitating MMVT. The morbidity and mortality associated with VT in this substrate cannot be overstated. Pharmacologic, and catheter-based approaches to treating VT specific to this substrate have evolved since the initial intraoperative mapping studies detailing critical elements of the ischemic substrate. While the structural elements of the ischemic heart disease substrate are well understood, the chronic functional changes that permit unidirectional block and other key electrophysiologic phenomena, as well as the acute functional changes that initiate MMVT remain poorly understood. Improving our understanding of this new frontier will undoubtedly improve or ability to prevent and care for patients with ischemic heart disease substrates suffering from recurrent MMVT.
Funding sources
This work was supported in part by NHLBI R01HL084261 to KS, and an A.P. Giannini Foundation Award to OAA.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors wish to thank the members and staff of the UCLA Cardiac Arrhythmia Center.
References
- 1.Setoguchi S., Glynn R.J., Avorn J. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol. 2008;51:1247–1254. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 2.Henkel D.M., Witt B.J., Gersh B.J. Ventricular arrhythmias after acute myocardial infarction: a 20-year community study. Am Heart J. 2006;151:806–812. doi: 10.1016/j.ahj.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Reimer K.A., Lowe J.E., Rasmussen M.M. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 4.Fenoglio J.J., Pham T.D., Harken A.H. Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation. 1983;68:518–533. doi: 10.1161/01.cir.68.3.518. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson W.G., Khan H., Sager P. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–1670. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 6.de Bakker J.M., van Capelle F.J., Janse M.J. Slow conduction in the infarcted human heart. 'Zigzag' course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 7.Pouliopoulos J., Chik W.W., Kanthan A. Intramyocardial adiposity post-myocardial infarction: new implications of a substrate for ventricular tachycardia. Circulation. 2013;128:2296–2308. doi: 10.1161/CIRCULATIONAHA.113.002238. [DOI] [PubMed] [Google Scholar]
- 8.Pinto J.M., Boyden P.A. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999;42:284–297. doi: 10.1016/s0008-6363(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 9.Peters N.S., Wit A.L. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 10.Peters N.S., Coromilas J., Severs N.J. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 11.Cao J., Fishbein M.C., Han J.B. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 12.Vaseghi M., Lux R.L., Mahajan A. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–H1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajijola O.A., Wisco J.J., Lambert H.W. Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1010–1116. doi: 10.1161/CIRCEP.112.972836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim R.J., Chen E.L., Lima J.A. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–3326. doi: 10.1161/01.cir.94.12.3318. [DOI] [PubMed] [Google Scholar]
- 15.Desjardins B., Crawford T., Good E. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644–651. doi: 10.1016/j.hrthm.2009.02.018. the official journal of the Heart Rhythm Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki T., Miller C.F., Hansford R. Myocardial structural associations with local electrograms: a study of postinfarct ventricular tachycardia pathophysiology and magnetic resonance-based noninvasive mapping. Circ Arrhythm Electrophysiol. 2012;5:1081–1090. doi: 10.1161/CIRCEP.112.970699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu Y., Cochet H., Jadidi A. Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: assessment of structural and electrical substrate. Circ Arrhythm Electrophysiol. 2013;6:342–350. doi: 10.1161/CIRCEP.112.000191. [DOI] [PubMed] [Google Scholar]
- 18.Stevens S.M., Tung R., Rashid S. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter defibrillators and ventricular tachycardia: late Gadolinium enhancement correlation with electroanatomical mapping. Heart Rhythm. Oct 2013 doi: 10.1016/j.hrthm.2013.10.032. the official journal of the Heart Rhythm Society. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J.F., Marchlinski F.E. Utility of intracardiac echocardiography in left heart ablation for tachyarrhythmias. Echocardiography. 2007;24:533–540. doi: 10.1111/j.1540-8175.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 20.Nazarian S., Kolandaivelu A., Zviman M.M. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118:223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassidy D.M., Vassallo J.A., Marchlinski F.E. Endocardial mapping in humans in sinus rhythm with normal left ventricles: activation patterns and characteristics of electrograms. Circulation. 1984;70:37–42. doi: 10.1161/01.cir.70.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Haqqani H.M., Marchlinski F.E. Electrophysiologic substrate underlying postinfarction ventricular tachycardia: characterization and role in catheter ablation. Heart Rhythm. 2009;6:S70–S76. doi: 10.1016/j.hrthm.2009.04.023. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 23.Callans D.J., Ren J.F., Michele J. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction. Correlation with intracardiac echocardiography and pathological analysis. Circulation. 1999;100:1744–1750. doi: 10.1161/01.cir.100.16.1744. [DOI] [PubMed] [Google Scholar]
- 24.Wrobleski D., Houghtaling C., Josephson M.E. Use of electrogram characteristics during sinus rhythm to delineate the endocardial scar in a porcine model of healed myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:524–529. doi: 10.1046/j.1540-8167.2003.02499.x. [DOI] [PubMed] [Google Scholar]
- 25.Fahmy T.S., Wazni O.M., Jaber W.A. Integration of positron emission tomography/computed tomography with electroanatomical mapping: a novel approach for ablation of scar-related ventricular tachycardia. Heart Rhythm. 2008;5:1538–1545. doi: 10.1016/j.hrthm.2008.08.020. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 26.Bello D., Kipper S., Valderrabano M. Catheter ablation of ventricular tachycardia guided by contrast-enhanced cardiac computed tomography. Heart Rhythm. 2004;1:490–492. doi: 10.1016/j.hrthm.2004.05.001. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 27.Wijnmaalen A.P., van der Geest R.J., van Huls van Taxis C.F. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011;32:104–114. doi: 10.1093/eurheartj/ehq345. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z.M., Pan H.C., Chen Y.P. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz P.J., Motolese M., Pollavini G. Prevention of sudden cardiac death after a first myocardial infarction by pharmacologic or surgical antiadrenergic interventions. J Cardiovasc Electrophysiol. 1992;3:2–16. [Google Scholar]
- 30.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 31.Waldo A.L., Camm A.J., deRuyter H. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 32.Cairns J.A., Connolly S.J., Roberts R. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997;349:675–682. doi: 10.1016/s0140-6736(96)08171-8. [DOI] [PubMed] [Google Scholar]
- 33.Julian D.G., Camm A.J., Frangin G. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–674. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 34.Boutitie F., Boissel J.P., Connolly S.J. Amiodarone interaction with beta-blockers: analysis of the merged EMIAT (European Myocardial Infarct Amiodarone Trial) and CAMIAT (Canadian Amiodarone Myocardial Infarction Trial) databases. The EMIAT and CAMIAT Investigators. Circulation. 1999;99:2268–2275. doi: 10.1161/01.cir.99.17.2268. [DOI] [PubMed] [Google Scholar]
- 35.Bunch T.J., Mahapatra S., Murdock D. Ranolazine reduces ventricular tachycardia burden and ICD shocks in patients with drug-refractory ICD shocks. Pacing Clin Electrophysiol: PACE. 2011;34:1600–1606. doi: 10.1111/j.1540-8159.2011.03208.x. [DOI] [PubMed] [Google Scholar]
- 36.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 37.Poole J.E., Johnson G.W., Hellkamp A.S. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz L.N., Josephson M.E., Harken A.H. Epicardial and endocardial activation during sustained ventricular tachycardia in man. Circulation. 1980;61:1227–1238. doi: 10.1161/01.cir.61.6.1227. [DOI] [PubMed] [Google Scholar]
- 39.de Bakker J.M., Janse M.J., Van Capelle F.J. Endocardial mapping by simultaneous recording of endocardial electrograms during cardiac surgery for ventricular aneurysm. J Am Coll Cardiol. 1983;2:947–953. doi: 10.1016/s0735-1097(83)80244-7. [DOI] [PubMed] [Google Scholar]
- 40.Cox J.L. Patient selection criteria and results of surgery for refractory ischemic ventricular tachycardia. Circulation. 1989;79:I163–I177. [PubMed] [Google Scholar]
- 41.Soejima K., Couper G., Cooper J.M. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110:1197–1201. doi: 10.1161/01.CIR.0000140725.42845.90. [DOI] [PubMed] [Google Scholar]
- 42.Michowitz Y., Mathuria N., Tung R. Hybrid procedures for epicardial catheter ablation of ventricular tachycardia: value of surgical access. Heart Rhythm. 2010;7:1635–1643. doi: 10.1016/j.hrthm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Josephson M.E., Harken A.H., Horowitz L.N. Endocardial excision: a new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60:1430–1439. doi: 10.1161/01.cir.60.7.1430. [DOI] [PubMed] [Google Scholar]
- 44.Guiraudon G., Fontaine G., Frank R. Encircling endocardial ventriculotomy: a new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thorac Surg. 1978;26:438–444. doi: 10.1016/s0003-4975(10)62923-2. [DOI] [PubMed] [Google Scholar]
- 45.Marchlinski F.E., Callans D.J., Gottlieb C.D. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 46.Reddy V.Y., Reynolds M.R., Neuzil P. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson W.G., Wilber D.J., Natale A. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 48.Kuck K.H., Schaumann A., Eckardt L. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 49.Berruezo A., Mont L., Nava S. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 50.Bazan V., Bala R., Garcia F.C. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006;3:1132–1139. doi: 10.1016/j.hrthm.2006.06.024. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida K., Liu T.Y., Scott C. The value of defibrillator electrograms for recognition of clinical ventricular tachycardias and for pace mapping of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2010;56:969–979. doi: 10.1016/j.jacc.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 52.Tung R., Mathuria N., Michowitz Y. Functional pace-mapping responses for identification of targets for catheter ablation of scar-mediated ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5:264–272. doi: 10.1161/CIRCEP.111.967976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma A., Marrouche N.F., Schweikert R.A. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:465–471. doi: 10.1046/j.1540-8167.2005.40443.x. [DOI] [PubMed] [Google Scholar]
- 54.Hsia H.H., Lin D., Sauer W.H. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identification of endocardial conducting channels. Heart Rhythm. 2006;3:503–512. doi: 10.1016/j.hrthm.2006.01.015. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 55.Di Biase L., Santangeli P., Burkhardt D.J. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–141. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 56.Mountantonakis S.E., Park R.E., Frankel D.S. Relationship between voltage map “channels” and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2013;61:2088–2095. doi: 10.1016/j.jacc.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 57.Tung R., Mathuria N.S., Nagel R. Impact of local ablation on inter-connected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol. 2013;6:1131–1138. doi: 10.1161/CIRCEP.113.000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arenal A., Glez-Torrecilla E., Ortiz M. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 59.Bogun F., Good E., Reich S. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–2019. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 60.Nakahara S., Tung R., Ramirez R.J. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakahara S., Tung R., Ramirez R.J. Distribution of late potentials within infarct scars assessed by ultra high-density mapping. Heart Rhythm. 2010;7:1817–1824. doi: 10.1016/j.hrthm.2010.07.032. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson W.G., Friedman P.L., Sager P.T. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–1189. doi: 10.1016/s0735-1097(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 63.Martinek M., Stevenson W.G., Inada K. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. J Cardiovasc Electrophysiol. 2012;23:188–193. doi: 10.1111/j.1540-8167.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 64.Tung R., Michowitz Y., Yu R. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013;10:490–498. doi: 10.1016/j.hrthm.2012.12.013. the official journal of the Heart Rhythm Society. [DOI] [PubMed] [Google Scholar]
- 65.Sacher F., Roberts-Thomson K., Maury P. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]


