Abstract
Background
Appropriate ICD programming is the key to prevent inappropriate shock delivery, that is closely associated to a negative patients' outcome.
Methods
Review of the literature on ICD therapy to generate ICD programmings that can be applied to the broad population of ICD and CRT-D carriers.
Results
Arrhythmia detection should occur with a detection time ranging 9″–12″ in the VF zone, and 15″–60″ in the VT zone. Discriminator should be applied at least up to 200 bpm. ATP therapy is applied to all VTs up to 250 bpm, with a success rate of 70%. Inappropriate shocks should occur in <3.6% of patients.
Conclusion
Tailored ICD programming can be achieved following evidence from large ICD trials. Pre-defined settings that are saved on the programmer and that can be uploaded at device implantation help to ensure optimal programming and to avoid random errors.
Keywords: ICD programming, Arrhythmia detection, Arrhythmia discrimination, VF/VT treatment
1. Introduction
Automatic Implantable Cardioverter-Defibrillators (ICD) are the cornerstone in the prevention of sudden death both in patients at risk of life-threatening ventricular arrhythmias with or without heart failure (primary prevention), and in patients rescued from non-tolerated ventricular tachycardia (VT) or ventricular fibrillation (secondary prevention).1–6 ICD efficacy is largely proven in several randomized controlled studies,1–6 however concern has arisen about the potentially harmful effect of shock therapy being delivered to terminate ventricular arrhythmias (appropriate therapy delivery), or inappropriately delivered because of misclassified supraventricular arrhythmias (SVT), self-terminating non-sustained ventricular tachycardia (NSVT), and oversensing of both cardiac and non-cardiac signals.7 Therapy delivered for causes other than VT/VF is termed “inappropriate”, and has been reported to occur in up to 17% of patients of heart failure patients.7 Shock delivery, whether appropriate or inappropriate, has been reported to negatively impact patient survival, being closely associated to progressive pump failure.7 In the recently published MADIT-RIT study, inappropriate delivery of anti-tachycardia pacing (ATP) because of SVT was found associated to increased mortality, although this finding has no pathophysiologically valid explanation.8,9 In several studies the chances of therapy delivery was higher in the sickest patients with multiple co-morbidities1,2,4 so it is speculative – but very likely – that therapy delivery is simply a marker of a more severe clinical scenario, the association to mortality thus being almost ineluctable.
This review will summarize the principles that may assist clinicians in defining ICD settings that may be suitable to the vast majority of patients as a generic framework to reduce inappropriate therapy delivery while maintaining the efficacy to detect and terminate VT and VF, and that can be used to provide an individualized programming for any specific patient.
In this review we will not consider device programming based on a primary or secondary prevention of sudden death setting. The major difference among these two patient populations is the burden of ventricular arrhythmia and the time of arrhythmia onset after ICD implantation, whereas the type of arrhythmia being detected and the efficacy of the therapy being delivered is similar.10 Indeed, these latter are related to the underlying disease and to the extent of myocardial damage, which are quite homogeneous among each specific disease etiology.
Device programming based on disease etiology is focused on the occurrence of VT that is ATP-terminable: a low chance favors a single, fast arrhythmia zone (VF), whereas a high chance favors a VF + VT zone.
The goals of ICD programming are:
-
-
detect high rate NSVT, as they are predictive of ICD discharge for life-threatening arrhythmias and all-cause mortality, and should prompt medical interventions to improve patients' outcome11
-
-
avoid unnecessary treatment of NSVT by delaying ICD intervention as tolerated by the patient
-
-
discriminate SVTs
-
-
terminate sustained VT and VF while minimizing shock therapy
-
-
monitor AF, SVTs, and slow VTs for stroke prevention management, ablative and/or drug therapy
-
-
provide alert on technical (lead integrity and device) and patient-related (atrial fibrillation, heart failure) medical issues
ICD programming is a comprehensive process that is based on the patient's clinical history and encompasses:
-
-
Choice of the device type
-
-Arrhythmia detection:
- Zones setting
- Detection duration of each programmed zone
- SVT discrimination
-
-
Termination of ventricular arrhythmias
-
-
Monitor zone programming
-
-
Device and clinical alerts
For the purpose of this review, the choice of device type will be discussed at the end, by the light of the observations that are relevant to this decision-making step.
2. Arrhythmia detection
Programming the features to achieve automatic arrhythmia diagnosis by the implanted device requires clinical as well technical knowledge, and may be hindered by several factors such as the frequent changes of the parameters presentation format across different device releases and different manufacturers, mismatch of the shipment programming compared to evidence stemming from clinical studies, shortage of time and multi-tasking during the clinical activity. Individualized programming is often not achieved in clinical practice, and this has a negative impact on patient outcome.
To avoid random errors in the set-up of ICD programming, we have found extremely helpful to save pre-defined custom-made settings in the programmer, that can suit a vast majority of patients and that are used to reach a refined, tailored setting for very specific patients with only a few changes.
This function is available for recent device releases in Biotronik, Medtronic, NayaMed, and St Jude Medical devices, and can be loaded after device interrogation (Fig. 1).
Fig. 1.
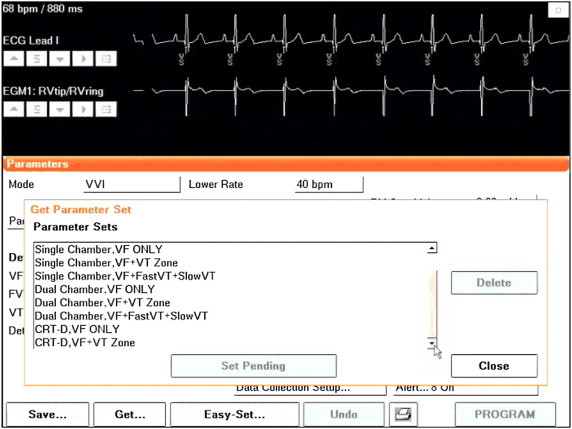
Example of pre-defined settings suitable for the majority of ICD and CRT-D patients, to be uploaded by the GET & SAVE function (Medtronic and NayaMed). Tailoring for individualized patient programming can be achieved with only minor changes. A similar function is also available in Biotronik and St Jude Medical devices.
2.1. Zones setting
Whatever the device chosen (single or dual chamber, CRT-D) the programming should be guided by the clinical knowledge of the patient's history. The key aspect is whether a patient can benefit from a “VF zone only” setting, or is eligible for a “VF zone + VT zone” programming.
Nowadays ATP delivery has become possible before and/or during capacitor charging, so a setting as “VF zone ≥ 200 bpm” seems a simple and effective programming. Labeling an arrhythmia zone as “VF” is crucial, as only Medtronic and NayaMed devices can discriminate SVT with fast ventricular rate in the VF zone: any SVT entering such a zone would be treated as VF with other manufacturers. Young patients, who can achieve very fast atrial rates or have SVTs with fast conduction to the ventricles, are at the highest risk of inappropriate shocks, especially when a subcutaneous ICD is considered.
The impact of VT zone programming on mortality is unclear. A high cut-off rate for VT/VF detection as 200 bpm or even 220 bpm has proved safe and capable to reduce inappropriate therapy delivery compared to shipment programming, but has to be traded off with a potentially harmful under-detection of monomorphic VT in the 170–220 bpm range.8,12 The tolerance of VT in patients with heart failure or low EF is unknown a priori, and, although anedoctically tolerated, is in general poor. Indeed, in the AVID trial the coexistence of VT slower than 180 bpm and heart failure identified a subgroup of VT patients at higher mortality risk.13 In the AVID trial patients with symptomatic VT or hemodynamically stable monomorphic VT and low EF had a poorer prognosis than patients presenting with VF, underlying that VT slower than 180 bpm is not a benign arrhythmia, and suggests the presence of a cardiac substrate at risk of both SCD and pump failure.14 Also in primary prevention patients therapy delivery for either VT or VF was associated to increased arrhythmic and heart failure-related mortality; VTs faster than 180 bpm were associated to an only slightly higher mortality rate compared to slower VTs.8,15 Moreover, the MADIT-RIT study was not powered to test the difference of a single zone programming against a VF + VT zone with prolonged detection, as proven effective in the PREPARE and in the RELEVANT studies,16,17 so it seems clinically appropriate to use a VF + VT zone with long detection times for the patients' safety.
Based on the fact that about 50% of clinical arrhythmias had a rate slower than 200 bpm in patients with structural heart disease and both primary and secondary indication to ICD therapy,10 we consider feasible an approach to programming that merges the evidences from all available studies, as summarized in Table 1 and Fig. 2:
-
-
patients with a primary arrhythmia or ion channel disease in the absence of structural heart disease should have a single VF zone faster than 200 bpm, since monomorphic VT that is ATP-terminable is not in the clinical scenario. When SVTs with fast ventricular rate coexist, a setting as VF ≥ 250 bpm + VT 200–250 bpm allows discrimination of SVT in this latter range, preserving the sensitivity of VT/VF detection
-
-
patients with a substrate prone to monomorphic VT should have a VT (170–200 bpm) and a VF zone, both with a long detection time.
-
-
patients with known monomorphic VTs slower than 170 bpm that cause significant hemodynamic compromise and are resistant or not amenable to drug/ablation therapy can benefit from a “dual VT zone” programming
Table 1.
Pragmatic approach at VF and VT detection and ATP treatment in the broad ICD/CRT-D population (primary and secondary prevention of sudden death), based on evidence stemming from several clinical studies.8,10,12–18,31,33,34
| Primary and secondary prevention, ALL ICD types | ||||||
|---|---|---|---|---|---|---|
| Clinical setting | VT zone | VF zone | Detection duration | Discrimination | ATP for VT zone | ATP for VF zone |
| No structural heart disease, No VT substrate No fast SVTs |
≥200 bpm | 9–12 s (30–40 intervals) |
None, apart Medtronic and NayaMed |
During the charge or before charging 8 pulses, 85–88% RR interval |
||
| No structural heart disease, No VT substrate Known fast SVTs |
200–250 bpm | ≥250 bpm Setting as previous for Medtronic & NayaMed |
9–12 s (30–40 intervals) |
VT zone up to 230 bpm for Medtronic & NayaMed, SQICD |
Single ATP 8 pulses, 85–88% RR interval |
Debatable clinical usefulness, but possible during charge in Medtronic, NayaMed, Biotronik, St Jude Medical |
| Structural Heart disease, Possible VT substrate |
170–200 bpm | ≥200 bpm | 9–12 s (30–40 intervals) VT detection>12 s |
VT zone up to 230 bpm for Medtronic & NayaMed, SQICD |
2 ATP burst*, 20 ms scan 8 pulses, 85–88% RR interval Single ATP ramp* 8 pulses 81% RR interval |
During the charge or before charging* 8 pulses, 85–88% RR interval |
| Structural heart disease, VT < 170 bpm unresponsive to ablation/drugs |
Dual VT 170–200 bpm 140–170 bpm |
≥200 bpm | 9–12 s (30–40 intervals) VT detection>12 s Slow VT > 60 s |
VT zone up to 230 bpm for Medtronic & NayaMed, SQICD |
Same as above for VT * 170–200 bpm Multiple individualized * ATP schemes for slow VT |
During the charge or before charging* 8 pulses, 85–88% RR interval |
* BIV ATP in CRT-D with ischemic heart disease.
Fig. 2.
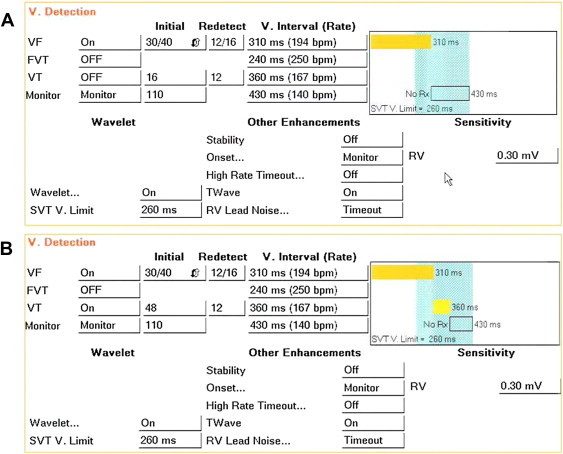
Suggested programming of Detection in Medtronic/NayaMed single chamber ICDs, as per Tables 1 and 2 recommendations. Panel A is a “VF Only” setting, panel B is “VF + VT zone”. Note that in these manufactures the cut-off interval belongs to the slower zone, thus VF is ≥300 ms (200 bpm), and VT is set at 350 ms (171 bpm). Detection is 30/40 intervals, Wavelet discrimination up to 260 ms (231 bpm) in the VF zone (light blue shaded area). Onset is set to monitor and stability OFF. Features to avoid T-Wave oversensing and “noise” are active by shipment. A Monitor zone for symptoms evaluation or slow arrhythmias detection is available.
The Detection Rate is the primary determinant of the arrhythmia being detected.
VF labeling means that no SVT arrhythmia can be discriminated in that zone (apart Medtronic and NayaMed), hence therapy will be delivered when the detection time is reached. It is common practice to consider “true VF” a rhythm faster than 250 bpm, very unlikely to be ATP-terminable and usually requiring shock therapy.
A rhythm in the range 200–250 bpm is considered a fast VT (FVT).
Nowadays, the treatment for FVT has been incorporated in the VF zone, since ATP has been made available either before or during the capacitor charge, thus obviating the need to program 3 detection zones. Nowadays, a “lean” ICD programming can be achieved with a 2 zones setting that ensures ATP delivery in the broad VT range and also into the VF zone (Figs. 2 and 3).
Fig. 3.
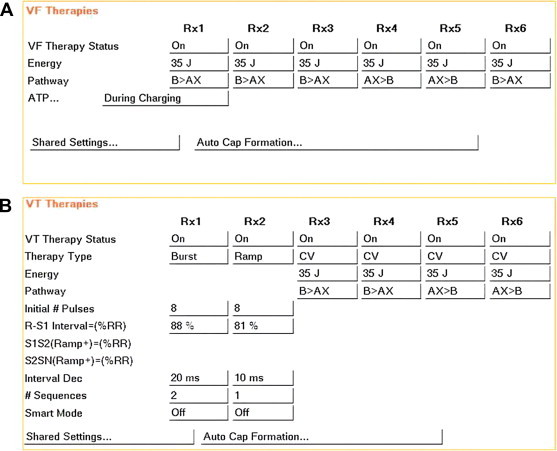
Therapy setting for both a “VF Only” (Panel A) and a “VF + VT zone” (Panel A + B) setting. When effective, ATP in the VF zone automatically switches to ”before charging” to improve device longevity. ATP sequences are based on published studies 10, 18, 31, 33. In CRT-Ds, biventricular ATP is preferred, especially in ischemic heart disease patients.34
2.2. Detection duration
Arrhythmia detection occurs when a fast rate exceeding the arrhythmia cut-off rate persists for the programmed detection time, that can be indicated more commonly as intervals, or time in seconds.
Detection duration is markedly different across manufacturers at shipment programming. This parameter is crucial, as a long detection time has proven as a very useful intervention to decrease inappropriate ICD therapy delivery.8,16–18
Reprogramming detection duration from shipment is a necessary step, as only Medtronic provides a shipment VF detection as 30/40 intervals (9 s at VF = 200 bpm) stemming from evidence based on available literature.16–18
Detection can be programmed in intervals or in seconds. The counters are probabilistic (the arrhythmia is declared present when a fraction of VT/VF intervals over a programmed sampling window is reached) around a 70–80% value, depending on the different manufacturers. This strategy ensures timely detection despite the occurrence of a signal amplitude drop at high ventricular rate during polymorphic VT or VF, potentially causing missed intervals.
It has to be remembered that, unlike other manufacturers, Medtronic and NayaMed have a consecutive counter in the VT zone and in the Monitor zone: one beat exceeding the detection cycle will reset the VT counter to 0, thus delaying the detection process.
A long detection decreases the chances of an unnecessary therapy delivery due to NSVT. This was firstly reported in a mixed ICD population in the PREPARE study: detection prolongation as 30/40 intervals for VF held a 43% reduction of appropriate shocks compared to an 18/24 intervals detection time.16 Since 2009, 3 studies of a long detection vs a conventional one have been published: the RELEVANT (primary prevention CRT-D patients), the MADIT-RIT (primary prevention dual chamber and CRT-D patients), the ADVANCE III (mixed ICD population). In RELEVANT, the long detection duration (30/40) reduced by 90% the number of treated VT and VF episodes compared to a 12/16 intervals (VF) and a 16 intervals (VT) detection.17 In the MADIT-RIT study, detections as 12″ in the range 200–250 bpm and 60″ in the 170–200 bpm range decreased ATP delivery by 67% against detection durations as 1 s and 2.5″, respectively.8 The ADVANCE III trial demonstrated a highly significant reduction of both appropriate and inappropriate therapy occurrence by means of a 30/40 vs 18/24 intervals detection, at no excess of either syncope or mortality.18
It has to be noted that in the MADIT-RIT the reduction of treated VT/VF episodes translated in a significant reduction of ATP delivery only, but not of shocks for life-threatening long-lasting arrhythmias.8 On the contrary, the RELEVANT and the ADVANCE III trial respectively reported a significant and a nearly significant (p = .06) reduction of appropriate shocks by a long detection time, at no compromise with mortality or syncope.17,18 Moreover, the RELEVANT study and the ADVANCE III study respectively reported a decrease of heart failure-related and all-cause hospitalizations in the long detection groups.
Compared to the RELEVANT and the MADIT-RIT, the ADVANCE III trial results can be generalized to all patients, primary and secondary prevention, and to all ICD types.
Detection duration in the control arm was longer in the ADVANCE III (18/24) than in the MADIT-RIT (1 s and 2.5″ respectively for VF and VT), which may account for some of the unwanted effect of ATP therapy delivery on VTs possibly going to be self-terminating.
In the subcutaneous ICD (SQICD), detection is non-programmable as 18/24, that means that charge delivery occurs in about 16 s, unlike transvenous ICDs (18″–21″), thus exposing the patients to some unnecessary therapy delivery on non-sustained VT, unless delayed detection occurs because of missed intervals. On the other hand, when a non-sustained VT is detected and charge delivery is aborted, the detection is prolonged by 1 s in the next detection; this process can repeat on consecutive episodes up to a maximum of 5 added seconds, thus leading to a smart detection prolongation up to 21″.
A long detection duration may also help to prevent inappropriate therapy delivery due to SVTs, although an important role is also played by SVT discriminators (Figs. 2 and 4, Table 2).
Fig. 4.
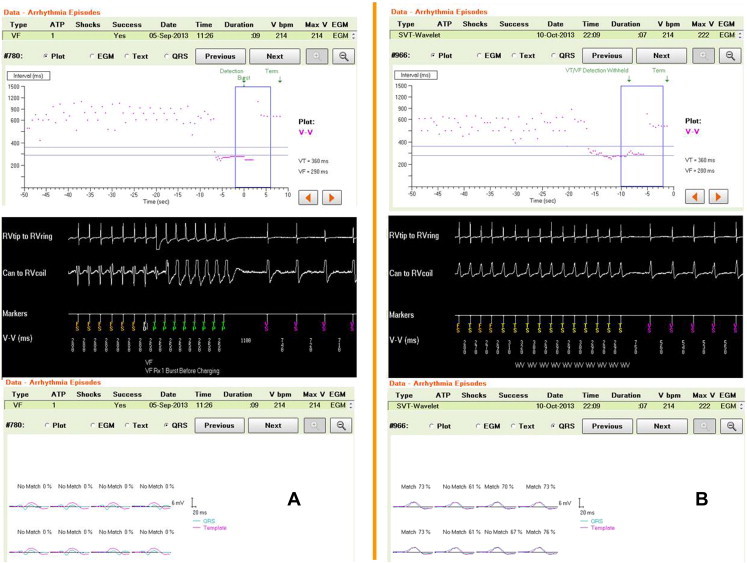
Benefit of SVT discrimination in the VT and VF zone added to a long Detection duration. Primary prevention patient with new-onset heart failure 1 year after ICD implantation. From top to bottom : interval plot, episode EGM, Wavelet snapshot. The patient was in sinus rhythm with very frequent premature beats (see plots). Panel A is VT at 280 ms treated by ATP before charging in the VF zone: no match with template. Panel B is an SVT (see template match and interval stability) on the border zone 280–290 ms: detection is withheld by Wavelet for 12″, longer than Detection Duration, until self termination. Long detection alone would have caused inappropriate ATP delivery.
Table 2.
Suggested programming of Discriminators in clinical practice, as from their performance.20–30
| ICD type | SVT limit | First line discriminator | Stability | Onset | |
|---|---|---|---|---|---|
| Medtronic & NayaMed |
Single chamber Dual/CRT-D |
260 ms 260 ms |
Wavelet, 70% match PR logic (+wavelet) (Medtronic only) |
50 ms; keep OFF unless proven to be helpful based on EGM recordings | 81%, Monitor Caution if VT/VF are triggered by sinus tachycardia or SVTs |
| St Jude Medical | Single chamber | VT zone | Morphology, 60% match (60 to 90% in Ellipse) | 50 ms, works in 2/3 logic | 100 ms, works in 2/3 logic |
| Dual/CRT-D | VT zone | Rate branch + Morphology | 50 ms, works in V < A branch (ANY or ALL logic) | 100 ms, works in V = A branch (ANY or ALL logic) | |
| Boston Scientific | Single chamber | VT zone | Rhythm ID, 94% match (programmable in Incepta) | 20 ms, works with onset | 9%, works with stability |
| Dual/CRT-D | VT zone | Rhythm match (V/A + Rhythm ID) | 20 ms, works in V ≤ A with A rate > 170 bpm | Not used | |
| Biotronik | Single chamber | VT zone | Smart Detection available also in single lead, Single chamber ICD | Integrated in Smart Detection: programmable % of cycle or absolute value, respectively in | Used only in V = A, stable V and A intervals that have no monotony: sudden Onset |
| Dual/CRT-D | VT zone | Single chamber and dual chamber | Confirms VT | ||
| Sorin Ela | Single chamber | VT zone | Stability | Onset when a stable rhythm is detected | |
| Dual/CRT-D | VT zone | PARAD+ | First step of PARAD+ | Chamber of origin of fast rhythms when A/V = 1 | |
| Cameron Health | Single chamber | Conditional shock zone | Discrimination based on: signal width, amplitude, stability |
Indeed, whereas inappropriate shock delivery was significantly reduced in both the RELEVANT and in the ADVANCE III study, no effect was observed in the MADIT-RIT trial, possibly owing to the absence of SVT discriminators in the VF zone in Boston Scientific devices. Indeed, inappropriate ATP delivery on AF or SVTs inducing VT/VF was hypothesized as a possible explanation of ATP being associated with increased mortality in the MADIT-RIT trial.8,9
A long detection is also helpful to decrease inappropriate shocks due to non-cardiac signals such as noise or lead insulation defects/fractures, that are observed to have typically a <20″ duration time.
Programming the detection duration based on available studies is reported in Table 1.
2.3. SVT discrimination
Discriminators have been implemented in the detection process in a view to withhold VT therapy delivery on sinus tachycardia and supraventricular arrhythmias. They cannot work in the VF zone, with exception for Medtronic and NayaMed.
Discriminators may have different approaches at arrhythmia discrimination:
-
-
analysis of the interval patterns (onset, stability, atrium to ventricle relationship)
-
-
morphologic analysis of the EGM entering the arrhythmia zones
Arrhythmia discrimination is applied at VT detection (VF also for Medtronic and NayaMed), whereas it is not applied during re-detection (after the first therapy has been delivered) apart from Medtronic, NayaMed, and Boston Scientific, that can apply Stability during re-detection. Although some similarities exist, the functioning of discriminators is different across manufacturers.
A description of discriminators functioning is reported in the Appendix.
2.4. Single-chamber ICD
Dual chamber arrhythmia discrimination is nowadays possible in a single-lead single-chamber ICD to increase specificity compared to onset + stability.19,20 In general, Morphologic discriminators “stand alone”, without any further discriminator, have been reported to correctly classify 75–90% of SVTs with a sensitivity for VT around 99%, that is superior to stability and onset working together. They are to be used as single discriminators, with the others in “monitor mode” (passive) where available, so that they can be turned ON only when proven to add significant value to arrhythmia discrimination.21
In the single study reporting a head to head comparison, Medtronic wavelet was observed as superior to Boston Scientific Rhythm ID (old release) in discriminating SVTs, at no compromise with VT detection.22
The reliability of detection with subcutaneous ICDs is actually suboptimal, with 13% of patients receiving inappropriate shocks; the use of a Conditional Shock Zone dramatically decreases inappropriate shocks due to SVTs.23 Technological advancement of the SQICD will most likely lead to consistent improvements in future releases. A clinical study is ongoing to evaluate the performance of transvenous against subcutaneous ICD discrimination.24
2.5. Dual chamber ICD and CRT-D
A slightly superior accuracy in arrhythmia classification has been reported with dual chamber ICDs, but this has never translated into a decrease of inappropriate therapy delivery nor in a better patient outcome.25–28 Indeed, more complications related to the atrial lead have been observed.26 Whether the unique Biotronik device using dual chamber detection with a single lead will provide better results, it awaits to be proven.
As a general rule, each discrimination algorithm has to be used at the best of its clinical performance as reported in literature, and needs to be tailored to the individual patient, although this is time consuming. For instance, in the event of rate-dependent aberrancy during SVT, morphologic discriminators should be reprogrammed to allow matching the template at a lower percentage, or the template should be collected during aberrancy, and template update be disabled.29 No inference of the performance of an algorithm (for instance, stability) can be made in a different manufacturer device, as they work differently and with non-comparable diagnostic pathways, as reported in the Appendix.
These considerations are of key importance when interpreting the data of comparisons among manufacturers.22,24
Based on actual knowledge of Detection Duration and Discriminators programming, the minimum target to be achieved nowadays is fewer than 3.6% of patients receiving inappropriate shocks.8,16–18
For the purpose of a pragmatic approach to arrhythmia detection, Discriminators programming is reported in Table 2.
Discrimination based on hemodynamic tolerance of a fast rhythm, whatever the chamber of origin, can be a possible approach in the next future. Some of the already existing technologies for intracardiac pressure or stroke volume measurements are apt to work in conjunction with nowadays ICDs as connected devices. It is speculative that such system could be implemented in either transvenous or non-transvenous devices.
2.6. T-wave oversensing
Double counting and delivery of inappropriate shocks due to T wave oversensing occurs in less than 4% of transvenous ICD patients. This is usually related to any of these situations:
-
-
small R waves (ventricular arrhythmogenic cardiomyopathy, new-onset right bundle branch block, fall of the RV signal amplitude due to changes in the lead-tissue interface, use of anti-arrhythmic drugs)
-
-
tall or delayed T waves (hypertrophic cardiomyopathy, long QT, post-paced T waves in CRT-D when sinus rhythm exceeds the UTR, electrolytes imbalance).
It is usually managed by delaying the increase of ventricular sensitivity towards its maximum value (St Jude Medical and Biotronik), or by detecting the T wave with a dedicated algorithm (Medtronic, NayaMed), or keeping the UTR as fast as the individual maximum sinus rate in CRT-Ds to prevent the loss of biventricular pacing. Decreasing ventricular sensitivity should be regarded as the last resort, to minimize the risk of ventricular undersensing during VF. Unacceptably low R wave signal (<1.5 mV) associated with device under-performance usually require lead revision.
In the subcutaneous ICD, 7% of patients had inappropriate shocks because of T-wave oversensing, that required re-intervention in 20% of cases.23 In a young population (mean age 20 years) inappropriate shocks due to T-wave oversensing were 50% of totally delivered shocks.30
2.7. Non-cardiac signal detection
Signals arising from the external environment or stemming from lead insulation defects/fractures (termed “noise”) account for up to 4% of delivered shocks in large trials.5,31 A unique feature of Medtronic and NayaMed can help to avoid this threatened occurrence, by withholding VF therapy delivery for up to 45 s when noise is detected, as “noise” is rarely observed for longer than 20 s. Therapy delivery is resumed automatically if “noise” persists more than 45 s. Moreover, the algorithm automatically prolongs VF detection to 30/40 intervals to decrease the chances of inappropriate “noise” detection; this feature can work with leads from any manufacturer.32
2.8. Termination of ventricular arrhythmias
2.8.1. VT and FVT termination
Several studies have demonstrated that both VT slower than 200 bpm and FVT (200–250 bpm) can be safely terminated with ATP sparing the painful and potentially harmful effect of ICD shocks.10,18,31,33,34 This approach not only enables a superior patient comfort, but also improves the ICD service of life, each capacitor charge impacting about 15 days of battery longevity.
Following the pilot PAINFREE study, several randomized studies have consistently demonstrated that:
-
-
ATP effectively terminates nearly 70% of VTs and FVTs10,18,31
-
-
ATP efficacy is maintained with a long detection duration18
-
-
Physician-tailored programming of ATP is as effective as an empiric one, thus up-loading a pre-defined setting enables an optimal outcome in the majority of patients31
-
-
long ATP bursts (15 pulses) are as effective as standard (8 pulses) at no excess of acceleration or syncope, a slightly superior efficacy being observed only in patients with an EF> 40%, therefore cannot be recommended as first choice33
-
-
in CRT-D patients, Biventricular (BIV) ATP has a significantly lower risk of arrhythmia acceleration compared to RV-only ATP, and is more effective in patients with ischemic heart disease34
A general approach is reported in Table 1, and can be recommended as:
-
-
single ATP attempt for 200–250 bpm VTs. At faster rates up to 300 bpm, ATP is unlikely to have a similar effectiveness, however it causes no harm when delivered during charging
-
-
2 bursts and one ramp can be considered in the 170–200 bpm VT range
-
-
more burst and ramp attempts on VT slower than 170 bpm can be useful/needed in individual patients to achieve tailored therapy, and as such cannot be recommended based on randomized trials
Early VT recurrences after a successful ATP – but before the episode is closed – may cause unnecessary shock delivery. This pitiful event can be avoided by decreasing the intervals to detect normal rhythm resumption from 5 (shipment) to 3 in St Jude Medical devices. Medtronic features “Confirmation+” (shipment, non-programmable): VT/VF termination is detected when the cycle is longer than the average treated arrhythmia +60 ms. In the event of consecutive VT/VF recurrences in a few beats, delivery of therapy 1 will occur again, thus sticking to an effective, painless, and battery-saving ATP treatment.
2.8.2. VF termination
Shock delivery is the main therapy of true VF, defined as a rhythm faster than 250 bpm. True VF episodes account for about 10–15% of arrhythmia episodes recorded in ICD patients.5,10,16
ICDs are reported to decrease sudden death mortality by about 70%. Whereas many events can cause sudden death, unresponsiveness to defibrillation is the cause in probably about 5% of patients nowadays.35–37 In recent years significant improvements have occurred in ICD technology, enabling delivery of about 35J in a reliably short charging time and programmability of the shocking pathway, such that shock failure should be viewed more as a patient-related issue than as a device issue. Indeed, the probabilistic nature of defibrillation is such that success is highly dependent on the clinical situation at the time of arrhythmia occurrence, with heart failure, myocardial ischemia, respiratory acidosis, playing a key role in the appraisal of shock failure. Changing the shocking pathway allows greater possibilities of arrhythmia termination, owing to the probabilistic nature of the defibrillation process, hence reversal of the shocking pathway should occur at some point in the sequence of charges delivered for a single VF episode (Fig. 3). The SQICD reverses shocking pathway automatically after a delivered charge failure, and keeps the successful pathway as the first therapy to be delivered in a newer arrhythmia episode.
Although several studies have reported a similar success rate whether an only minimal safety margin is used as opposed to a full energy shock, it has become common practice to program a full charge first shock after a long detection duration, owing to the short and reliably stable capacitor charging time across the full span of ICD life service.38
The choice of a dual vs a single coil shocking lead is largely debated, since no trial has ever been conducted to solve any of the controversial issues still pending after 20 years of transvenous ICD therapy. In favor of dual-coil leads stands a lower energy requirement to terminate induced VF, that is not supported by any trial in terms of survival benefit. In favor of single coil leads stands a perceived benefit for lead extraction, that is not supported by any trial reporting fewer complications during lead extraction. Indeed, the predictors of both lead extraction failure and catastrophic complications are older age of lead in service and infection as cause of lead extraction regardless of lead type (pacemaker or ICD), and use of powered sheaths.39
As a general approach, dual-coil leads can be recommended in patients at risk of a high defibrillation threshold such as hypertrophic cardiomyopathy, ventricular arrhythmogenic cardiomyopathy, operated Tetralogy of Fallot with severe ventricular enlargement. Primary ion channel disease patients (Brugada for instance) are occasionally found to have higher than average defibrillation thresholds.
2.9. Monitor zone programming
Programming a monitoring zone may be helpful to investigate patients with symptoms, or to detect asymptomatic AF that has clinical relevance for stroke management.40,41 In our practice we program a Monitor zone from 140 to the VT/VF cut-off rate: Discriminators are active to help the diagnostic process (Fig. 2).
2.10. Device and clinical alerts
Device alerts on battery longevity and lead integrity issues are on by shipment. In the view of the widespread adoption of remote device and patient monitoring, automatic alerts should also be used to enable a prompt response to changing medical conditions. Beyond fast NSVT,11 this is particularly important for and AF, where stroke prevention and rate/rhythm control strategies are concerned, and for heart failure management.40–46 Composite heart failure scores based on the clinical profile and on device data are built to predict clinical worsening and hospitalization risk, and as such are made available to clinicians. Moreover, when alerts are automatically issued to the device clinic and/or the referring physician, clinical decisions are made in the appropriate time frame to prevent untoward events, thereby decreasing in-hospital clinical visits.47 This appears to translate also in a significant all-cause and cardiovascular mortality benefit in heart failure patients, as from the preliminary data disclosure of the IN-TIME trial.48
2.11. Choice of the device type
Based on available studies, no advantage has been observed in the use of Dual Chamber vs Single Chamber ICDs, although the former enable a more appropriate arrhythmia detection and superior detection of AF occurrence and AF burden.25–28 Dual chamber devices should be nowadays preferred in a minority of patients, when cardiac stimulation is needed in the setting of a normal left ventricular systolic function. The availability of a single chamber ICD capable of dual-chamber detection could possibly further decrease the use of dual chamber devices.20 SQICD fit into this trend towards single chamber devices, and their reliability is already very close to transvenous ICDs, although the rate of inappropriate shock delivery (13% patients)23 is still unacceptable compared to actual transvenous ICD (2.4–3.6% patients).8,16–18 However, some unmet needs still prevent the broad adoption of the SQICD in the general ICD population: lack of ATP, bulky big size that poses skin issues in young or thin habit patients, subcutaneous lead infections, cost (twice a single chamber ICD), and limited device longevity (5 years) compared to the past generation transvenous units (close to or >10 years).23,30,49,50 Its usage is actually appropriate for a minority of patients with lead infection who need a fast re-implantation, or when a venous access issue is the key point to be managed.
3. Conclusions
ICD programming can be made safe and effective in preventing inappropriate therapy delivery (fewer than 3% of patients), by implementing a custom-made programming stemming from evidence by clinical trials, that can be applied to the broad ICD/CRT-D population and used for tailoring individualized therapy. Such settings can be uploaded from the programmer to increase efficiency and minimize the risk of random errors.
Appendix.
SVT discrimination
Algorithms functioning across different manufacturers.
Onset is intended to rule out sinus tachycardia because of its gradual rate acceleration. Failure to detect VT/VF because of onset may occur when the arrhythmia occurs during sinus tachycardia or it is triggered by an ongoing SVT or atrial fibrillation (AF). It has to be remembered that onset works differently across manufacturers:
-
-
in Medtronic/NayaMed devices onset prevents the VF/VT counter to reach detection, thus overrules all other discriminators. Arrhythmia detection cannot occur if onset misclassifies VT;
-
-
in St Jude Medical devices, onset works at Detection as part of the detection logic, together with stability and Morphology Discrimination in single chamber ICDs;
-
-
in Boston Scientific devices onset and stability cannot work together with the morphologic discriminator (Rhythm ID). Onset and stability work together in single chamber ICDs, each overruling the other in favor of sensitivity for VT;
-
-
in Biotronik devices a single chamber, single-lead device can provide dual chamber detection owing to a highly reliable P wave sensing.19 Its decisional tree makes minimal use of onset20;
-
-
in Sorin devices onset is applied sequentially to rule out sinus tachycardia only after stability has detected a fast regular rhythm.
In general, Onset is of little use, unless coupled to stability or other discriminators as in St Jude Medical devices.
Stability is intended to rule out Atrial Arrhythmias with irregular atrio-ventricular conduction. Failure of Stability may occur with SVTs and highly regular AF at fast ventricular rates, that mimic the spontaneous cycle length variability of VT (10–40 ms).
As Onset, Stability works differently across manufacturers:
-
-
in Medtronic/NayaMed devices stability works as soon as VT counter reaches 3, and resets the VT counter to 0 when at least 1 interval in a window of 4 differs more than the programmed stability value; arrhythmia detection can thus be indefinitely delayed until a regular rhythm develops (VT) or the probabilistic VF counter is filled. Stability can work after therapy delivery during re-detection;
-
-
in St Jude Medical devices stability is part of the detection logic, and is applied once the detection time has elapsed, together with onset and Morphology Discrimination. Stability compares the difference of the 2nd shortest and longest intervals of a programmable sampling window (8–20 intervals) to a defined programmable value, and declares SVT when the former exceeds the latter;
-
-
in Boston Scientific devices onset and stability cannot work together with the morphologic discriminator (Rhythm ID). Stability and onset override each other in favor of VT diagnosis. Intervals entering the detection rate are compared to the variability of the last 5 beats: the weighted average is then calculated to be compared with the programmed stability. Stability can be used during re-detection after therapy delivery;
-
-
in Biotronik devices a single lead device can provide dual chamber detection, whose decisional tree makes extensive use of stability in both scenarios of atrial rate equal or faster than ventricular rate. Stability is a programmable percentage of cycle length in single chamber devices, or an absolute programmable value in dual chamber. Each beat is weighted differently depending on the specific scenario of the decisional tree19,20;
-
-
in Sorin devices, stability is the first step in the discrimination pathway. Sophisticated computation of long intervals – though sporadic – is used to unmask regular AF. Arrhythmias with a regular interval are further analyzed by onset to rule out sinus tachycardia;
-
-
In the subcutaneous ICD, interval stability is part of the Conditional Shock Zone detection process, together with 3 other algorithms that work to avoid sensing the T wave, and check for changes in the QRS width and QRS amplitude to confirm VT.
Morphologic discriminators
They work by comparing the EGM of the rhythm entering the VT zone (VF also for Medtronic and NayaMed) with a reference template that is acquired during the patient's normal rhythm, and is periodically, automatically updated. None of these discriminators can be used after therapy has been delivered, because the residual voltage (polarization) on the coil causes marked changes (widening, polarity change) to the EGM being recorded, making the comparison with the template unreliable. There are marked differences across manufacturers:
-
-
Wavelet is used in Medtronic and NayaMed devices. The template is collected from the can to RV Coil EGM; it is automatically updated, but the amplitude range needs to be manually programmed to avoid signal clipping. VT is declared when 6 of the 8 beats before detection do not match with the template. The percentage of match with the template is programmable; shipment is 70% and reported to be optimal in the majority of cases, but reprogramming is needed in specific cases (less than 5% in my experience). From the Protecta release onward, Wavelet has been added to dual chamber discrimination on top of PR Logic;
-
-
Morphology Discrimination is used in St Jude Medical devices. Template was collected tip to ring in former releases, whereas it is available either from can to RV coil or tip to ring in the Ellipse release. The amplitude range is automatically set. In the old tip to ring releases VT is declared when 6 of the 12 beats before detection do not match the template. The percentage of match with the template is programmable; shipment is 60% and reported to be optimal in the majority of cases, but reprogramming was needed in the tip to ring only releases (10% of patients in my experience). With can to RV coil, VT is diagnosed when 8/10 intervals do not match; shipment matching percentage is 90%: both parameters are programmable. It has to be remembered that when all discriminators are turned ON, a logic as ANY, 2/3, or ALL needs to be defined for detection to occur: 2/3 has the best sensitivity/specificity ratio, although a single report found no difference compared to Morphology alone21;
-
-
Rhythm ID is used in Boston Scientific devices. It uses alignment of the can to coil EGM (eight points) to the Rate EGM to build the reference template. When alignment of the former is lost during a fast rhythm, non-match is detected. VT is declared when 8 of 10 beats match below 94% with the template. It used to be a non-programmable algorithm until the Incepta release, where match percentage is programmable: lowering the matching percentage from shipment is necessary to increase specificity for SVT detection;
-
-
Signal analysis is operated in the subcutaneous ICD in the “Conditional Shock zone” to confirm VT, based on changes of QRS width and amplitude. This zone should always be used, up to 240 bpm.
Atrial Rhythm analysis in dual chamber devices
The discrimination of SVTs from VT (VF also for Medtronic and NayaMed) is based on the relationship of atrial and ventricular EGMs. The functioning of combined chamber analysis is markedly different across manufacturers:
-
-
in Medtronic and NayaMed the algorithm named PR Logic consecutively classify beats relying on patterns that are based on the relative timing of the atrial EGM respect to the ventricular interval. By this way each beat is labeled as VT, VF, Sinus Tachycardia, SVT, Atrial Tachycardia, or Atrial Fibrillation. Detection of far-field R wave sensing is provided, and is part of the correct pattern identification. From the Protecta release onwards, wavelet has been added on top of PR Logic.
-
-
In St Jude Medical, Boston Scientific and Biotronik the relationship of atrial and ventricular rate is used for a rate branch analysis, where V rate > A rate qualifies VT, hence no other discrimination is applied. On the contrary, both V = A and V < A rate are managed stepwise by further discrimination with Morphologic discriminators, Stability, and Onset (not used in Boston, applied in a single scenario in Biotronik). Whereas in Boston Scientific and Biotronik these added algorithms work stepwise sequentially, in ST Jude Medical the discriminators work in pair with a programmable ANY or ALL logic in the 2 different settings: Onset and Morphology for V = A, Stability and Morphology for V < A.
-
-
In Sorin devices, stability is the entry discriminator to detect AF. Atrial to ventricular relationship is used for arrhythmia discrimination in the presence of a regular rhythm with an N:1 relationship to rule out VT. Onset is used in the setting of a 1:1 atrioventricular relationship, where sinus tachycardia is to be discriminated from VT with retrograde conduction. Chamber of origin is used to help discrimination of arrhythmias with sudden onset.
Conflicts of interest
The author has none to declare.
References
- 1.Moss A.J., Hall W.J., Cannom D.S. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.The AVID Investigators A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 3.Buxton A.E., Lee K.L., Fisher J.D. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 4.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [Erratum, N Engl J Med 2005;352:2146.] [DOI] [PubMed] [Google Scholar]
- 6.Bristow M.R., Saxon L.A., Boehmer J. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Poole J.E., Johnson G.W., Hellkamp A.S. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss A.J., Schuger C., Beck C.A. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 9.Kay G.N., Ellenbogen K.A. A clinical trial of ICD programming. N Engl J Med. 2013;368:964–966. doi: 10.1056/NEJMc1300614. [DOI] [PubMed] [Google Scholar]
- 10.Wathen M.S., DeGroot P.J., Sweeney M.O., PainFREE Rx II Investigators Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: pacing fast ventricular tachycardia reduces shock therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Johnson G., Hellkamp A.S. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation. J Am Coll Cardiol. 2013;61:2161–2168. doi: 10.1016/j.jacc.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clementy N., Pierre B., Lallemand B. Long-term follow-up on high-rate cut-off programming for implantable cardioverter defibrillators in primary prevention patients with left ventricular systolic dysfunction. Europace. 2012;14:968–974. doi: 10.1093/europace/eus028. [DOI] [PubMed] [Google Scholar]
- 13.Brodsky M.A., McAnulty J., Zipes D.P., AVID Investigators A history of heart failure predicts arrhythmia treatment efficacy: data from the antiarrhythmics versus implantable defibrillators (AVID) study. Am Heart J. 2006;152:724–730. doi: 10.1016/j.ahj.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Klein R.C., Raitt M.H., Wilkoff B.L., AVID Investigators Analysis of implantable cardioverter defibrillator therapy in the antiarrhythmics versus implantable defibrillators (AVID) trial. J Cardiovasc Electrophysiol. 2003;14:940–948. doi: 10.1046/j.1540-8167.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 15.Daubert J.P., Zareba W., Cannom D.S., MADIT II Investigators Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 16.Wilkoff B.L., Williamson B.D., Stern R.S., PREPARE Study Investigators Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Gasparini M., Menozzi C., Proclemer A. A simplified biventricular defibrillator with fixed long detection intervals reduces implantable cardioverter defibrillator (ICD) interventions and heart failure hospitalizations in patients with non-ischaemic cardiomyopathy implanted for primary prevention: the RELEVANT [Role of long dEtection window programming in patients with LEft VentriculAr dysfunction, non-ischemic eTiology in primary prevention treated with a biventricular ICD] study. Eur Heart J. 2009;30:2758–2767. doi: 10.1093/eurheartj/ehp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparini M., Proclemer A., Klersy C. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309:1903–1911. doi: 10.1001/jama.2013.4598. [DOI] [PubMed] [Google Scholar]
- 19.Stazi F., Mampieri M., Cardinale M. Implant and long-term evaluation of atrial signal amplification in a single-lead ICD. Pacing Clin Electrophysiol. 2012;35:1119–1125. doi: 10.1111/j.1540-8159.2012.03452.x. [DOI] [PubMed] [Google Scholar]
- 20.Sticherling C., Zabel M., Spencker S., for the ADRIA Investigators Comparison of a novel, single-lead atrial sensing system with a dual chamber implantable cardioverter-defibrillator system in patients without antibradycardia pacing indications. Circ Arrhythm Electrophysiol. 2011;4:56–63. doi: 10.1161/CIRCEP.110.958397. [DOI] [PubMed] [Google Scholar]
- 21.Boriani G., Occhetta E., Cesario S. Contribution of morphology discrimination algorithm for improving rhythm discrimination in slow and fast ventricular tachycardia zones in dual-chamber implantable cardioverter-defibrillators. Europace. 2008;10:918–925. doi: 10.1093/europace/eun146. [DOI] [PubMed] [Google Scholar]
- 22.Gold M.R., Ahmad S., Browne K. Prospective comparison of discrimination algorithms to prevent inappropriate ICD therapy: primary results of the Rhythm ID Going Head to Head Trial. Heart Rhythm. 2012;9:370–377. doi: 10.1016/j.hrthm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Weiss R., Knight B.P., Gold M.R. Safety and efficacy of a totally subcutaneous implantable cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 24.Gold M.R., Theuns D.A., Knight B.P. Head-to-Head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012;23:359–366. doi: 10.1111/j.1540-8167.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 25.Diemberger I., Martignani C., Biffi C. Arrhythmia discrimination by physician and defibrillator: importance of atrial channel. Int J Cardiol. 2012;154:134–140. doi: 10.1016/j.ijcard.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Bänsch D., Steffgen F., Grönefeld G. The 1+1 trial: a prospective trial of a dual-versus a single-chamber implantable defibrillator in patients with slow ventricular tachycardias. Circulation. 2004;110:1022–1029. doi: 10.1161/01.CIR.0000140259.16185.7D. [DOI] [PubMed] [Google Scholar]
- 27.Theuns D.A., Rivero-Ayerza M., Boersma E. Prevention of inappropriate therapy in implantable defibrillators: a meta-analysis of clinical trials comparing single-chamber and dual-chamber arrhythmia discrimination algorithms. Int J Cardiol. 2008;125:352–357. doi: 10.1016/j.ijcard.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 28.Friedman P.A., McClelland R.L., Bamlet W.R. Dual-chamber versus single-chamber detection Enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113:2871–2879. doi: 10.1161/CIRCULATIONAHA.105.594531. [DOI] [PubMed] [Google Scholar]
- 29.Theuns D.A., Rivero-Ayerza M., Goedhart D.M. Morphology discrimination in implantable cardioverter–defibrillators: consistency of template match percentage during atrial tachyarrhythmias at different heart rates. Europace. 2008;10:1060–1066. doi: 10.1093/europace/eun194. [DOI] [PubMed] [Google Scholar]
- 30.Jarman J.W.E., Lascelles K., Wong T. Clinical experience of entirely subcutaneous implantable cardioverter–defibrillators in children and adults: cause for caution. Eur Heart J. 2012;33:1351–1359. doi: 10.1093/eurheartj/ehs017. [DOI] [PubMed] [Google Scholar]
- 31.Wilkoff B.L., Ousdigian K.T., Sterns L.D., EMPIRIC Trial Investigators A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the prospective randomized multicenter EMPIRIC trial. J Am Coll Cardiol. 2006;48:330–339. doi: 10.1016/j.jacc.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Kneller J., Delacey W., Wood M.A. Detection of a Riata™ insulation failure by the Medtronic Lead Integrity Alert™. Europace. 2012;14:1215–1216. doi: 10.1093/europace/eus021. [DOI] [PubMed] [Google Scholar]
- 33.Santini M., Lunati M., Defaye P. Prospective multicenter randomized trial of fast ventricular tachycardia termination by prolonged versus conventional anti-tachyarrhythmia burst pacing in implantable cardioverter-defibrillator patients-Atp DeliVery for pAiNless ICD thErapy (ADVANCE-D) Trial results. J Interv Card Electrophysiol. 2010;27:127–135. doi: 10.1007/s10840-009-9454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasparini M., Anselme F., Clementy J., ADVANCE CRT-D Investigators BIVentricular versus right ventricular antitachycardia pacing to terminate ventricular tachyarrhythmias in patients receiving cardiac resynchronization therapy: the ADVANCE CRT-D Trial. Am Heart J. 2010;159:1116–1123. doi: 10.1016/j.ahj.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Anderson K.P. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians, industry and government. J Interv Card Electrophysiol. 2005;14:71–78. doi: 10.1007/s10840-005-4547-9. [DOI] [PubMed] [Google Scholar]
- 36.Kroll M.W., Schwab J.O. Achieving low defibrillation thresholds at implant: pharmacological influences, RV coil polarity and position, SVC coil usage and positioning, pulse width settings, and the azygous vein. Fundam Clin Pharmacol. 2010;24:561–573. doi: 10.1111/j.1472-8206.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow C.D., Russo A.M., Degroot P.J. The dilemma of ICD implant testing. Pacing Clin Electrophysiol. 2007;30:675–700. doi: 10.1111/j.1540-8159.2007.00730.x. [DOI] [PubMed] [Google Scholar]
- 38.Gold M.R., Higgins S., Klein R. Efficacy and temporal stability of reduced safety margins for ventricular defibrillation: primary results from the low energy safety study (LESS) Circulation. 2002;105:2043–2048. doi: 10.1161/01.cir.0000015508.59749.f5. [DOI] [PubMed] [Google Scholar]
- 39.Diemberger I., Mazzotti A., Biffi M. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices. 2013;10:551–573. doi: 10.1586/17434440.2013.811837. [DOI] [PubMed] [Google Scholar]
- 40.Ricci R.P., Morichelli L., Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–56. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 41.Boriani G., Santini M., Lunati M., Italian ClinicalService Project Improving thromboprophylaxis using atrial fibrillation diagnostic capabilities in implantable cardioverter-defibrillators: the multicentre Italian ANGELS of AF Project. Circ Cardiovasc Qual Outcomes. 2012;5:182–188. doi: 10.1161/CIRCOUTCOMES.111.964205. [DOI] [PubMed] [Google Scholar]
- 42.Boriani G., Gasparini M., Landolina M., ClinicalService cardiac centres Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:868–877. doi: 10.1093/eurjhf/hfr046. [DOI] [PubMed] [Google Scholar]
- 43.Whellan D.J., Ousdigian K.T., Al-Khatib S.M., PARTNERS Study Investigators Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. doi: 10.1016/j.jacc.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 44.Abraham W.T., Compton S., Haas G., FAST Study Investigators Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST) Congest Heart Fail. 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 45.van Veldhuisen D.J., Braunschweig F., Conraads V., DOT-HF Investigators Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 46.Landolina M., Perego G.B., Lunati M. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125:2985–2992. doi: 10.1161/CIRCULATIONAHA.111.088971. [DOI] [PubMed] [Google Scholar]
- 47.Boriani G., Da Costa A., Ricci R.P., MORE-CARE Investigators The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res. 2013;15:e167. doi: 10.2196/jmir.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hindricks G. ESC Meeting; Amsterdam, The Netherlands: 2013 September 1. The IN-TIME trial preliminary results. [Google Scholar]
- 49.Marquis VR. 7230CX Performance Report. [Data on file. Medtronic CRDM Product Performance eSource]
- 50.Analysis of Aggregate LATITUDE® Patient Management system data: From over 100,400 patients as of April 2013. [Boston Scientific Corporation]


